Short Communication
Effect of growth regulators on postharvest characteristics of
Zantedeschia aethiopica
Ewa Skutnik
a, Aleksandra Lukaszewska
a, Margrethe Serek
b,c,*,
Julita Rabiza
aaDepartment of Ornamental Plants,Faculty of Horticulture,Warsaw Agricultural Uni
6ersity,Nowoursynowska166,
02-787Warszawa,Poland
bDepartment of Agricultural Sciences,Horticulture,The Royal Veterinary and Agricultural Uni6ersity,Thor6aldsens6ej57,
1871Frederiksberg C.,Denmark
cDepartment of Horticulture,Floriculture,Uni6ersity of Hano6er,Herrenhauser Str.2,30049Hano6er,Germany Received 19 April 2000; accepted 31 July 2000
Abstract
Treating cut leaves of Zantedeschia aethiopica with aqueous solutions of gibberellic acid (GA3) considerably
extended their display life, whether applied as a 24 h pulse treatment, or as a brief postharvest dip. In contrast, a standard preservative solution used to prolong the longevity of cut flowers (8-HQC+sucrose) was deleterious to
Zantedeschia foliage, reducing display life several fold from that of the water control. After harvest, chlorophyll content of the leaves fell more or less rapidly depending on the postharvest treatment, falling most rapidly in leaves placed in the ‘preservative’ solution and relatively gradually in leaves that had been treated with GA3. Leaf senescence
was also associated with changes in characteristics of the cell sap, namely increased pH and electrical conductivity, parameters that may be a useful and rapid means of determining the senescence status of cut flowers during marketing. © 2001 Elsevier Science B.V. All rights reserved.
Keywords:BA; Benzyladenine; Cell sap pH; Cut leaves; Electric conductivity; GA3; Gibberellic acid; Longevity; Senescence; Total chlorophyll
www.elsevier.com/locate/postharvbio
1. Introduction
Plant species grown for florists’ greens are gain-ing importance as the foliage component becomes an increasingly important part of cut flower ar-rangements. Not only are new plants being tested but also researchers are seeking treatments to * Corresponding author. Tel./fax: +48-22-843-1980.
E-mail address: [email protected] (M. Serek).
improve their keeping qualities (Roberts et al., 1995). Senescence of cut leaves and other vegeta-tive tissues is distinct from the senescence of cut flowers, and commercial preservatives used to prolong the vase life of cut flowers may not always be suitable for cut foliage. In cut roses, senescence has been associated with changes in cellular properties including vacuolar pH, conduc-tivity, and osmolarity (Barthe et al., 1991). Changes in these parameters have been observed even before the onset of visible symptoms of senescence.
Like other developmental processes, senescence is strongly modulated by plant hormones. For example, the cytokinins have long been known to strongly inhibit the senescence of leaves and some floral tissues (Van Staden et al., 1988; Wingler et al., 1998). Similarly, gibberellic acid (GA) has been shown to retard leaf yellowing in lilies (Nowak and Mynett, 1985), prolong longevity of leaves and bracts of poinsettia, and delay senes-cence of alstroemeria spikes (Hicklenton, 1991) and nasturtium leaves (Beevers, 1966).
The leaves ofZ.aethiopicaare a potential new florist green whose use is limited by their rela-tively rapid postharvest yellowing, particularly in the preservative solutions recommended for ex-tending the life of cut flowers. We report here the effect of different preservative solutions on the timing of leaf senescence, biochemical changes during senescence, and the effects of GA and BA (benzyladenine) on senescence of cut leaves of Z. aethiopica. We also tested application of the hor-mones as a pulse treatment and as a whole-leaf dip.
2. Material and methods
Cut leaves ofZ. aethiopicagrown in the green-houses of the Department of Ornamental Plants of the Warsaw Agricultural University were used in all experiments. Mature, healthy, undamaged leaves were harvested in the morning, graded for uniformity, treated with growth regulators and then placed in vases with water or preservative containing 8-hydroxyquinoline citrate (8-HQC 200 mg l−1) and sucrose (20 g l−1) in a room with
controlled conditions: temperature 20°C, relative humidity 60% and 12 h photoperiod (25 mmol
m−2 s−1 PAR).Growth regulators were applied
in two ways:
1. Pulse conditioning: leaves were placed for 24 h in aqueous solutions containing 0.25 mM GA3
or 0.1 mM BA.
2. Immersion: leaves were immersed for several seconds in solutions containing 1 mM GA3or
1 mM BA and 0.01% Tween 20 as a surfactant.
