Statins and cardiovascular diseases: the multiple effects of
lipid-lowering therapy by statins
U. Rauch
a,b, J.I. Osende
a,b, J.H. Chesebro
b, V. Fuster
b, D.A. Vorchheimer
b,
K. Harris
b, P. Harris
b, D.A. Sandler
b, J.T. Fallon
b,c, S. Jayaraman
a,
J.J. Badimon
a,b,*
aThe Cardio
6ascular Biology Research Laboratory,The Zena and Michael A.Wiener Cardio6ascular Institute, Mount Sinai School Of Medicine,One Gusta6e L.Le6y Place,Box1030,New York,NY-10029,USA
bThe Zena and Michael A.Wiener Cardio6ascular Institute,New York NY-10029,USA cDepartment of Pathology,Mount Sinai School of Medicine,New York NY-10029,USA
Received 12 October 1999; received in revised form 20 December 1999; accepted 19 January 2000
Abstract
Cholesterol lowering involving different therapies improves the clinical outcome of patients. To define the underlying pathomechanism, we studied whether treatment with statins was associated with changes in blood thrombogenicity, endothelial dysfunction and soluble adhesion molecule levels. Fifty hypercholesterolemic patients were treated with pravastatin (40 mg/day, n=24) or simvastatin (20 mg/day,n=26). Lipid profile and blood thrombogenicity were assessed in all patients before and after 3 months of cholesterol reducing therapy. Blood thrombogenicity was assessed as thrombus formation, perfusing non-anticoagu-lated blood directly from the patients’ vein through the Badimon perfusion chamber (shear rate 1690/s). Endothelial-dependent vasomotor response was tested by laser-Doppler flowmeter. Soluble adhesion molecule level were measured by ELISA. Total and LDL cholesterol were reduced in the two treatment groups by statin therapy. Statin therapy was associated with a significant reduction in blood thrombogenicity and endothelium-dependent vasoresponse. No differences were observed between simvastatin or pravastatin treatment. Lipid lowering by statins had no effect on plasma levels of fibrinogen, sL-selectin, sP-selectin and sICAM-1 antigen. Cholesterol lowering by both statins reduced the increased blood reactivity and endothelial dysfunction present under hypercholesterolemia. The multiple effects of lipid lowering therapy by statins may explain the benefits observed in recent epidemiological trials. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Statin; Hyperlipidemia; Thrombosis; Endothelium; Atherosclerosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
Hypercholesterolemia — the major risk factors for the development of atherosclerotic disease [1 – 4] is asso-ciated with increased deposition of lipids and mono-cytes/macrophages within the arterial wall, endothelial dysfunction and vasoconstriction, enhanced platelet re-activity, and hypercoagulability [5,6]. Reduction of serum cholesterol levels by different interventions in-cluding diet, exercise, lifestyle changes or pharmacolog-ical approaches is associated with a significant
reduction in cardiovascular mortality and morbidity [3 – 6]. Recently, the effectiveness of a new class of powerful hypolipidemic agents, the statins, has been tested in several large clinical trials. These trials showed significant improvement in clinical outcomes of patients with and without coronary artery disease [7 – 12]. These observations were applicable to primary and secondary prevention of coronary events in both, hyper- and normocholesterolemic populations [4,6 – 12]. Statin treatment was associated with a consistent reduction in coronary events of :30 – 40% [13,14].
Angiographic trials assessing the effect of lipid lower-ing treatment on coronary atherosclerosis showed only a small reduction in coronary stenosis [14,15]. The clinical benefits observed in these trials significantly * Corresponding author. Tel.: +1-212-2418484; fax: +
1-212-4266962.
