Summary Soluble and cell-wall-bound phenolics in mycor-rhizas of Larix decidua--Laccaria amethystea and in nonmy-corrhizal fine roots of larch grown in sterile culture were analyzed by HPLC. The soluble phenolics p -hydroxybenzoyl-glucose, p-hydroxybenzoic acid glucoside, picein, catechin, and epicatechin were identified in nonmycorrhizal fine roots. The same phenolics also occurred in mycorrhizas, but only in very small quantities compared with those in nonmycorrhizal fine roots. The amount of cell-wall-bound ferulic acid was much lower in mycorrhizas than in nonmycorrhizal fine roots. Pure mycelia of Laccaria amethystea (Bull.) Murr. contained none of the identified phenolics. We conclude that L. amethys-tea induced a large decrease in soluble and cell-wall-bound phenolics in fine roots of Larix decidua Mill. that may explain their rapid mycorrhization.
Keywords: antifungal compounds, catechin, epicatechin, ferulic acid, flavonoids, Laccaria amethystea, larch, mycor-rhiza, p-hydroxybenzoylglucose, p-hydroxybenzoic acid glu-coside, picein.
Introduction
Low molecular weight phenolics are involved in resistance of forest trees to attack by parasitic fungi (Jorgensen 1961, Shain 1967, Alcubilla 1970, Prior 1976, Shain 1979, Alcubilla et al. 1987). After fungal infection, the concentration of constitutive phenolics in stems and woody roots of trees is enhanced. The phytoalexin-like phenolic defense chemicals are mainly stil-benes such as piceatannol, isorhapontigenin, resveratrol and their glucosides, pinosylvin and its monomethyl ether, and flavonoids such as quercetin, taxifolin, catechin and epicate-chin. p-Hydroxyacetophenone, an acetophenone in needles of Norway spruce, exhibits fungitoxic activity toward Cladosporium cucumerinum and the forest pathogen Rhizo-sphaera kalkhoffii (Osswald et al. 1987).
Fine roots of tree species forming ectomycorrhizas also contain constitutive soluble phenolics such as p -hydroxyben-zoic acid glucoside, piceatannol and its glucoside, pinosylvin and its monomethyl ether, isorhapontin, picein and catechin (Hillis et al. 1968, Hillis and Ishikura 1969, Münzenberger et al. 1990, Bonello et al. 1993), and cell-wall-bound phenolics such as ferulic acid (Münzenberger et al. 1990).
Nonmycorrhi-zal fine roots of spruce (Picea abies (L.) Karst.) contain higher concentrations of soluble and cell-wall-bound phenolics than mycorrhizas of Picea abies--Laccaria amethystea and Picea abies--Lactarius deterrimus (Münzenberger et al. 1990). Be-cause most of the identified phenolic compounds exhibit strong antifungal activity against mycorrhizal fungi (Münzen-berger 1991), we hypothesized that a reduction in the concen-trations of soluble and cell-wall-bound phenolics is a prerequisite for mycorrhization.
To test this hypothesis, we analyzed the amounts of soluble and cell-wall-bound phenolics in both mycorrhizal and non-mycorrhizal roots of Larix decidua Mill. cultivated under ster-ile conditions. The mycelium of Laccaria amethystea (Bull.) Murr. was also analyzed for phenolic compounds. We chose the tree species L. decidua because mycorrhizas are more rapidly established with fine roots of this species (Kottke and Oberwinkler 1988) than with fine roots of P. abies.
Materials and methods
Fungal material
The fungal strain of L. amethystea was isolated from a fruiting body in a Norway spruce stand near Oberjoch, Bavarian Alps, Germany in 1987. The isolate is stored in the type collection in Tübingen, Spezielle Botanik/Mykologie (strain LA 977).