Leaves not treated with growth regulators and placed directly into water or preservative served as controls. No effect of Tween 20 on leaf postharvest performance was observed (data not shown), and no water-dipped control was there-fore included in the experiments described here. Vase life was considered terminated when 30% of the leaf surface showed yellowing and/or wilting. Ten leaves were individually tagged and treated as replications for each test.
Samples for chlorophyll determinations as well as cell sap pH and electrical conductivity mea-surements were collected from replicate leaves treated similarly to those kept for longevity evalu-ation. Material from three leaves was pooled on each sampling date. Three samples were weighed for each chlorophyll analysis and three replica-tions of each extract were made for colour read-ings (nine readread-ings per data point in the figures). Chlorophyll was determined after DMF extrac-tion (Moran and Porath, 1980) according to the modification of Inskeep and Bloom (1985). Three replicate samples were taken for dry weight deter-minations; after determining fresh weight, the ma-terial was dried at 105°C until a constant weight was achieved. For determining cell sap parameters the plant material (3 samples per treatment) was frozen on each sampling date. Frozen tissue was placed in a syringe and after thawing the cell sap was expressed by gently pressing the piston, thus avoiding cell disruption. Acidity was measured directly with an ORION Sure-Flow 616500 pH electrode (Research Inc., Boston, USA) and con-ductivity after diluting the sap (50 times) with ultra-pure water (purity 18.2 MQ cm−3=0.055
mS) using an ELMETRON CX-721 conductivity
Fig. 1. Changes in chlorophyll a+b in cut leaves of Zant-edeschia aethiopica(initial value 17.55 mg g−1 DW).
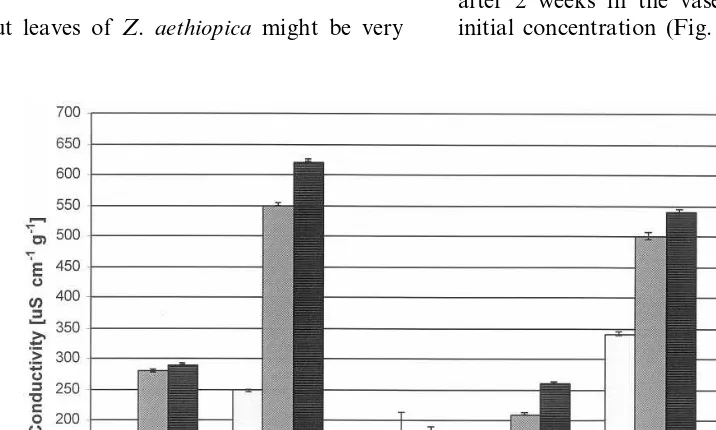
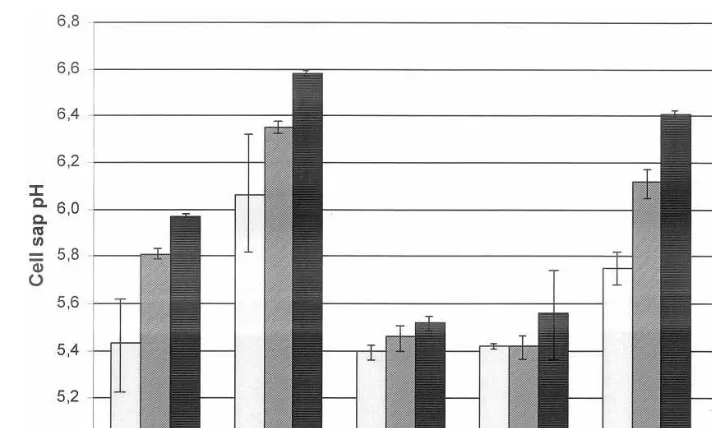
attractive green elements accompanying flowers in bouquets and other floral compositions. However, in our experiments leaves held in water lost chlorophyll relatively rapidly (Fig. 1); after 9 days, only 50% of the chlorophyll remained in the tissue. Concomitantly with chlorophyll loss, there were significant increases in the electrical conduc-tivity and pH of the cell sap (Figs. 2 and 3). Extending postharvest life of Z. aethiopicaleaves would increase their use, enable cold storage to extend the season, and facilitate their long dis-tance transport.
3.2. Effect of floral preser6ati6es on leaf senescence
A standard floral preservative, containing 2% sucrose and 200 ppm 8-HQC had a dramatic and deleterious effect on the senescence of Z. aethiopica leaves. After 6 days in the vase, the conductivity of the cell sap had risen over 60% that of fresh leaves, and pH of the cell sap had increased to 6.05 (Figs. 2 and 3). Concomitantly, there was a dramatic loss of chlorophyll, which, after 2 weeks in the vase was one sixth of the initial concentration (Fig. 1).