E-mail address:[email protected] (J.J. Badimon).
exceed the expectations based on alterations in vessel lumen diameter. This suggested that lipid-reducing ther-apy might have additional effects that would explain the discrepancy between clinical benefits and lack of significant lesion regression [3 – 6]. Experimental and clinical studies have indicated a relationship between hyperlipidemia and increased blood thrombogenicity [15 – 21]. It was suggested that correction of hyper-cholesterolemia by pravastatin could normalize blood thrombogenicity [20]. However, conflicting findings on the effect of different statins on thrombosis have been reported by other authors [22,23]. In addition, hyper-lipidemia has been associated with an impaired en-dothelium-dependent vasoresponse [24] and cholesterol lowering by the use of specific statins has been reported to improve endothelial dysfunction [3,24]. The goal of our study was to delineate whether lipid lowering by statins, irrespective of the specific statin used, was associated with changes in blood thrombogenicity and endothelial dysfunction in the same hyperlipidemic pa-tient population. Furthermore, we studied whether changes in thrombogenicity and endothelial function were associated with alterations in circulating soluble adhesion molecule levels.
The working hypothesis of this study was that (1) the reduction of plasma lipid levels reduces blood thrombo-genicity and endothelial dysfunction and (2) that the decreased thrombogenicity of blood and endothelium is associated with a reduction in circulating soluble adhe-sion molecule levels. Given the number of pathogenic processes modulated by plasma lipid levels, we further hypothesized that the significant benefits observed in clinical trials with statins might rather be a result of the significant reduction of plasma cholesterol level than a specific effect associated with the administration of one specific statin. To prove this hypothesis, hyperlipidemic patients were randomized into two groups receiving one of two most frequently prescribed statins for lipid reduction.
This study demonstrates multiple effects of choles-terol reducing therapy by the statin class on blood thrombogenicity and endothelial dysfunction in the same patient group under hyperlipidemic conditions. The combined reduction of blood thrombogenicity and endothelial dysfunction observed after lipid lowering by statins may be responsible for the clinical improvements reported in several large epidemiological trials.
2. Material and methods
2.1. Patient population
The study population included hypercholesterolemic patients (n=50). Hypercholesterolemia was defined as LDL-cholesterol level \120 mg/dl. Twenty-six of the
patients had documented coronary artery disease and/
or peripheral artery disease. The presence of coronary artery disease was documented by coronary angiogra-phy (stenosis \50%), history of previous myocardial infarction or typical angina pectoris with typical changes in treadmill ECG. Exclusion criteria included malignant diseases, hematological disorders, severe hy-pertension, heparin or warfarin therapy and exposure to any lipid lowering therapy within the 8 weeks prior to randomization. The selected patients were instructed to follow a low cholesterol, low fat diet and then randomized into two treatment groups. Group I was assigned to receive pravastatin, 40 mg per day and Group II simvastatin, 20 mg per day for a period of 3 months. All patients were advised to take the medica-tion at evening time. Other drugs the patients were currently receiving remained unchanged. The study was approved by the Institutional Review Board, and all patients signed informed consent.
The following routine laboratory parameters were determined at baseline and at the follow-up visits: lipid profile, white blood cell count, platelet count, partial thromboplastin time (aPTT), and fibrinogen level. In addition, citrated plasma samples, obtained pre and post treatment with simvastatin or pravastatin, were aliquoted and frozen for the analysis of sLselectin, sP-selectin and sICAM-1 (R&D systems, Minneapolis, MN). The coefficient of variation for all these parame-ters was B6% in all instances. All assays were per-formed according to the manufacturer’s instructions.