Fungal culture
The mycelium of L. amethystea (977) was pre-cultivated on Modified Melin Norkrans (MMN) agar modified according to Kottke et al. (1987): CaCl2, 0.05 g; NaCl, 0.025 g; KH2PO4, 0.5 g; (NH4)2HPO4, 0.25 g; MgSO4⋅7H2O, 0.15 g; FeCl3⋅6H2O, about 1 mg; thiamine hydrochloride, 100 µg; glucose mono-hydrate, 10 g; malt extract, 5 g; casein hydrolysate, 1 g; 10 ml of stock solution of trace elements (see below); and 2% (w/v) Agar-Agar (Merck, Darmstadt, Germany); per liter. The stock solution of trace elements contained: KCl, 3.728 g; H3BO3, 1.546 g; MnSO4⋅H2O, 0.845 g; ZnSO4⋅7H2O, 0.575 g; CuSO4⋅5H2O, 0.125 g; and (NH4)6Mo7O24⋅4H2O, 0.018 g; per liter. Five small plugs (7 mm i.d.) of vigorously growing mycelium were used as inoculum for mycorrhizal synthesis. For liquid culture of the fungus, 10 small plugs of mycelium were transferred from agar plates to 500-ml Erlenmeyer flasks
Reduction of phenolics in mycorrhizas of
Larix decidua
Mill.
BABETTE MÜNZENBERGER,
1INGRID KOTTKE
2and FRANZ OBERWINKLER
21 Zentrum für Agrarlandschafts- und Landnutzungsforschung (ZALF) e.V., Institut für Wald- und Forstökologie, Dr.-Zinn-Weg,
16225 Eberswalde, Germany
2 Institut für Botanik, Spezielle Botanik und Mykologie, Universität Tübingen, Auf der Morgenstelle 1, 72076 Tübingen, Germany
Received January 24, 1994
with one indent containing 150 ml of modified MMN solution (medium as described, but without agar). The fungal plugs were homogenized for several seconds with an Ultra-Turrax apparatus (Janke and Kunkel, IKA Labortechnik, Staufen, Germany). The flasks were sealed with cotton stoppers and incubated on a rotary shaker (100 rpm). After one month the pellets that formed were homogenized again to keep the fun-gus in a growing state. One month later the mycelia were harvested, immediately frozen in liquid nitrogen and freeze-dried.
Plant culture
Seeds of L. decidua from the Hess. Staatsdarre Wolfgang (Hanau, Germany) were surface sterilized for 2 h in hydrogen peroxide (30%) and germinated in petri dishes on modified Benecke agar according to Frohnmeyer: KH2PO4, 0.1 g; NH4NO3, 0.2 g; MgSO4⋅7H2O, 0.1 g; CaCl2, 0.1 g; FeCl3⋅6H2O, about 1 mg; 10 ml of stock solution of trace elements (see above); and 10 ml of PIPES (Serva, Heidelberg, Germany); per liter. The pH was adjusted to 5 with H2SO4 and 10 g of Agar-Agar (Merck) was added. The seedlings were transferred to Erlenmeyer flasks when cotyledons were well developed.
Mycorrhizal synthesis
Axenic cultures were established in 500-ml Erlenmeyer flasks containing 250 ml of a 9/1 (w/w) mix of perlite and Sphagnum moistened with 150 ml of modified MMN nutrient solution (Kottke et al. 1987): CaCl2, 0.05 g; NaCl, 0.025 g; KH2PO4, 0.5 g; (NH4)2HPO4, 0.5 g; MgSO4⋅7H2O, 0.15 g; FeCl3⋅6H2O, about 1 mg; thiamine hydrochloride, 100 µg; glucose mono-hydrate, 1 g; and 10 ml of stock solution of trace elements (see above); per liter. Each flask contained one seedling without inoculum for nonmycorrhizal roots or with added fungal in-oculum (five plugs) for mycorrhizal synthesis. Two series were cultivated for about 5 months (Series 1: December 1990--May 1991; Series 2: May 1991--October 1991). For each series, 30 inoculated and 30 noninoculated flasks were prepared, each sealed with a cotton stopper and incubated in a growth cham-ber (day/night temperature of 20/10 °C; 18-h photoperiod, lamps: Universal-White, Osram, Augsburg, Germany).
