Levels of mRNAs Encoding Synaptic Vesicle and
Synaptic Plasma Membrane Proteins in the Temporal
Cortex of Elderly Schizophrenic Patients
Boris P. Sokolov, Andrew A. Tcherepanov, Vahram Haroutunian,
and Kenneth L. Davis
Background: Electron microscopy and biochemical
stud-ies indicate that developmental abnormalitstud-ies in synaptic organization may be present in brains of schizophrenic patients. This study determined whether these synaptic abnormalities are reflected in differential or uniform alterations in the expression of various synaptic protein genes in the left superior temporal gyrus of schizophrenic patients.
Methods: Levels of mRNAs encoding four synaptic vesicle
proteins (synaptotagmin I [p65], rab3a, synaptobrevin 1, and synaptobrevin 2) and two synaptic plasma membrane proteins (syntaxin 1A and SNAP-25) were measured post-mortem in the left superior temporal gyrus from elderly (58 –95 years) schizophrenic patients (n5 14) and age-matched control subjects (n5 9).
Results: There were significant negative correlations
between age and levels of synaptotagmin I (p65), rab3a, synaptobrevin 1, SNAP-25, and syntaxin 1A mRNAs in schizophrenic patients (2.692,r, 2.517, .003,p,
.030) but not in control subjects. Levels of all six synaptic mRNAs studied were increased in the younger (58 –79 years) subgroup of schizophrenic patients compared to control subjects and older (80 –95 years) subgroup of schizophrenic patients.
Conclusions: That similar abnormalities were found for
mRNAs encoding different synaptic vesicle and synaptic plasma membrane proteins suggests that they reflect overall neurodevelopmental abnormalities in synaptic connectivity in the temporal cortex of schizophrenic pa-tients rather than changes in the number of synaptic vesicles per synapse or abnormalities in a specific synap-tic function. Biol Psychiatry 2000;48:184 –196 © 2000 Society of Biological Psychiatry
Key Words: Schizophrenia, temporal cortex, synaptic proteins, gene expression, synaptic organization, mRNA
Introduction
S
ynaptic abnormalities may be involved in various sensory processing deficits associated with schizo-phrenia (Feinberg 1982). Electron microscopy studies have revealed ultrastructural changes in the synaptic or-ganization of some brain regions of schizophrenic patients (Miyakawa et al 1972; Ong and Garey 1993; Soustek 1989). Consistent with these data are biochemical findings indicating that region, age, and gene-specific abnormali-ties in expression of some synaptic proteins may be present in brains of schizophrenic patients. These studies have shown a pattern of synaptic protein disregulation with the levels of different synaptic proteins increased, decreased, or unchanged in different brain regions (Browning et al 1993; Eastwood et al 1995; Eastwood and Harrison 1995; Glantz and Lewis 1997; Karson et al 1999; Thompson et al 1998).In one series of studies increased concentrations of synaptophysin, as well as two other synaptic proteins (SNAP-25 and syntaxin), were revealed in cingulate cor-tex from a group of chronically hospitalized elderly schizophrenic patients (Gabriel et al 1997). A subsequent mRNA study in the same cohort of elderly schizophrenic patients provided further evidence that the expression of synaptic protein genes may be increased in some cortical regions of schizophrenic patients, but only in a subgroup of patients younger than 75 years (Tcherepanov and Sokolov 1997). Levels of mRNAs encoding three synaptic vesicle-associated proteins (synaptophysin, synapsin 1A, and synapsin 1B) were increased in the temporal cortex (Brodmann’s areas 21 and 22) of 52–73-year-old schizo-phrenic patients, compared to age matched control sub-jects, and declined significantly with age in schizophrenic patients, but not in control subjects. Coordinated age-associated alterations in expression of mRNAs encoding three major synaptic proteins (synaptophysin and synapsin 1A and synapsin 1B) were hypothesized to indicate overall age-related alterations in synaptic function in the temporal cortex of elderly schizophrenic patients (Tcherepanov and Sokolov 1997). It remains unclear, however, whether these alterations reflect overall abnormalities in synaptic
densi-From Molecular Neurobiology Branch, National Institute on Drug Abuse, National Institutes of Health, Baltimore, Maryland (BPS) and the Department of Psychiatry, Mount Sinai School of Medicine, New York, New York (BPS, AAT, VH, KLD).