2.2. Assessment of blood thrombogenicity
Blood thrombogenicity was evaluated at baseline and 3-months after lipid-lowering therapy in both groups of patients. This experimental design allows for each pa-tient to serve as his/her own control. Effect of lipid lowering on blood thrombogenicity was assessed as changes in the thrombus area formed in an ex-vivo perfusion chamber [25]. The ex-vivo perfusion chamber mimics the in vivo local rheologic conditions that de-velop in a mildly stenosed coronary artery. In this perfusion system, thrombus formation is triggered by the exposition of components of the subendothelial vessel wall to the flowing blood. Blood from hyperc-holesterolemic patients, prior to and after statin treat-ment, was allowed to circulate through the perfusion system. Blood reactivity was assessed by morphometric analysis of thrombus formed on the thrombogenic sub-strate. The description, validity and reliability of the Badimon perfusion system to study thrombus forma-tion on defined thrombogenic substrates under stable rheological conditions have been previously reported [25,26].
brief, a 19-gauge needle was carefully inserted into an antecubital vein. After discarding the first 5 ml of blood, the needle was connected to three Badimon perfusion chambers placed in series to minimize blood loss. Native, non-anticoagulated, blood was directly perfused from the antecubital vein through the perfu-sion chambers at a constant flow maintained by using a peristaltic pump (Masterflex, Cole- Parmer Instru-ments) distal to the chambers. The perfusion chamber consists of a cylindrical flow channel (1 mm diameter and 2.5 cm length) that allows the blood to flow over the thrombogenic substrate. All the perfusions were performed for a period of 5 minutes at a flow rate of 10 ml/min (calculated shear rate of 1690/s; Reynolds num-ber 60; average blood velocity 21.2 cm/s). The selected dynamic conditions modeled the local rheology devel-oping on mildly stenosed coronary arteries. Our previ-ous work demonstrated that these rheologic conditions result in consistent levels of platelet deposition.
To mimic the in vivo situation of severe arterial injury associated with coronary interventions, porcine aortic tunica media was used as the substrate to trigger thrombus formation. Segments of porcine aorta were cut into segments (2.8×0.8cm) and surgically prepared to expose the deeper components of the arterial wall as previously described [25,26].
The histological assessment of thrombus formation was performed by histomorphometry as previously de-scribed [25]. In brief, the perfused media segments were fixed in 4% paraformaldehyde. Six 2 mm cross sections were removed from the perfused media segment: two in the proximal, middle, and distal thirds of the exposed vessel segments. These tissue sections were then embed-ded in paraffin and processed for histology. Sections (5 mm) were cut and stained with a combined Masson’s trichrome-elastin to visualize the total thrombus formed on the exposed substrates.
Morphometric analysis of thrombus was conducted at 400-fold magnification. Images were digitized with a Sony DKC-5000 camera using Adobe Photoshop 4.0 software on a PowerMacIntosh 8500 computer. Throm-bus area on each section was measured by computer-ized planimetry using NIH Image 1.6 software. The results are given as the average of the analyzed sections per perfused media segments, and the results from the three perfusion chamber were averaged to obtain the overall thrombus formation for each patient. The mea-surements were independently done by two of the inves-tigators, who were blinded to the therapy assignment.
2.3. Endothelium-dependent 6asomotor response
Skin blood flow is controlled both by reflexes and local factors. Cutaneous vascular response to local warming is an endothelium-mediated mechanism [27]. Therefore, measurements of microcirculatory blood
flow to heat have been used as marker of endothelium function. Vascular responses to local heat were recorded in a subgroup of 11 hyperlipidemic patients at baseline and after 3-months on lipid lowering therapy. A laser-Doppler flowmeter with a combined laser Doppler and thermostatic probe (Perimed, model PF 4001 Master, Perimed, Ja¨rfa¨lla, Sweden) was used to assess the microcirculatory blood flow [28]. Measure-ments were made on the dorsum of the hand with the probe fixed to the skin by a double-sided adhesive ring. This technique shows an intraindividual variability of B2%. Results are expressed as arbitrary perfusion units (PU) and are an index of the blood flow [29].