Harvest of root material
After mycorrhizal synthesis, the root systems of the seedlings were washed quickly to remove the substrate, and mycorrhizas or nonmycorrhizal fine roots were sorted under a stereomicro-scope. Only vital mycorrhizal systems were harvested: fungus and host are vital when the hyphal mantle of the mycorrhiza is lavender in color and the root tips are light in color (Münzen-berger et al. 1990). Only fine roots with light-colored root tips were harvested from root systems of noninoculated seedlings. The material was frozen in liquid nitrogen and freeze-dried.
Extraction of soluble phenolics from lyophilized samples Lyophilized samples of 20 mgDW of fine roots, mycorrhizas or mycelia were homogenized in liquid nitrogen in a mortar with a pestle. The powders were each suspended in 4 ml of 80%
aqueous MeOH and extracted for 1 h with continuous stirring. After centrifugation, each pellet was re-extracted twice with 2 ml of 80% aqueous MeOH for 10 min each time. The com-bined extracts were evaporated to dryness in vacuo at 40 °C and then re-dissolved in 0.5 ml of 50% aqueous MeOH, cen-trifuged, and used for HPLC analysis.
Extraction of insoluble phenolics
The pellets from the preparation of soluble phenolics were treated successively with water (5 ml), twice with methanol (5 ml each time), and finally with acetone (1 ml). Each extrac-tion was carried out for 15 min with continuous stirring, and the extract was centrifuged each time. The remaining insoluble material was dried at 60 °C for 2 h, suspended in 1 ml of hot (80 °C) 1 M NaOH, and incubated with continuous stirring for 16 h at room temperature. Hydrolysates were centrifuged and aliquots (500 µl) of the supernatants were acidified with 50 µl of H3PO4. After centrifugation, 20 µl aliquots of the clear supernatants were analyzed by HPLC.
Isolation and purification of soluble phenolics from root tissue
Root systems of nonmycorrhizal seedlings (5.5 gDW) were homogenized with an Ultra-Turrax apparatus and extracted with 150 ml of 80% aqueous MeOH for 1 h with continuous stirring. After centrifugation, the pellet was re-extracted twice with 50 ml of the same solvent for 15 min. The combined extracts were concentrated under vacuum. The residue was re-dissolved in 10 ml of 50% aqueous MeOH and applied to a polyamide column (SC 6, 60 × 3 cm i. d., Macherey-Nagel, Düren, Germany). The column was eluted with H2O (elution of p-hydroxybenzoylglucose), 40% MeOH (elution of p -hy-droxybenzoic acid glucoside and picein) and 80% MeOH (elution of catechin and epicatechin). These fractions were concentrated in vacuo and purified on a Sephadex LH-20 column (100 × 2 cm i.d., Fluka, Neu-Ulm, Germany). The column was eluted with H2O (elution of p -hydroxybenzoylglu-cose and p-hydroxybenzoic acid glucoside), 50% MeOH (elu-tion of picein) and 80% MeOH (elu(elu-tion of catechin and epicatechin). Final purification of phenolic compounds was achieved by preparative HPLC.
Preparative HPLC was carried out with a liquid chroma-tograph (two-pump system, Waters, Millipore, Eschborn, Ger-many) on prepacked Silica C18 (10 µm, 300 mm long, 40 mm i.d., Latek, Eppelheim, Germany). The elution system was a linear gradient from solvent A (1% HCOOH) to 100% solvent B (80% MeOH) in [A+B] in 200 min. The flow rate was 20 ml min−1 and the detection wavelength was 265 nm.
Identification of phenolics
Thin-layer chromatography was carried out on microcrys-talline cellulose (‘Avicel’, Macherey-Nagel) with a water-satu-rated chloroform/acetic acid solvent (3/2 v/v). Absorbance was measured at 254 nm and the Rf-values were: p -hydroxyben-zoic acid, 0.78; picein, 0.62; p-hydroxybenzoic acid glucoside, 0.42; catechin, 0.14; and epicatechin, 0.11.