Address reprint requests to Boris P. Sokolov, National Institute on Drug Abuse, Molecular Neurobiology Branch, NIH, 5500 Nathan Shock Dr., Baltimore MD 21224.
Received October 13, 1999; revised January 28, 2000; revised February 16, 2000; accepted March 6, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
ty/activity or whether they are restricted to some specific synaptic functions or structures. For example, synaptophy-sin and synapsynaptophy-sin 1 are localized solely to synaptic vesicles; thus, changes in their expression may reflect abnormalities in the number of synaptic vesicles per synapse rather than in the number of synapses.
One way to address these questions is to examine expression of other synaptic protein genes in brains of schizophrenic patients. Different synaptic proteins have different functions and localization in nerve terminals (synaptic vesicles or synaptic plasma membrane) and are differentially expressed in different neurons (Jahn and Su¨dhof 1994; Oyler et al 1989; Su¨dhof 1995; Ullrich and Su¨dhof 1995). Therefore, differential alterations of differ-ent synaptic protein genes may reveal abnormalities in a specific synaptic function/structure and in specific sub-classes of synapses. Alternatively, similar abnormalities in expression of many synaptic protein genes may indicate a general failure of synaptic function or a generalized reduction in synaptic specializations.
This study used measurements of mRNAs encoding four synaptic vesicle proteins (synaptotagmin I [p65], rab3a, synaptobrevin 1, synaptobrevin 2) and two synaptic plasma membrane proteins (syntaxin 1A and SNAP-25) to address the question of whether levels of these synaptic protein mRNAs were differentially or uniformly altered in elderly schizophrenic patients who had previously been found to have abnormal levels of some other synaptic protein mRNAs and synaptic proteins (Gabriel et al 1997; Tcherepanov and Sokolov 1997). The superior temporal gyrus was chosen for study because of multiple studies showing structural abnormalities in this region in schizo-phrenia and suggesting a role for this region in the mediation of thought disorders and auditory hallucinations (Barta et al 1997; Hirayasu et al 1998; Levitan et al 1999; Menon et al 1995; Nestor et al 1993; Pearlson 1997; Pearlson et al 1996; Penfield and Perot 1963; Ross and Pearlson 1996; Shenton et al 1992).
Methods and Materials
Patients
Postmortem brain specimens derived from elderly chronically institutionalized schizophrenic patients (n 5 14) and normal elderly control subjects (n 5 9) were obtained through the Schizophrenia Brain Bank of the Department of Psychiatry at the Mount Sinai School of Medicine, New York. Each case satisfied DSM-III-R criteria for schizophrenia. Eight of the 14 schizo-phrenic patients had been assessed and diagnosed antemortem within 18 months of death by a team of research psychiatrists; the diagnoses for the remaining six cases were based on extensive chart review by the same diagnosis and assessment team. The antemortem assessment battery and assessment procedures have been described in detail previously (Davidson et al 1995). The
general neuropathological characteristics of this cohort of sub-jects have been described in detail (Purohit et al 1998). On neuropathological examination, evidence of an old infarct was found in the temporal cortex of one of the control subjects (case 109) contralateral to the side used for the current study. Three patients (cases 163, 106, and 170) had senile plaque counts in temporal cortex ranging from 3.6 to 6.8 per mm2
. One of these patients (case 170) also had sparse neurofibrillary tangles in temporal cortex. The densities of these neuropathological lesions were normal for age and did not approach density criteria for Alzheimer’s disease (Khachaturian 1985; Mirra et al 1991). Exclusion of these three schizophrenic cases (163, 106, and 170) from analysis produced essentially the same results as the analysis of the total group of schizophrenic cases (data not shown). Other cases were free of neuropathological lesions. There were no histories of drug or alcohol abuse for any of the cases. Demographic characteristics of the cases studied are shown in Table 1. Five schizophrenic cases had very long postmortem intervals (above 60 hours); however, exclusion of these cases from analyses produced essentially the same results as the analyses of the total schizophrenic group (see Results). In addition, there were no significant correlations between postmor-tem interval (PMI) and synaptic protein mRNA levels (see Results). All patients with schizophrenia had been treated with antipsychotic drugs some time in their lives. Ten patients with schizophrenia were free of neuroleptic drugs at least 4 weeks (up to 5 years) prior to death. Four other patients were receiving antipsychotic drugs at the time of death or until 72 hours prior to death. The superior temporal gyrus was dissected from coronal sections at the level of the anterior mammillary body at a level roughly corresponding to coronal section 6 of the atlas published in Damasio and Damasio (1989).
