Influence of maturity and bagging on the relationship
between anthocyanin accumulation and phenylalanine
ammonia-lyase (PAL) activity in ‘Jonathan’ apples
Hongqing Wang * , Osamu Arakawa , Yoshie Motomura
Faculty of Agriculture and Life Science,Hirosaki Uni6ersity,Bunkyo-machi3,Hirosaki,Aomori036-8561,Japan
Received 2 August 1999; accepted 19 February 2000
Abstract
The influence of maturing and ripening on phenylalanine ammonia-lyase (PAL) activity and anthocyanin accumulation was examined using bagged, non-bagged and shaded ‘Jonathan’ (Malus domestica) apples. Bagged fruit were covered with paper bags from mid-June at an early stage of fruit growth. Shaded apples were insulated from sunlight by newspaper at the beginning of colouring (middle September). The apples were harvested at different maturity stages and were irradiated with UV+white light for 96 h at 15°C after which anthocyanin content and PAL activity were measured. In non-bagged and shaded apples, anthocyanin accumulation and maximal PAL activity increased as the apples developed from the immature to the initial ripe stage, then decreased at full ripe stage. A positive linear relationship between the maximum PAL activity and the amount of anthocyanin accumulated suggested that the change in PAL activity is involved in the change of anthocyanin accumulation. In the bagged apples in the immature stage, anthocyanin accumulation and peak PAL activity after irradiation was higher than that in non-bagged apples. However, as the apples ripened, anthocyanin accumulation was less than for less mature fruit, even though PAL activity was relatively high. Therefore, PAL is not the only regulating factor for anthocyanin accumulation in bagged mature and ripe fruit. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Maturity; Bagging; Anthocyanin; Phenylalanine ammonia-lyase (PAL)
www.elsevier.com/locate/postharvbio
1. Introduction
The development of the red pigmentation with advancing maturity in apples still on the tree was thought to be dependent on an increase of their
ability to produce anthocyanin during the matur-ing period (Arakawa, 1988, 1990a,b). Faust (1965) suggested that at that time, glycolysis shifts to-ward the pentose phosphate pathway and sec-ondary metabolism, resulting in an increase in anthocyanin synthesis. Moreover, it has been known that bagging promotes anthocyanin syn-thesis, and Arakawa, (1988, 1991) reported that bagged fruit which had been covered with paper
* Corresponding author: Tel.:+81-172-393809; fax:+ 81-172-393750.
E-mail addresses: [email protected] (H. Wang), [email protected] (O. Arakawa)
bags for about one month after flowering pro-duced much higher levels of anthocyanin at im-mature and im-mature stages than did shaded apples. However, this anthocyanin synthesis remarkably decreased during ripening.
So, this result indicated that the change in anthocyanin synthesis in bagged fruit was largely different from that in shaded apples during ripening.
Anthocyanin synthesis is a process that involves many steps from the primary precursor (phenyl-alanine) to the end products (glycosides of cyanidin) (Lancaster, 1992). Phenylalanine ammo-nia-lyase (PAL) is the first enzyme to catalyse the elimination of NH3 from L-phenylalanine to give trans-cinnamate (Hanson and Havir, 1981). PAL activity has been reported to positively correlate with anthocyanin synthesis in grapes (Kataoka et al., 1983), strawberries (Given et al., 1988) and apples (Faragher and Chalmers, 1977; Tan, 1979, 1980; Arakawa et al., 1986), but its role in regu-lating anthocyanin formation in apples remains controversial (Blankenship and Unrath, 1988; Ju et al., 1995).
The purpose of this study is to examine the function of PAL in anthocyanin accumulation in bagged, non-bagged and shaded apples of varying levels of maturity.
2. Materials and methods
Apple fruit were harvested from ‘Jonathan’ (Malus domestica Borkh, cv. Jonathan) trees at the experimental orchard of the Faculty of Agri-culture and Life Science, Hirosaki University.
Some apples were covered by two-layer-paper bags (Kobayashi seitai, Japan; 13×16 cm; blue paper with the inner layer coated with wax) in the middle of June. Apples covered by bags are re-ferred to as ‘bagged fruit’. The bagged apples had very low chlorophyll content when the experi-ments started. At the beginning of colour develop-ment (middle September), non-bagged apples were covered by bags made from newspaper to shade them from direct sunlight effectively in-hibiting pigmentation (referred to as ‘shaded ap-ples’). This bagging treatment had little effect on
chlorophyll content of the fruit. The bagged ap-ples were harvested on 2nd August (very imma-ture), 23rd August (immaimma-ture), 12th September (mature non-ripe), and 29th September (partially ripe). Bags were removed before subsequent light treatment.