(two-pump system, Pharmacia LKB, Freiburg, Germany) with a data processor (Chromatopac C-R3A, Shimadzu, Kyoto, Japan) using prepacked Nucleosil C18 (5 µm, 250 mm long, 4 mm i.d., Macherey-Nagel). The elution system for soluble phenolics was a two-step linear gradient from solvent A (1.5% H3PO4 in H2O) to 40% solvent B (H2O/CH3OH/CH3CN, 1/1/1, (v/v)) in A in 60 min and then to solvent B in 40 min. The elution system for ferulic acid was a linear gradient from 25% to 60% solvent B in [A+B] in 20 min. The flow rate was 1 ml min−1 and the detection wavelengths were 265 nm (soluble phenolics) and 320 nm (ferulic acid). For identification, we used authentic p-hydroxybenzoic acid glucoside and p -hy-droxybenzoylglucose from previous isolation of larch roots, p-hydroxybenzoic acid (Sigma, Deisenhofen, Germany), picein (Sarsynthèse, Mérignac, France), catechin (Serva), epi-catechin (Aldrich, Steinheim, Germany) and ferulic acid (Serva). p-Hydroxybenzoic acid and picein have identical re-tention times under the chromatographic conditions described, but could be separated by TLC (see above). p-Hydroxybenzoic acid, picein, catechin and ferulic acid were used for quantifi-cation (external standardization).
Structures of purified phenolics were identified by mass spectrometry (MS) and by nuclear magnetic resonance spec-troscopy (NMR).
Results and discussion
Soluble phenolics
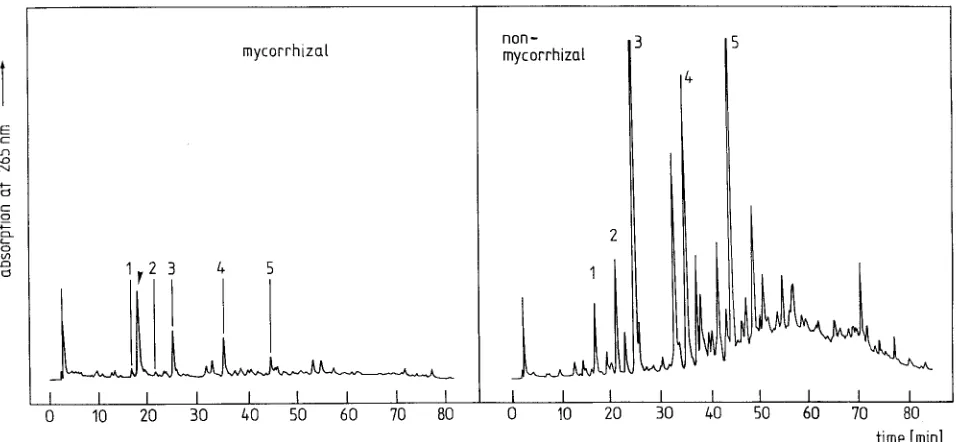
The constitutive soluble phenolics p-hydroxybenzoic acid glu-coside, p-hydroxybenzoylglucose, picein, catechin and epi-catechin were identified in fine roots of larch. Representative
HPLC profiles of soluble phenolics of mycorrhizal and non-mycorrhizal fine roots are shown in Figure 1. Mycorrhizal and nonmycorrhizal fine roots contained the same phenolic com-pounds. No phenolic phytoalexins were found. The amounts of all identified phenolics were lower in mycorrhizas than in nonmycorrhizal fine roots. Only a trace of the aglycone of picein, p-hydroxyacetophenone, was detected in both mycor-rhizal and nonmycormycor-rhizal fine roots. No stilbenoid com-pounds were found in either mycorrhizal or nonmycorrhizal fine roots.