RNA Isolation
Total RNA was isolated from 200 mg of postmortem human brain by the guanidinium isothiocyanate method (Chomczynsky and Sacchi 1987). To remove DNA contamination, the RNA samples were treated with 30 units of DNAse I (5 Prime-3 Prime, Boulder, CO) in a 200mL reaction mixture containing 5 mmol/L MgCl2, 30 mmol/L TrisHCl, pH 7.5; and 100 units of RNase inhibitor (Clontech Laboratories, Palo Alto, CA) for 1 hour at 37°C, extracted twice with phenol/chloroform/isoamyl alcohol, precipitated by the addition of an equal amount of isopropanol and washed three times with cold 70% ethanol. The yield of total RNA was in the range between 48 and 180mg per 200 mg of brain tissue.
Cultured fibroblasts from human kidney were isolated and grown as described previously for skin fibroblasts (Sokolov et al 1995). Approximately 107
cells were used for RNA isolation. RNA extraction and analysis were carried using the same methods as for postmortem brain tissue.
Assay for mRNAs Encoding Synaptic Proteins
mRNAs. This highly specific and sensitive method incorporates controls for possible variability in differential levels of RNA degradation among samples, as well as for variations in efficiency of the RT-PCR and pipetting errors (Ma et al 1994a, 1994b; Sokolov 1998; Stanta and Bonin 1998; Zamorano et al 1996).
Several studies reported on possible effects of PMI, brain pH, or sample storage time on measurements of some mRNAs in postmortem brain tissue (Barton et al 1993; Harrison et al 1995; Johnston et al 1997). Accordingly, the following important factors have been considered and accounted for in the design of mRNA measurements in the current study. First, prolonged PMI, changes in pH, or prolonged sample storage time may indeed potentially lead to changes in the postmortem brain tissue, possibly causing activation of ribonucleases that can non-specif-ically degrade RNA. Ribonucleases are normally compartmen-talized within the cells, however, therefore degradation of mRNA occurs mainly when the ribonucleases begin to diffuse and access RNA molecules. The most critical step here is when the tissue is being removed from the storage at280°C, thawed
and homogenized for RNA extraction or prepared for in situ hybridization. Thawing and homogenization disrupts cellular membranes, enabling access of ribonucleases to RNA and subsequently enabling RNA degradation. This process of degra-dation is very fast and mRNA may be degraded almost com-pletely within minutes, even in freshly prepared tissue. Thus, the level of mRNA degradation depends not only on the amount or activity of ribonucleases, but by and large on the time of ribonucleases’ action, which is the interval between the time of disruption of cellular membranes and time of physical isolation of RNA or inactivation of ribonucleases. Accordingly, our protocol was developed to minimize RNA degradation in the process of RNA isolation. The pulverized brain tissue stored at 280°C was immediately transferred into preheated solution of guanidinium isothiocyanate and homogenized. Guanidinium iso-thiocyanate is a denaturing agent and strong ribonuclease inhib-itor, which disrupts cells, releases RNA, and simultaneously inactivates ribonucleases, thus preventing RNA degradation (Chomczynsky and Sacchi 1987). After RNA isolation, all Table 1. Demographic Data for Autopsy Cases