All measurements were made in a temperature con-trolled room (19 – 22°C) with the patients lying in supine position. Skin blood flow was continuously recorded during a baseline period with the probe set to 33°C (neutral skin temperature) and during local skin heating to 44°C. After 3 min of monitoring baseline perfusion, blood flow was measured for 10 min over the heating period. The typical response is a maximum vasodilatation response after 3 – 5 min of skin heating over 42°C. Comparisons were made between the mean baseline flow and the area under the curve of the time course recorded during 3 min at 33°C (baseline perfu-sion) and 5 min of heating over 42°C (endothelium-de-pendent vasomotor response to heat) after 5 min heating period. Results (mean9SEM) are expressed as perfusion units (PU) and perfusion units x min.
2.4. Data analysis
Differences between the treatment groups were as-sessed using ANOVA. Comparisons pre and post ther-apy within the same group were analyzed using Student’s paired t-test. If not otherwise indicated, data are given as mean9SD for independent measurements. The two tailed significance threshold was set at PB 0.05.
3. Results
3.1. Study population
Table 1
Patient population
Group I Group II
Age (years) 6292 5892
Hypertension 13 14
Diabetes mellitus 8 12
14 13
Smoking
12
Family history CHD 11
0 Cerebral ischemic disease 0
4 4
Peripheral artery disease
5
CAGB 3
Angioplasty 7 7
3.2. Blood thrombogenicity
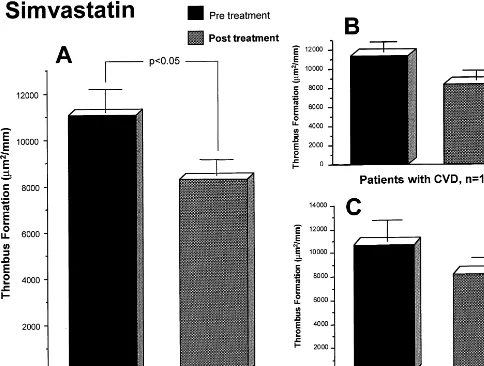
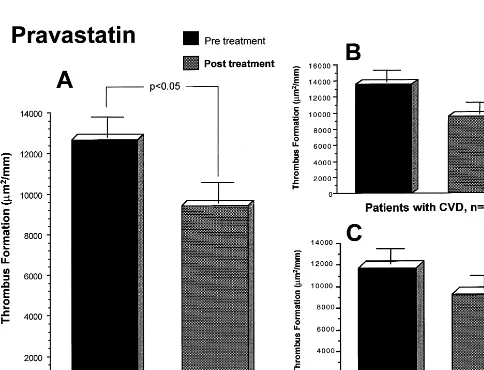
Baseline blood thrombogenicity, evaluated by the thrombus area formed on the perfused substrates, was similar in both groups. A representative photomi-crograph of thrombus formation at baseline and 3-months after statin treatment is shown in Fig. 1. The simvastatin group showed a significant reduction in thrombus formation (11 0679980 vs 82769728 mm2/ mm baseline and post-treatment, respectively; PB 0.05). Similar reduction in thrombus formation took place in the pravastatin group (12 64891023 vs 94119992 mm2/mm at baseline and 3-months post treatment, respectively; PB0.05). No significant differ-ence in thrombus formation was found between the two
Table 2
Plasma lipid profile and plasma levels of fibrinogen, sP-selectin, sL-selectin, CAM-1 of the two patient groups at baseline and after 3-months with statinsa
Sinvastain group Pravastatin group
Baseline Post treatment Baseline Post treatment
20298* 251910
Total cholesterol (mg/dl) 17199* 24698
151916 141911
Triglycerides (mg/dl) 123913 165919
4191.9 4192.0 4492.2
HDL cholesterol (mg/dl) 4091.9
17197 12897* 11097*
LDL cholesterol (mg/dl) 184910
4.890.3 6.390.4
4.490.3* Total/HDL cholestrol ratio 6.690.4
LDL/HDL cholesterol ratio 4.890.3 2.890.2* 4.490.3 3.090.2*
263910 243916
Fibrinogen (mg/dl) 255913 239919
105917 115915 130930 129922
sP-selectin (ng/ml)
738931
sL selectin (ng/ml) 834942 778932 753930
5493 4893 4795
sICAM-1 (ng/ml) 5395
aData is expressed as mean9SD.