Laccaria amethystea contained none of the identified phe-nolics; however, a nonphenolic compound from the fungus, which appeared in mycorrhizas of Picea abies--Laccaria amethystea (Münzenberger et al. 1990), was also detected in mycorrhizas of larch (Figure 1, arrow). On the basis of HPLC analysis of this unidentified substance from extracts of pure mycelium and of mycorrhizas, we calculated that the fungal contribution to the dry weight was 43% of the total. This was taken into account when the quantitative differences were compared. The values of soluble and insoluble phenolics in mycorrhizas in the tables and figures were corrected accord-ingly.
The concentrations of soluble phenolics for both series of cultures are shown in Table 1. In Series 1, the values of the two extractions were similar for mycorrhizal and nonmycorrhizal fine roots, respectively. However, the concentrations of soluble phenolics in mycorrhizal and nonmycorrhizal fine roots of seedlings cultivated at different times of the year showed fluctuations (cf. values for soluble phenolics for Series 1 and 2). It seems likely that an endogenous annual rhythm regulates the amount of phenolic compounds in the soluble pool even when the plants are grown under controlled
tions. Large fluctuations in concentrations of soluble phenolics were also observed in cultures from Norway spruce (Münzen-berger et al. 1990).
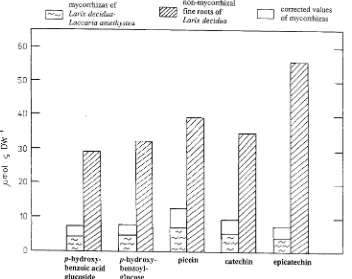
The flavonoid epicatechin was the major component of the phenolic compounds (Figure 2). The derivatives of p- hydroxy-benzoic acid, picein and catechin were also present in high quantities. The concentrations of all identified phenolic com-pounds were less in mycorrhizas than in nonmycorrhizal fine roots, especially epicatechin, whose concentration in mycor-rhizal extracts was only 13% (corrected value) of that extracted
from nonmycorrhizal fine roots. The concentrations of the remaining phenolic compounds present in mycorrhizas were between 25 and 32% (corrected values) of those present in nonmycorrhizal fine roots.
The identified soluble phenolics were present in higher concentrations in nonmycorrhizal fine roots of larch than in nonmycorrhizal fine roots of Norway spruce (Münzenberger et al. 1990), whereas the concentrations of soluble phenolic com-pounds were lower in the mycorrhizas of larch than in the mycorrhizas of Norway spruce. The finding that the decrease
Table 1. Concentrations (µmol gDW−1) of soluble phenolics and insoluble ferulic acid from mycorrhizas of Larix decidua--Laccaria amethystea
and nonmycorrhizal fine roots. Corrected values in parenthesis.
SampleExtractSoluble phenolic compoundsFerulic acid
p-HBAp-HBLPiceinCatechinEpicatechin glucoside1glucose2
Series 1
Nonmycorrhizal fine 113.8524.4826.540.0255.910.11 roots of Larix decidua214.4025.4527.8737.5260.480.092
Larix decidua--Laccaria1TraceTrace 2.66 (4.63) 5.59 (9.74) 3.15 (5.49)0.024 (0.042)
amethystea mycorrhizas2 3.33 (5.80) 4.50 (7.84) 2.88 (5.02) 4.42 (7.70) 2.55 (4.44)0.021 (0.037) Series 2
Nonmycorrhizal fine159.7340.7763.0328.7051.770.079 roots of Larix decidua
Larix decidua--Lacaria1 5.09 (8.87) 4.95 (8.62)15.77 (27.47) 6.22 (10.84) 7.19 (12.53)0.032 (0.056) amethystea mycorrhizas
Figure 2. Comparison of soluble phenolics from mycorrhizas of
in the concentration of these fungitoxic compounds in the fine roots of larch during mycorrhization is greater than the de-crease that occurs in the fine roots of Norway spruce may account for the faster mycorrhization of larch fine roots than of Norway spruce fine roots. The results support the hypothesis that phenolic compounds are effective in regulating the mycor-rhizas of forest trees.