The non-bagged apples were harvested on 2nd August, 23rd August and 12th September, and shaded apples were harvested on 29th September and 17th October (full ripe). Harvested apples were irradiated for 96 h at 15°C with mixed light from a white fluorescent lamp (FL20-S. W, Toshiba, Tokyo; 4 W m−2
) and an ultraviolet fluorescent lamp with a filter eliminating radiation below 290 nm in wavelength (FL20-S. E., Toshiba, Tokyo; 1.3 W m−2). Skin disks, 18 mm
in diameter and 1 – 2 mm in thickness including cortical tissue, were prepared every 24 h and stored at −40°C.
Anthocyanin was extracted from skin disks with methanol containing 1% HCI, and ab-sorbency was measured at 530, 620, and 650 nm by spectrophotometer (U-2000S Spectrophotome-ter, Hitachi, Japan). The anthocyanin content was calculated as described previously (Arakawa, 1991).
An enzyme was prepared as described by Faragher and Chalmers (1977) with some modifi-cation. To assay the PAL activity, the enzyme was made of 100 mM borate buffer (pH8.8) and an
L-phenylalanine concentration of 60 mM.
Sam-ples ware incubated for 1 h at 60°C. The protein concentration in the reaction mixture was from 30 to 60 ng l−1, and was quantified according to the
Bradford method (Bradford, 1976).
A unit of PAL activity was defined as the production of 1 mol of trans-cinnamic acid per min under the assay conditions.
All data reported throughout this paper are the averages of three to six replicates.
3. Results and discussion
3.1. Immature stage
days after full bloom, very immature fruit) in-creased linearly with light irradiation, and reached its highest level after 96 h (Fig. 1). The amount of
anthocyanin accumulated in bagged apples after 96 h light irradiation was about twice that of non-bagged apples. The PAL activity in both bagged and non-bagged apples increased rapidly up to 48 h under light irradiation, then decreased rapidly after 72 h. The highest level PAL activity in bagged apples was more than three times as high as that of non-bagged apples.
In the immature apples (23rd August, 99 days after full bloom), the anthocyanin accumulation in bagged apples was greater than that in non-bagged apples, and PAL activity in non-bagged apples was also higher than that of non-bagged apples (Fig. 2).
As indicated by Figs. 1 and 2, maximal PAL activity coincided with anthocyanin accumulation, which suggests that in immature apples, PAL activity is related to the larger anthocyanin accu-mulation in bagged apples, as indicated by its absence in non-bagged apples.
3.2. Mature stage
The difference in anthocyanin content between bagged and non-bagged mature non-ripe apples (12th September, 119 days after full bloom) be-came smaller compared with that of the immature fruit stage (2nd and 23rd August), though the anthocyanin accumulation in the bagged apples was still higher than that in non-bagged apples (Fig. 3). The PAL activity in the bagged apples was more than twice as high as that in non-bagged apples, and the maximal PAL activity was still higher than in the apples harvested in August. The PAL activity in bagged apples increased rapidly with light irradiation for 24 h, and reached a maximum after 48 h, then decreased rapidly, as shown in Figs. 1 and 2. On the other hand, the PAL activity in non-bagged apples in-creased rapidly and attained its maximum after 24 h, maintaining this level for 48 h. The value of the maximal PAL activity in the mature stage was higher than that in the very immature and imma-ture apples (2nd and 23rd August).
This suggests that the increased PAL activity took part in the promotion of anthocyanin accu-mulation in the non-bagged apples at this stage.
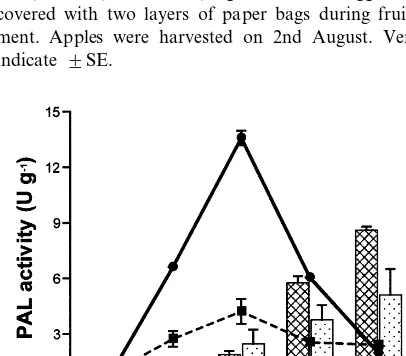
Fig. 1. Anthocyanin content and PAL activity in the skin of immature ‘Jonathan’ apples irradiated with white (2.6 W m−2)+UV (1.0 W m−2) light at 15°C. Bagged fruits were
covered with two layers of paper bags during fruit develop-ment. Apples were harvested on 2nd August. Vertical bars indicate 9SE.