*PB0.05 versus baseline values.
Fig. 2. Values of thrombus formation corresponding to the Simvastatin group. A.All patients.B. Subgroup of patients with cardiovascular disease and,C.Subgroup of patients without cardiovascular disease. Data expressed asmm2/mm
statin-treated groups. The antithrombotic effect of lipid reduction was still present when the groups were subdi-vided into patients with and without cardiovascular disease (CVD) for both groups (Figs. 2 and 3). The group of patients with CVD in the Simvastatin group (n=14) showed values of 11 37391087 mm2/mm at baseline and 836891061mm2/mm at 3-months, respec-tively, while the group of patients without CVD (n=
12) had 10 71091755 mm2/mm at baseline and 817091027mm2/mm at 3-months, respectively. Values of thrombus formation within the pravastatin group were 13 60791444 and 957391447 mm2/mm at base-line and 3-months, respectively for the CVD group; and 11 68991457 mm2/mm at baseline and 925191419 mm2/mm at 3-months in the non-CVD group. The lipid lowering effect of the two statins used in our study was associated with an :20 – 25% reduction in thrombus formation as compared with the baseline values.
A correlation between thrombus formation and total cholesterol/HDL cholesterol ratio was calculated to further assess the relation between circulating plasma
cholesterol and blood thrombogenicity. A significant relationship between amount of thrombus and total cholesterol/HDL cholesterol ratio was found consider-ing the measurements pre and post lipid lowerconsider-ing treat-ment (correlation coefficient r=0.410, P50.001, Fig. 4).
3.3. Endothelium-dependent 6asomotor response (see
Table 3)
respec-tively; P50.01). These observations suggest an im-provement of the endothelium-dependent vasoresponse by the hypolipidemic treatment. Similar observations were obtained when analyzing the areas under the curve at the different time/points and conditions.
3.4. Soluble adhesion molecule le6els
Treatment with statins did not induce any significant changes in the plasma levels of sP-selectin, sL-selectin or sICAM-1 between baseline and post-treatment mea-surements (Table 2).
In addition, the treatment with statins was not asso-ciated with any significant effect on white blood cell count, platelet count, activated partial thromboplastin time (aPTT) or plasma fibrinogen.
4. Discussion
This study demonstrates that the reduction of plasma cholesterol levels by statins reduces the high blood thrombogenicity observed in the same group of patients under hyperlipidemic conditions. We conclude that the reduction of blood thrombogenicity and endothelial activity may be mechanisms by which cholesterol lower-ing through statins accounts for the beneficial effects on CHD observed in several large epidemiological trials. Epidemiological evidence has shown the relationship between plasma total cholesterol and mortality rate from CHD [2 – 12]. A variety of mechanisms have been proposed to understand the effect of hypercholes-terolemia on atherosclerosis and thrombosis [2 – 6]. Acute thrombus formation depends not only on the
Fig. 4. Correlation between total cholesterol/HDL cholesterol ratio and amount of thrombus formation. Lipid levels and thrombus formation were measured pre and post lipid reducing therapy (n= 100 measurements for 50 patients, respectively). R, correlation coeffi-cient
as lipid lowering agent. The effect of placebo treatment on cholesterol level and thrombus formation has also been tested in the same ex vivo model system and shear stress conditions that we used here [35]. Lipid plasma levels and thrombus formation were not altered when a placebo instead of pravastatin was given [35]. In our study, both statins, simvastatin and pravastatin, in-duced a significant hypolipidemic effect that was associ-ated with similar reduction of blood thrombogenicity in the respective group. Therefore, we conclude that the reduction of blood thrombogenicity could be one of the mechanisms by which cholesterol lowering by statins reduces the incidence of acute thrombotic events.