Cell-wall-bound ferulic acid
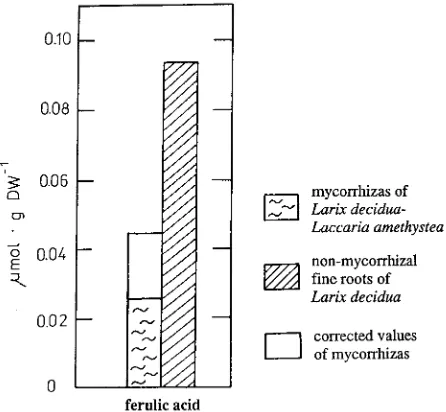
Extracts of mycorrhizas contained 52% less ferulic acid (cor-rected value) than those of nonmycorrhizal fine roots (Fig-ure 3). Unlike the concentrations of soluble phenolics, the concentrations of insoluble ferulic acid did not vary between Series 1 and 2 (Table 1). Ferulic acid was not present in cell walls of the pure mycelium of L. amethystea.
The biological function of cell-wall-bound hydroxycin-namic acids is to make the wall less extensible and to prevent digestion by fungal enzymes (Fry 1982, 1986, 1987, Tan et al. 1992). Thus, a decrease in the concentration of ferulic acid in mycorrhizas enhances cell wall extensibility, facilitating inter-cellular penetration by the fungal symbiont. The lower concen-trations of insoluble ferulic acid in cell walls of nonmycorrhizal fine roots of larch and the larger decrease in the concentration of this monophenol in larch mycorrhizas compared with Norway spruce may explain the faster mycor-rhization of the fine roots of larch.
The present results confirm previous findings that none of the mechanisms underlying pathogenic plant--fungus interac-tions function in mature ectomycorrhizas. That is, in mature ectomycorrhizas, there is no accumulation of phenolic com-pounds, no release of aglyca, such as p-hydroxyacetophenone from picein, and no de novo synthesis of phenolic inhibitors (phytoalexins).
The regulatory mechanism underlying the decrease in con-centrations of phenolic compounds in ectomycorrhizas is not known. Several processes could explain the low concentrations of soluble and cell-wall-bound phenolic compounds in ec-tomycorrhizas. For example, altered physiology of fine roots as a result of mycorrhization could affect the routes for phenol synthesis. Alternatively, the synthesis of enzymes catalyzing the phenolic production could be suppressed by compounds released by the mycorrhizal fungus. A third possibility is that the low molecular weight phenols are polymerized to high molecular weight phenols. This mechanism is known to occur for catechin, which can be polymerized to condensed tannins (Harborne 1980). A fourth possibility is that the phenolic compounds are degraded by enzymes released either by the fungus or the plant (e.g., peroxidases, phenoloxidases). How-ever, cytochemical tests of Norway spruce mycorrhizal tissue showed no peroxidase or phenoloxidase activity by the plant or fungus (Münzenberger 1991). Furthermore, we have been unable to detect degradation products of phenolic compounds in mycorrhizas of P. abies and L. decidua (unpublished obser-vations).
Wyss et al. (1991) found differences in the regulation of phenolic compounds in mycorrhizal systems compared with pathogenic systems in a study of vesicular arbuscular mycor-rhiza. They found no accumulation of the phytoalexin glyceol-lin, an isoflavonoid, by the mycorrhizal fungus Glomus mosseae in soybean roots, but the pathogen Rhizoctonia solani caused glyceollin to accumulate. Wyss et al. (1991) concluded that the fungus was not able to induce the enzymes for biosyn-thesis of phytoalexins. This is consistent with the results of Harrison and Dixon (1993) who found a suppression of the isoflavone reductase (IFR) transcripts that encode an enzyme specific for phytoalexin biosynthesis in Medicago truncatula roots during colonization with the vesicular arbuscular mycor-rhizal fungus Glomus versiforme.
Acknowledgments
We are grateful to the Deutsche Forschungsgemeinschaft (Schwer-punktprogramm ‘‘Physiologie der Bäume’’) for financial support. We thank Ortrun Ebinger for technical assistance.
References
Alcubilla, M. 1970. Pilzhemmstoffe in der Fichtenrinde. Landw. Fo. 25:96--101.