Group (case)
Interval of time between the last antipsychotic medication and death
Other drugs Gender Age (years)
PMI
(hours) pH Storage
Cause of death Weeks Neuroleptic
Schizophrenic patients
163 .260 Thioridazine Hydroxizine pameate F 84 32 6.2 50 CPF
309 .260 NRb Notriptyline F 65 5.8 5.9 30 CPF
271 .260 NR Buspirone F 82 111.0 6.2 33 CPF
106 250 Chlorpromazine F 86 6.9 5.8 62 ARI
170 176 Thioridazine F 95 60.0 6.7 49 MI
193 106 Haloperidol M 84 6.2 6.5 45 CPF
283 45 Haloperidol Benadryl M 80 91.3 6.4 32 Sepsis, PN
426 9 Thiothixene F 79 20.0 7.1 12 CPF
195 6 Haloperidol M 69 4.5 6.4 37 MI
337 4 Thioridazine F 69 14.0 6.2 25 CPF
199 ,0.4 Thioridazine F 76 8.5 6.1 43 CPF
254 ,0.4 Haloperidol Iorazepam
Hydroxizine pameate, desaril, dilantin
M 58 30.0 6.9 35 CPF
265 ,0.4 Haloperidol M 73 72.0 6.5 34 CPF
287 ,0.4 Haloperidol Iorazepam F 84 111.0 6.4 32 CPF Meansa F9:M5 77.462.6 41.0610.7 6.460.1 37.163.3
Range 58 –95 4.5–111.1 5.8 –7.1 12– 62
Control subjects
232 F 74 3.0 6.0 38 CPF
230 F 96 3.2 6.7 38 CPF
82 F 86 4.7 6.5 73 Unknown
192 F 79 3.0 5.5 45 CPF
22 M 69 6.0 5.8 97 Sepsis
46 M 88 4.8 5.9 86 Unknown
97 M 55 10.0 5.7 65 Lymph
93 M 70 8.0 6.0 67 LGIB
109 M 77 4.3 6.3 61 MI
Meansa F4:M5 77.164.0 5.260.8 6.060.13 63.3620.6
Range 55–96 3–10 5.5– 6.7 38 –97
Cases were partially described previously (Hernandez and Sokolov 1997a; Sokolov 1998). PMI, postmortem interval; NR, no record on neuroleptic treatment within the last 5 years prior to death; CPF, cardiopulmonary failure; ARI, acute respiratory insufficiency; MI, myocardial infarction; PN, pneumonia; Lymph, lymphoma; LGIB, Lower gastrointestinal bleeding.
subsequent manipulations with RNA were carried out in the presence of RNase inhibitor from placenta (Clontech Laborato-ries). The second important consideration is that the relative levels of synaptic mRNAs in the samples were estimated based on amplification of short (below 600 bases) mRNA sequences. Thus, even partially degraded mRNAs were accounted in the measurements. Importantly, random hexamers were used in the reverse transcriptase reaction instead of oligo-dT primers. There-fore, preservation of only those short sequences of mRNAs, which are used for amplification, is necessary for detection, whereas in the case of oligo-dT primers a larger sequence including also a sequence between the amplified fragment and polyA tail is necessary. Additionally, normalization with endog-enously expressedb-actin mRNA was used to further account for differential levels of RNA degradation among the samples (Ma et al 1994a, 1994b; Sokolov 1998; Stanta and Bonin 1998; Zamo-rano et al 1996).