It has been suggested that endothelial dysfunction plays a major role, not only in the pathogenesis of atherosclerosis but also in its ischaemic manifestations. Therefore, normalization of the dysfunctional endothe-lium may restore its antiatherogenic properties. There are evidences that endothelial dysfunction is often im-proved by cholesterol lowering [24,36] and it seems to be mediated by an increased bioavailability of nitric oxide [37]. To define whether the described lipid lower-ing effect of statins was the only mechanism responsible for the observed antithrombotic effect, we studied the effects of lipid reduction by statins on endothelial de-pendent vasoresponse in the same patient population. As described by others, we observed a significantly impaired endothelium-dependent vasoresponse in our hyperlipidemic population after lipid lowering therapy compared to baseline. Furthermore, a marked improve-ment of the endothelial-dependent vasoresponse was associated to the lipid lowering effect induced by the statins.
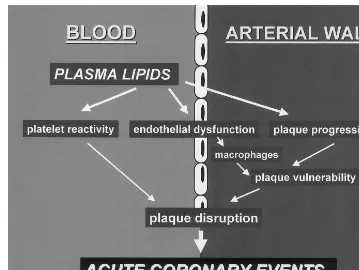
An additional objective of the study was to assess whether the observed normalization of blood thrombo-genicity and endothelial function by statin therapy would be associated with changes in plasma levels of adhesive proteins involved in the interactions between blood cells and endothelium. We determined plasma levels of sP-selectin, sL-selectin and sICAM-1 at base-line and 3-months after statin administration. Treat-ment with statins did not modify levels of adhesive proteins in the circulating blood. We speculate that the study population tested here may have been to small to identify differences. Since only the circulating soluble antigen levels were determined in this study, it cannot be excluded that surface-associated selectin and ICAM-1 expression on endothelial cells and/or circulating blood cells may have been affected by statin therapy. As indicated by Fig. 5, hyperlipidemia is associated with an increased platelet reactivity, endothelial dys-function and plaque progression, altogether leading to an increased risk of acute thrombotic events. Our data on blood thrombogenicity and endothelial function were simultaneously obtained from the same individu-als before and after lipid lowering therapy with two different statins. Our data and the observations of Table 3
Effect of lipid lowering by statins on endothelial activity at baseline and after 3-months with statinsa
Pre-treatment 3-month statins
Hyperlipidemic patients 6.590.9
Basal values 8.190.9
81.5912.3*,**
Heat induced 49.795.7*
* PB0.05 versus basal values. ** PB0.05 versus pre-treatment.
aResults expressed as PU.
local alterations of the arterial wall, but it is also modulated by the thrombogenicity of the circulating blood [30 – 33]. Our group and others have recently shown that the circulating platelets are activated and hyperactive in hypercholesterolemia [16 – 20]. In addi-tion, it has been reported that reducing cholesterol level by pravastatin decreases thrombus formation on deeply injured arterial wall [20]. Whether this antithrombotic effect was a result of the cholesterol reduction or a specific effect of the specific statin used was questioned [21,22]. On the other hand, it has recently been demon-strated that lipid reducing therapy by simvastatin markedly depressed thrombin generation and that this effect on thrombin generation was not dependent on the presence of aspirin in patients with hypercholes-terolemia [34].
Fig. 5. Multiple effects of hyperlipidemia on blood and arterial wall. Implications for acute coronary events.
others suggest that multiple biological systems may be affected by lipid lowering therapy with statins. This study strongly supports the hypothesis that the pleiotropic effects of lipid lowering therapy by statins may explain the significant clinical benefits of statin therapy observed in several large epidemiological trials [4 – 6,38 – 41]. Thus, the observed improvement in clini-cal outcome after statin therapy seems rather to be a result of interfering with multiple pathogenic parame-ters present under hypercholesterolemic conditions (Fig. 5) than the intrinsic effect of one specific statin. The major limitation of our study is that we can not rule out whether all statins as a drug class have a direct effect on thrombogenicity and endothelial function or whether the observed changes are mediated by the lipid reduction only.