Alcubilla, M., R. Heibl and K.-E. Rehfuess. 1987. Chemische Zusam-mensetzung und fungistatische Wirkung gegenüber Heterobasidion annosum von Wurzelbast und -holz der Fichte (Picea abies [L.] Karst.) in Abhängigkeit vom Standort. Mitt. Ver. Forstl. Standort-skde Forstpflanzenzüchtung 33:81--92.
Bonello, P., W. Heller and H. Sandermann, Jr. 1993. Ozone effect on root-disease susceptibility and defence responses in mycorrhizal and nonmycorrhizal Scots pine (Pinus sylvestris L.) seedlings. New Phytol. 124:653--663.
Fry, S.C. 1982. Phenolic components of the primary cell wall: feruloy-lated disaccharides of D-galactose and L-arabinose from spinach polysaccharide. Biochem. J. 203:493--504.
Fry, S.C. 1986. Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu. Rev. Plant Physiol. 37:165--186. Figure 3. Comparison of insoluble ferulic acid from mycorrhizas of
Fry, S.C. 1987. Intracellular feruloylation of pectic polysaccharides. Planta 171:205--211.
Harborne, J.B. 1980. Plant phenolics. In Encyclopedia of Plant Physi-ology, New Series Vol. 8: Secondary Plant Products. Eds. E.A. Bell and B.V. Charlwood. Springer, Berlin, Heidelberg, New York, pp 329--402.
Harrison, M. and R. Dixon. 1993. Induction and suppression of tran-scripts encoding enzymes of phenylpropanoid and isoflavonoid biosynthesis in vesicular arbuscular mycorrhizae. In Abstracts of the 9th North American Conference on Myorrhizae. Guelph, On-tario, Canada, 150 p.
Hillis, W.E. and N. Ishikura. 1969. The extractives of the mycorrhizas and roots of Pinus radiata and Pseudotsuga menziesii. Aust. J. Biol. Sci. 22:1425--1436.
Hillis, W.E., N. Ishikura, R.C. Foster and G.C. Marks. 1968. The role of extractives in the formation of ectotrophic mycorrhizae. Phyto-chemistry 7:409--410.
Jorgensen, E. 1961. The formation of pinosylvin and its monomethyl ether in the sapwood of Pinus resinosa Ait. Can. J. Bot. 39:1765--1772.
Kottke, I. and F. Oberwinkler. 1988. Comparative studies on the mycorrhization of Larix decidua and Picea abies by Suillus gre-villei. Trees 2:115--128.
Kottke, I., M. Guttenberger, R. Hampp and F. Oberwinkler. 1987. An
in vitro method for establishing mycorrhizae on coniferous tree seedlings. Trees 1:191--194.
Münzenberger, B. 1991. Lösliche und zellwandgebundene Phenole in Mykorrhizen und nicht mykorrhizierten Wurzeln der Fichte (Picea abies [L.] Karst.) und des Erdbeerbaumes (Arbutus unedo L.) und ihre Bedeutung in der Pilz-Wurzel-Interaktion. Ph.D Thesis. Uni-versity of Tübingen, Tübingen, 145 p.
Münzenberger, B., J. Heilemann, D. Strack, I. Kottke and F. Ober-winkler. 1990. Phenolics of mycorrhizas and nonmycorrhizal roots of Norway spruce. Planta 182:142--148.
Osswald, W.F., S. Zieboll, W. Schütz, J. Firl and E.F. Elstner. 1987.
p-Hydroxyacetophenone a fungitoxic compound in spruce needles. Z. Pflanzenkr. Pflanzenschutz 94:572--577.
Prior, C. 1976. Resistance by Corsican pine to attack by Hetero-basidion annosum. Ann. Bot. 40:261--279.
Shain, L. 1967. Resistance of sapwood in stems of loblolly pine to infection by Fomes annosus. Phytopathology 57:1034--1045. Shain, L. 1979. Dynamic responses of differentiated sapwood of
Norway spruce to infection by Fomes annosus. Phytopathology 69:1143--1147.