The conditions for RT-PCR were designed to allow simulta-neous amplification of six synaptic mRNAs together withb-actin mRNA in the same reaction with the same efficiency, permitting the precise measurements of mRNA levels relative to one another.b-Actin mRNA is a housekeeping mRNA that is widely used as an internal standard in gene expression studies (Ma et al 1994a, 1994b; Sokolov 1998; Stanta and Bonin 1998; Zamorano et al 1996). Previous studies have shown that its abundance does not differ in postmortem brains of schizophrenic patients as compared to control brains, is independent of the age of the donors, and is not affected by postmortem delay (Sokolov 1998; Tcherepanov and Sokolov 1997). In accordance with previous studies (Sokolov 1998; Tcherepanov and Sokolov 1997), mea-surements of the rawb-actin mRNA levels in the current study revealed no significant correlation with PMI (r5 2.079, n523,
p5.72) Importantly, no significant correlations with PMI were found also for the raw (not normalized withb-actin) levels of any of the synaptic protein mRNAs studied here (2.262 , r , 2.055, .228,p,.802).
Two mg of total RNA were used in 20 mL of reverse transcription reaction to synthesize cDNA using a commercial kit (SuperScript, GIBCO BRL, Grand Island, NY) and random hexanucleotides as primers. Then 0.05mg of the diluted cDNA were amplified in 10mL of PCR reaction using a mixture of seven pairs of primers. Primers STGF (59 -AATAGCCAT-AGTCGCAGTCC-39) and STGR (59 -CCAATTCCGAGTATG-GTACC-39) were designed to amplify a 473– base pair (bp) fragment of synaptotagmin 1 mRNA using published sequence of the gene (Perin et al 1991). Primers S25F (59 -TGAGTCGCT-GGAAAGCACC-39) and S25R (59 -ATCAGCCTTCTCCAT-GATCC-39) were designed to amplify a 490 bp fragment of SNAP-25 mRNA (Zhao et al 1994). Primers STX1F (59 -AGCTGGAAGAACTCATGTCC-39) and STX1R (59 -GAA-CATGTCGTGTAGCTCAC-39) were designed to amplify a 428 bp fragment of syntaxin 1 mRNA (Zhang et al 1995). Primers Syb1F (59-TGCTCCAGCTCAGCCACCT-39) and Syb1R (59 -AACTACCACGATGATGGCAC-39) were designed to amplify a 331 bp fragment of synaptobrevin 1 mRNA (Archer et al 1990). Primers Syb2F (59-CCTCACCAGTAACAGGAGAC-39) and Syb2R (59-GAGGATGATGGCGACAATCA-39) were designed to amplify a 249 bp fragment of synaptobrevin 2 mRNA (Archer
et al 1990). Primers Rab3F (59 –TCGACTTCAAGGTCAA-GACC-39) and Rab3R (59-TCTCGCAGATGACATCCACC-39) were designed to amplify a 387 bp fragment of Rab3a mRNA (Zahraoui et al 1989). Primers BA1 (59 -ACGAAACTACCT-TCAACTCC-39) and BAR (59 -CTTCCTGTAACAATG-CATCTC-39) were designed to amplify a 585 bp fragment of b-actin mRNA using published sequence (Ponte et al 1984). All RT-PCR products were verified by sequencing. The conditions for PCR were: 30 sec at 94°C, 1 min at 54°C, and 1 min at 72°C in a 9600 (Perkin Elmer) temperature cycler. Primers for PCR were 59-end labeled with32
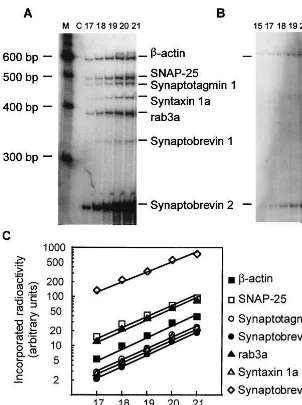
P. The kinetics of amplification were assessed by withdrawing aliquots of the reaction mixture after successive PCR cycles. The PCR products were separated on a 6% polyacrylamide gel containing 2 mol/L urea (Figure 1A). The incorporated radioactivity in each DNA fragment was measured using a phosphostimulable storage plate (Molecular Dynamics, Sunnyvale, CA). As illustrated on Figure 1, amplification kinet-ics for synaptic protein mRNAs andb-actin mRNA were in the exponential phase and were similar for different mRNAs up to 21 cycles of PCR reaction. In subsequent experiments, 20 cycles of amplification were chosen for quantitative assays.