In summary, we conclude that the reduction of blood thrombogenicity and endothelial activity may be mech-anisms by which cholesterol-reducing therapy with statins improves the clinical outcome of patients with hypercholesterolemia.
Acknowledgements
This work was supported in part by grants from the Spanish Society of Cardiology (to Dr. J Osende) and the followings grants from the National Institutes of Health (NIH P50 HL54469 to Dr J.J. Badimon and Dr J.T. Fallon) and (NIH 5 MO1 RR 00071) to the Mount
Sinai General Clinical Research Center. The authors would like to express their appreciation to the excellent work, care and dedication of the nurse coordinators Stella Palencia and Ida Guzman.
References
[1] National Heart, Lung, and Blood Institute Fact Book, Fiscal Year 1997. Bethesda, Md: National Heart, Lung, and Blood Institute, National Institute of Health; 1998.
[2] Tracy RP, Tracy PB. New views on the relationship of plasma lipids to cardiovascular disease. Circulation 1997;95:1347 – 8.
[3] Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. National cholesterol education program: second report of the expert panel on detection, evalua-tion, and treatment of high blood cholesterol in adults (adult treatment panel II). Circulation 1994;89:1329 – 445.
[4] Cleeman JI, Lenfant C. The national cholesterol education pro-gram: progress and prospects. J Am Med Acad 1998;280:2099 – 104.
[5] Archbold RA, Timmis AD. Cholesterol lowering and coronary artery disease: mechanisms of risk reduction. Heart 1998;80:543 – 7.
[6] Gould AL, Rossouw JE, Santanello NC, Heyse JF, Furberg CD. Cholesterol reduction yields clinical benefit. Impact of statin trials. Circulation 1998;97:946 – 52.
[7] Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383 – 9.
[9] Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravas-tatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001 – 9.
[10] Downs JR, Clearfield M, Weis S, et al. AM for the AFCAPS/ TexCAPS Research Group. Primary prevention of acute coro-nary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. J Am Med Am 1998;279:1615 – 22.
[11] The Long-Term Intervention with Pravastatin in Ischaemic Dis-ease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;1995(333):1301 – 7.
[12] Jukema JW, Bruschke AV, vanBowen AJ, et al. Effect of lipid lowering by pravastatin on progression and regression of coro-nary disease in symptomatic men with normal to moderately elevated cholesterol levels. The Regression Growth Evaluation Statin Study. (REGRESS). Circulation 1995;91:2528 – 40. [13] Herbert PR, Gaziano JM, Chan KS, Hennekens CH.
Choles-terol lowering with statin drugs, risk of stroke, and total mortal-ity: an overview of randomized trials. J Am Med Acad 1997;278:313 – 21.
[14] Gotto AM. Cholesterol management in theory and practice. Circulation 1997;96:4424 – 30.
[15] Fuster V, Badimon JJ. Regression or stabilization of atheroscle-rosis means regression or stabilization of what we don’t see in the arteriogram. Eur Heart J 1995;16:6 – 12.
[16] Carvalho AC, Colman RW, Lees RS. Platelet function in hyper-lipoproteinemia. New Engl J Med 1974;290:434 – 8.
[17] Badimon JJ, Badimon L, Turitto VT, Fuster V. Platelet deposi-tion at high shear rates is enhanced by high plasma cholesterol levels: in vivo study in the rabbit model. Arterioscler Thromb 1991;11:395 – 402.
[18] Cadroy Y, Lemozy S, Diquelou A, et al., Human type IIa hyperlipoproteinemia enhances platelet-collagen adhesion in flowing nonanticoagulated blood, Arterioscler Thromb 1993;13:1650 – 3.