Zabrze-Grzybowice, Poland). All the results were subjected to statistical analyses (ANOVA 1 or ANOVA 2) and the means compared using Dun-can’s multiple ranget-test.
3. Results and discussion
3.1. Changes during Z. aethiopica leaf senescence
The cut leaves of Z. aethiopica might be very
Fig. 3. Changes in pH of cell sap from cut leaves ofZantedeschia aethiopica(initial value pH 5.42).
‘Preservatives’, such as the mixture of 8-HQC and sucrose used in the present experiment, are often used to prolong the vase life of cut flowers (Halevy and Mayak, 1981). These materials con-tain a bacteriocide, materials to improve water flow, and sugar as an osmoticum and respiratory substrate. As with some other flowers (notably Alstroemeria) the preservative accelerated senes-cence in the cut leaves of Z. aethiopica, pre-sumably because the presence of sugar in the xylem stream changes source/sink relationships in leaves under the low illumination of the vase life room.
3.3. Response to pulse treatments with growth regulators
A pulse treatment with 0.25 mM GA3 greatly
improved the postharvest performance of Z. aethiopicaleaves, reducing the rate of chlorophyll loss so that it took 4 weeks for the chlorophyll A+B concentration to fall to 50% of that in the freshly-harvested leaves (Fig. 1), and dramatically reducing the normal increase in pH and conduc-tivity of the cell sap. In contrast, pulsing with 0.1 mM BA had no beneficial effects on changes in
the cell sap, and significantly accelerated the loss of chlorophyll from the leaves (Figs. 1 – 3).
Cytokinins are known to retard senescence of detached leaves, delaying proteolysis, chlorophyll degradation and increase in activity of many hy-drolases (Gan and Amasino, 1997; Wingler et al., 1998). A several-fold increase in longevity of cut leaves of Hosta plantaginea was obtained follow-ing BA application (Skutnik et al., 1999). This classic response was not observed inZantedeschia. In contrast, as inAlstroemeria(Hicklenton, 1991), GA was more active in prolonging longevity of cut leaves. These findings indicate the need to test the response of florists’ greens to different growth regulators, as well as their optimal concentration and method of application (Bosse and Van Staden, 1989).
3.4. Effects of dipping in growth regulator solutions
Application of growth regulators by brief im-mersion in solutions containing the regulator and a surfactant amplified the effects of pulse treat-ment. Dipping in 1 mM GA3even further reduced
contained almost 50% of the initial concentra-tion of chlorophyll at the last sampling date. In contrast, dipping in 1 mM BA accelerated the increases in sap pH and conductivity, and loss of chlorophyll (Figs. 1 – 3). In case of Z. aethiopica, as in Hosta (Skutnik et al., 1999), dipping the leaves gave better results than pulse treatment, probably by providing direct local contact of the foliar tissues with the growth reg-ulator while avoiding transport difficulties through petioles.
3.5. Effect of different treatments on display life
Loss of decorative value in Z. aethiopica leaves was due to leaf yellowing associated with chlorophyll degradation. The display life of leaves treated in the different ways described above was closely correlated with the effects of the treatments on chlorophyll loss, and increases in pH and conductivity. Placing the leaves in a normal preservative solution reduced the life of the leaves to less than a week, while those pre-treated with a GA3 dip lasted more than six
times longer (Table 1).
Barthe and co-workers (1991) showed that rose petal ageing was associated with a number of changes in the properties of the cell sap. They suggested that parameters such as sap pH, osmolarity and electrical conductivity are ‘senes-cence indicators’, and our findings with cut fo-liage are consistent with this hypothesis. The loss in chlorophyll in Z. aethiopica was corre-lated, for the different treatments, with increases
in sap pH and sap conductivity. Presumably the catabolism of proteins and other macro-molecules that accompanies the onset of senes-cence generates increased concentrations of amino acids, organic acids and phosphate esters as well as anions and cations that result in the increase in sap conductivity. Decrease in acidity of cell sap in flower petals has been shown to be the result of proteolysis and ammonia accu-mulation (Paulin, 1971; Borochov et al., 1976) and reflects a balance between the vacuolar and cytoplasmic compartsmentalisation of the cells as shown by direct measurements of vacuolar and cytoplasmic pH (Barthe and Vaillant, 1993). These readily determined parameters may allow identification of the physiological condition of tissues before the appearance of visual senes-cence symptoms. They have been used to provide a rapid and objective evaluation of treatments applied to determine postharvest quality of cut roses (Durkin et al., 1991) and even to predict flower longevity when new culti-vars are being tested.