Statistical Analysis
The primary hypothesis (levels of synaptic protein mRNAs decrease with age in schizophrenic patients) was examined using Pearson product moment correlation analysis. One-tailed signif-icance was used, as the hypothesis was directional and derived from earlier findings (Tcherepanov and Sokolov 1997). Note, however, that similar conclusions would have been reached even if two-tailed tests had been used. The correlation between levels of various synaptic protein mRNAs, as well as the effects of PMI, brain pH, and sample storage time were examined using Pearson’s correlation analysis (two-tailed). The effects of with-drawal from antipsychotic medication were examined using a logarithmic model (synaptic protein mRNA vs. logarithm(10)of continuous neuroleptic-free interval before death controlling for age). The logarithmic model was used because of the wide range of neuroleptic free interval (from 72 hours to 260 weeks) and in view of logarithmic modes of correlations with neuroleptic-free interval observed for a number of serotonin and glutamate receptor mRNAs (Hernandez and Sokolov 1997a, 1997b; Sokolov 1998). Differences among groups were examined using two-way analysis of variance (ANOVA) with diagnosis and age group (below or above 79.5 years) as the main factors. Statistical analyses were performed using SPSS 7.5 and Statistica statistical programs. A modification of the Newman–Keuls procedure (Begun and Gabriel 1981; Ryan 1960) was used for post hoc comparisons among means.
Results
Age-Related Differences between Schizophrenic Patients and Control Subjects
temporal gyrus of schizophrenic patients correlated nega-tively and significantly with the age of subjects at time of death (2.692, r , 2.517, .003, p,.03; Figure 2). Similarly, there were significant negative correlations between age of schizophrenic patients and levels of mRNAs encoding the synaptic plasma membrane pro-teins—syntaxin 1A (r5 2.672, p5 .004) and SNAP25 (2.639, p5 .007). No correlation with age was revealed for mRNA encoding another synaptic vesicle protein— synaptobrevin 2 (r 5 .127, p 5 .333). In contrast to schizophrenic patients, there were no significant correla-tions between age and levels of any of synaptic vesicle or synaptic plasma membrane protein mRNAs in control subjects (2.102,r,.520, .099,p,.397; Figure 2). Comparisons of the slopes of the regression lines for these synaptic proteins against age revealed significant slope differences between schizophrenic patients and control subjects for synaptotagmin [F(1,190) 5 6.87, p5 .02],
and nearly significant differences for SNAP25 and syn-taxin 1A [Fs(1,19) . 3.95, ps , .65], confirming the impressions gleaned from Figure 2.
Examining of the raw (not normalized with b-actin) levels of the synaptic protein mRNAs confirmed signifi-cant negative correlations with age in the schizophrenic patients (2.549,r, 2.499, .019,p,.035), but not in the control subjects (2.391,r, 2.152, .149,p, .348). The raw levels ofb-actin mRNA did not correlate significantly with age in both schizophrenic patients (r5 2.395, p5 .081) and control subjects (.332, p5 .192).