[19] Davi G, Averna M, Catalano I, et al. Increased thromboxane biosynthesis in type IIa hypercholesterolemia. Circulation 1992;85:1792 – 8.
[20] Lacoste L, Lam JYT, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease: correction of the increased thrombogenic potential with cholesterol reduction. Circulation 1995;92:3172 – 7.
[21] Broijersen A, Hamsten A, Eriksson M, Angelin B, Hjemdahl P. Platelet activity in vivo in hyperlipoproteinemia — importance of combined hyperlipidemia. Thromb Haemost 1998;79:268 – 75. [22] Aviram M, Hussein O, Rosenblat M, Schlezinger S, Hayek T, Keidar S. Interactions of platelets, macrophages, and lipo-proteins in hypercholesterolemia: antiatherogenic effects of HMG-CoA reductase inhibitor therapy. Cardiovasc Pharmacol 1998;31:39 – 45.
[23] Broijersen A, Eriksson M, Leijd B, Angelin B, Hjemdahl P. No influence of simvastatin treatment on platelet function in vivo in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol 1997;17:273 – 8.
[24] Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on
endothelium-dependent coronary vasomotion. N Engl J Med 1995;332:488 – 93.
[25] Dangas G, Badimon JJ, Coller BS, et al. Administration of abciximab during percutaneous coronary intervention reduces both ex vivo platelet thrombus formation and fibrin deposition. Implications for a potential anticoagulant effect of abciximab. Arterioscler Thromb Vasc Biol 1998;18:1342 – 9.
[26] Badimon L, Turitto V, Rosemark J, Badimon JJ, Fuster V. Characterization of a tubular flow chamber for studying platelet interaction with biological and prosthetic materials: deposition of indium-labeled platelets on collagen, subendothelium, and goretex. J Lab Clin Med 1987;110:706 – 18.
[27] Pergola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol 1993;265:H785 – 92.
[28] Nilsson GE, Tenland T, Oberg P. Evaluation of a laser Doppler flow meter for measurement of tissue blood flow. EEE Trans Biomed Engl 1980;27:597 – 604.
[29] Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature 1975;254:56 – 8.
[30] Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogen-esis of coronary artery disease, parts 1 and 2. N Engl J Med 1992;326:310 – 8.
[31] Badimon JJ, Fuster V, Chesebro J, Badimon L. Coronary atherosclerosis: a multifactorial disease. Circulation 1993;87:3 – 16.
[32] Falk E, Shah PK, Fuster V. Coronary Plaque disruption. Circu-lation 1995;92:657 – 71.
[33] Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276 – 82.
[34] Szczeklik A, Musial J, Undas A, et al. Jankowski M. Inhibition of thrombin generation by simvastatin and lack of additive effects of aspirin in patients with marked hypercholesterolemia. J Am Coll Cardiol 1999;33:1286 – 93.
[35] Dangas G, Badimon JJ, Smith DA, Unger AH, Levine D, Shao JH, Meraj P, Fier C, Fallon JT, Ambrose JA. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. J. Am. Coll. Cardiol. 1999;33:1294 – 304.
[36] O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coen-zyme A reductase inhibitor improves endothelial function within 1 month. Circulation 1997;95:1126 – 31.
[37] John S, Schlaich M, Langelfeld M, et al. Increased bioavailabil-ity of nitric oxide after lipid-lowering therapy in hypercholes-terolemic patients: a randomized, placebo-controlled, double-blind study. Circulation 1998;98:211 – 6.
[38] Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet 1996;348:1079 – 82.
[39] Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins. Implications for cardiovascular event reduction. J Am Med Acad 1998;279:1643 – 50.
[40] Libby P, Aikawa M. New Insights into plaque stabilisation by lipid lowering. Drugs 1998;56:9 – 13.
[41] Bellosta S, Via D, Canavesi M, et al. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscl Thromb Vasc Biol 1998;18:1671 – 8.