Fig. 2. Anthocyanin content and PAL activity in the skin of ‘Jonathan’ apples irradiated with white (2.6 W m−2)+UV
(1.0 W m−2) light at 15°C. Bagged fruits were covered with
Fig. 3. Anthocyanin content and PAL activity in the skin of ‘Jonathan’ apples irradiated with white (2.6 W m−2)+UV
(1.0 W m−2) light at 15°C. Bagged fruits were covered with
two layers paper of bags during fruit development. Apples were harvested on the 12th September. Vertical bars indicate 9SE.
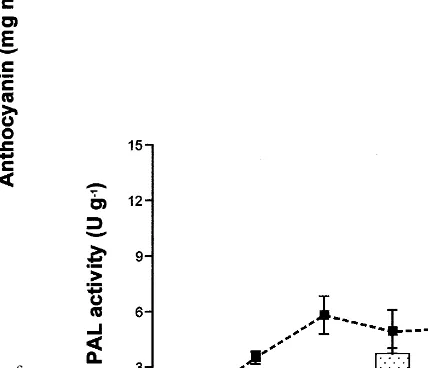
stage), the shaded apples had twice as much an-thocyanin as the bagged apples; in fact, the bagged apples harvested on 29th September pro-duced less anthocyanin than did the bagged ap-ples harvested on 12th September (Fig. 4). The PAL activity in shaded apples increased linearly until 72 h and decreased slightly after 96 h. Maximal PAL activity in the bagged apples was higher than that in shaded apples. Therefore, the decrease in anthocyanin accumulation (when it is compared with that immature or mature stage) in bagged apples at this stage did not correspond to a change in PAL activity.
In the apples harvested on 17th October (154 days after full bloom, ripening stage), the PAL activity in shaded apples increased linearly until 48 h and then remained relatively constant, and the anthocyanin accumulation was increased lin-early until 96 h (Fig. 5). The maximal PAL ac-tivity and anthocyanin accumulation were lower than those of apples harvested on 29th Septem-ber. Thus the decreased PAL activity in shaded apples was coincided with decreased an-thocyanin accumulation.
Fig. 4. Anthocyanin content and PAL activity in the skin of ‘Jonathan’ apples irradiated with white (2.6 W m−2)+UV
(1.0 W m−2) light at 15°C.Bagged fruits were covered with 2
layers of paper bags during fruit development. Apples were harvested on 29th Sep. Vertical bars indicate 9SE.
Fig. 5. Anthocyanin content and PAL activity in the skin of ‘Jonathan’ apples irradiated with white (2.6 W m−2)+UV
(1.0 W m−2) light at 15°C. Apples were harvested on the 17th
October. Vertical bars indicate 9SE.
3.3. Ripening stage
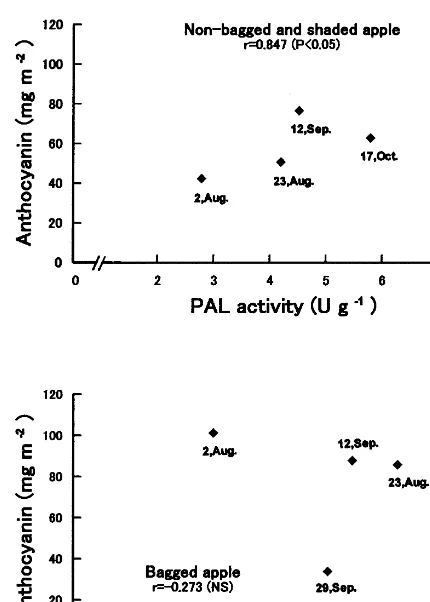
Fig. 6. Distribution plot showing a correlation between PAL activity and anthocyanin content in the skin of ‘Jonathan’ apples irradiated with white (2.6 W m−2)+UV (1.0 W m−2)
light at 15°C for 96 h.
According to Ju et al. (1995), changes in an-thocyanin accumulation can occur independently of changes in PAL activity; the increase of an-thocyanin during maturation involved additional enzymes between leucocyanidin and cyanidin gly-cosides. In our experiment, harvested apples were exposed to UV+white light, a process that can reveal the ability of the fruit to produce an-thocyanin. This method differs from those of Ju et al. (1995) and Lister and Lancaster (1996a,b) who investigated the relationship between anthocyanin accumulation and PAL activity using fruit on the tree. PAL activity in apples has been reported to be induced by light irradiation (Arakawa et al., 1985), while anthocyanin synthesis in apples also requires long light exposure. So it is unlikely that anthocyanin accumulation is initiated without PAL when the fruit is exposed to light.