4. Conclusions
Our findings indicate a similar value for these measurements in assessment of the progress of senescence in cut foliage. Since they are easy to perform, they could serve as objective measures of the quality of cut greens at every stage of the marketing chain. We have also shown the con-siderable benefits of applying GA3 to cut leaves
of Z. aethiopica in increasing their postharvest life.
Acknowledgements
The study was supported by a grant from the EU Individual Mobility Grant 1996 – 97 no. IMG-96-PL-1046 (ES), KBN no. PB 0339/P06/
96/10 (AL, ES, JR) and from the Danish Min-istry of Agriculture, Grant no. 93S-2466-A, 97-01023 (MS). The authors would like to thank Professor Michael S. Reid for critical re-view of the manuscript.
Table 1
Effect of BA and GA3 on postharvest longevity of leaves of Zantedeschia aethiopica 0.1mM BA condit.24 h/water
34.2 e 0.25mM GA3 condit.24 h/water
1 mM BA dipping/water 17.5 b 38.8 f 1 mM GA3dipping/water
8HQC+2%S 6.3 a
References
Barthe, P., Vaillant, V., Gudin, S., 1991. PH of cell sap and vacuolar pH during senescence of the rose petals. Acta Hortic. 298, 141 – 144.
Barthe, P., Vaillant, V., 1993. Changes in the buffering capac-ity of cell sap in senescing rose petals. Scientia Hortic. 54, 165 – 174.
Beevers, L., 1966. Effect of gibberellic acid on the senescence of leaf discs of nasturtium,Tropaeolum majus. Plant Phys-iol. 41, 1047 – 1076.
Borochov, A., Tirosh, T., Halevy, A.H., 1976. Abscisic acid content of senescing petals on cut rose flowers as affected by sucrose and water stress. Plant Physiol. 58, 175 – 178. Bosse, A., Van Staden, J., 1989. Cytokinins in cut carnation
flowers. Effect of cytokinin type, concentration and mode of application of flower longevity. J. Plant Physiol. 135, 155 – 159.
Durkin, D., Barthe, P., Vaillant, V., Arene, L., 1991. Effect of preservative solutions on some indicators of senescence in cut rose flowers. Acta Hortic. 298, 141 – 144.
Gan, S., Amasino, R.M., 1997. Making Sense of Senescence. Molecular genetic regulation and manipulation of leaf senescence. Plant Physiol. 113, 313 – 319.
Halevy, A.H., Mayak, S., 1981. Senescence and postharvest physiology of cut flowers. Hort. Rev. 3, 59 – 143 Part 2. Hicklenton, P.R., 1991. GA3 and benzyloaminopurine delay
leaf yellowing in cut Alstroemeria stems. HortScience 26, 1198 – 1199.
Inskeep, W.P., Bloom, P.R., 1985. Extinction coefficients of chlorophyll a and b inN,N-dimethylformamide and 80% acetone. Plant Physiol. 77, 483 – 485.
Moran, R., Porath, D., 1980. Chlorophyll determination in intact tissues usingN,N-dimethylforamide. Plant Physiol. 65, 478 – 479.
Nowak, J., Mynett, K., 1985. The effect of growth regulators on post-harvest characteristics of cut lilium ‘Prima’ infl-orescences. Acta Hortic. 167, 109 – 116.
Paulin, A., 1971. Influence de la composition de la solution nutritive sur la teneur en divers acides amines libres et en ammoniac des petales de fleurs coupees. Ann. Tech. Agr. 20, 283 – 303.
Roberts, C.M., Serek, M., Andersen, A.S., 1995. Supplemental irradiance and STS improve the display life of Dicentra species forced as flowering potted plants. Scientia Hort. 62, 121 – 128.
Skutnik, E., Lukaszewska, A., Tyborowska, K., 1999, Retard-ing senescence of cut leaves ofHosta plantagineaby growth regulators. Annals of Warsaw Agricultural University No 20, 3 – 8.
Van Staden, J., Cook, E.L., Nooden, L.D., 1988. Cytokinins and senescence. In: Nooden, L.D., Leopold, A.C. (Eds.), Senescence and Aging in Plants. Academic Press, San Diego, pp. 281 – 328.
Wingler, A., Von Schaewen, A., Leegood, R.C., Lea, P.J., Quick, W.P., 1998. Regulation of leaf senescence by cytokinin, sugars and light. Plant Physiol. 116, 329 – 335.