In bagged apples, the relationship between PAL activity and anthocyanin accumulation was not significant (Fig. 6). PAL activity in the bagged apples was always relatively high, but it was not always related to the induction of higher levels of anthocyanin accumulation. Ju et al. (1995) sug-gested that precursors of anthocyanin synthesis after the PAL stage in bagged apples were defi-cient, and that PAL was critical for pigment formation (with ‘Delicious’ apples 120 days after full bloom). Our experimental methods differed with those of Ju et al. (1995), and we found that the induction of higher levels of anthocyanin ac-cumulation during the immature stage does coin-cide with an increase in PAL activity. But as the apples ripened, the anthocyanin accumulation de-creased, even though PAL activity was relatively high. Therefore, maximal PAL activity is not the only regulating factor for anthocyanin accumula-tion in bagged mature and ripe apples.
References
Arakawa, O., Hori, Y., Ogata, R., 1985. Relative effectiveness and interaction of ultraviolet-B, red and blue light in anthocyanin synthesis of apple fruit. Physiol. Plant. 64, 323 – 327.
Arakawa, O., Hori, Y., Ogata, R., 1986. Characteristics of color development and relationship between anthocyanin synthesis and phenylalanine ammonia-lyase activity in
3.4. Relationship between PAL acti6ity and
anthocyanin accumulation
‘Starking Delicious’, ‘Fuji’ and ‘Mutsu’ apple fruits. J. Jpn. Soc. Hort. Sci. 54 (4), 424 – 430.
Arakawa, O., 1988. Characteristics of color development in some apple cultivars: changes in anthocyanin synthesis during maturation as affected by bagging and light quality. J. Jpn. Soc. Hort. Sci. 57 (3), 373 – 380.
Arakawa, O., 1990a. Anthocyanin synthesis in apple fruit. Agr. Hort. 65 (2), 246 – 250.
Arakawa, O., 1990b. Anthocyanin synthesis in apple fruit. Agr. Hort. 65 (3), 363 – 366.
Arakawa, O., 1991. Effect of temperature on anthocyanin accumulation in apple fruit as affected by cultivar, stage of fruit ripening and bagging. J. Hort. Sci. 66 (6), 763 – 768. Bradford, M.M., 1976. A rapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248 – 254.
Blankenship, S.M., Unrath, C.R., 1988. PAL and ethylene content during maturation of Red and Golden Delicious apples. Phytochemistry 27, 1001 – 1003.
Faragher, J.D., Chalmers, D.J., 1977. Regulation of an-thocyanin synthesis in apple skin. III: Involvement of phenylalanine ammonia-lyase. Aust. J. Plant Physiol. 4, 133 – 141.
Faust, M., 1965. Physiology of anthocyanin development in Mcintosh apple I. participation of pentose phosphate path-way in anthocyanin development. Proc. Amer. Soc. Hort. Sci. 87, 1 – 9.
Given, N.K., Venis, M.A., Grierson, D., 1988. Phenylalanine
ammonia-lyase activity and anthocyanin synthesis in ripen-ing strawberry. J. Plant Physiol. 133, 25 – 30.
Hanson, K.R., Havir, E.A., 1981. Phenylalanine ammonia-lyase. Biochem. Plants 7, 577 – 625.
Ju, Z.G., Yuan, Y.B., Liou, C.L., Xin, S.H., 1995. Relation-ships among phenylalanine ammonia-lyase activity, simple phenol concentrations and anthocyanin accumulation in apple. Sci. Hort. 61, 215 – 226.
Kataoka, I., Kubo, Y., Sugiura, A., Tomana, T., 1983. Changes in L-phenylalanine ammonia-lyase activity and anthocyanin synthesis during berry ripening of three grape cultivars. J. Jpn. Soc. Hort. Sci. 52 (3), 273 – 279. Lancaster, J.E., 1992. Regulation of skin color in apples. Crit.
Rev. Plant Sci. 10, 487 – 502.
Lister, C.E., Lancaster, J.E., 1996a. Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J. Sci. Food Agric. 71, 313 – 320. Lister, C.E., Lancaster, J.E., 1996b. Phenylalanine
ammonia-lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand-grown apple cultivars. J. Amer. Soc. Hort. Sci. 121 (2), 281 – 285.
Tan, S.C., 1979. Relationships and interactions between phenylalanine lyase, phenylalanine ammonia-lyase inactivating system, and anthocyanin in apples. J. Amer. Soc. Hort. Sci. 104 (5), 581 – 586.
Tan, S.C., 1980. Phenylalanine ammonia-lyase and the pheny-lalanine ammonia-lyase inactivating system : Effects of light, temperature and mineral deficiencies. Aust. J. Plant Physiol. 7, 159 – 167.