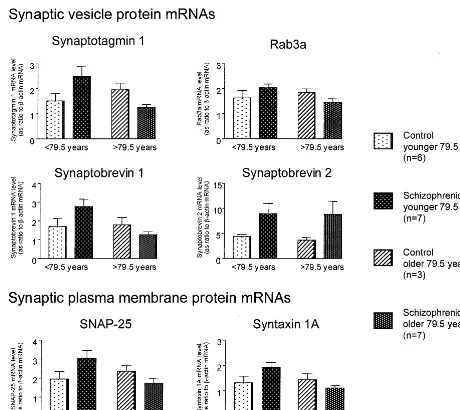
was used as the cutoff age. This cutoff age produces relatively equal group sizes for ANOVA and is higher than those we used previously (75 years) for other synaptic proteins (Tcherepanov and Sokolov 1997). Note, however, that similar conclusions would have been reached if the age of 75 years had been used as the cutoff point. Consistent with our previous findings for mRNAs encod-ing synaptophysin, synapsin 1A, and synapsin 1B, levels of all six synaptic protein mRNAs studied here were higher in the left superior temporal gyrus of “young” (58 –79 years) schizophrenic patients compared to “young” control subjects (55–79 years; Figure 3). In “old” (80 –95 years) schizophrenic patients, levels of five syn-aptic mRNAs were slightly, but not statistically signifi-cantly, lower than in the “old” (86 –96 years) control
subjects, whereas the levels of synaptobrevin 2 mRNA were increased. Two-way ANOVAs revealed significant diagnosis by age– group (below or above 79.5 years) interactions for synaptotagmin 1, rab3a, synaptobrevin 1, SNAP-25, and syntaxin 1A [5.550, F(1,19) , 11.304; .003 , p , .029] but not for synaptobrevin 2 mRNA [F(1,19) 5 0.014; p 5 .907]. Comparisons between groups revealed that the levels of synaptotagmin 1, rab3a, synaptobrevin 1, SNAP-25, and syntaxin 1A mRNAs were significantly (ps,.05) higher in the left superior temporal gyrus of young schizophrenic patients relative to the older schizophrenic group. Furthermore, similar analyses re-vealed that the levels of synaptotagmin 1, synaptobrevin 1, SNAP-25, and syntaxin 1A, but not rab3a, mRNAs were significantly higher in the left superior temporal gyrus of
Figure 3. Levels of synaptic protein messenger RNAs (mRNAs) in the left superior temporal gyrus (Brodmann’s area 22) from control subjects and schizophrenic patients. Levels of synaptic protein mRNAs are represented as ratios tob-actin mRNA. Values are means6
young schizophrenic patients than in the young normal control subjects (ps , .05). Caution should be applied, however, to the results of these latter statistical analyses because the small sample sizes.
Confounding Factors
GENDER. The schizophrenic group had a higher fe-male to fe-male ratio (F9:M5) than the control group (F4: M5), which might potentially have contributed to the differences in correlations with age observed between schizophrenic patients and control subjects; however, separate analyses in gender subgroups revealed that cor-relations with age for synaptotagmin 1, rab3a, synaptobre-vin 1, SNAP-25, and syntaxin 1A mRNAs were direction-ally similar in male (2.945 , r , 2.873, .008 , p, .027, n55) and female schizophrenic patients (2.534,
r, 2.395, .069,p,.171, n59) and were of similar magnitude as the correlations observed in the group as a whole. In addition, no differences in synaptic protein mRNA levels were found between males and females in both the schizophrenic and control groups using analysis of covariance (ANCOVA) with age as a covariate [0.248,F(1,10),1.012, .336,p,.628 and 0.006,
F(1,5), 1.288, .300,p,.942, correspondingly).
POSTMORTEM INTERVAL. The PMI was greater in
the schizophrenic group (4.5–111 hours, mean 5 41.0, SEM 5 10.7) than in the control group (3–10 hours, mean 5 5.2, SEM 5 0.8; p , .001); however, no significant correlations between PMI and synaptic protein mRNAs measured as ratios tob-actin mRNA were found (control subjects,2.481 , r , .040, .190, p, .919; schizophrenic patients,2.377,r, 2.195, .109,p, .504). Furthermore, no significant differences in levels of synaptic protein mRNAs between schizophrenic patients with long PMIs (60 –111 hours) and schizophrenic patients with short PMIs (4.5–32 hours) were found using AN-COVA with age as a covariate [0.005,F(1,13), 3.74, .079,p,.994]. These data indicate that measurements of synaptic protein mRNAs as ratios to b-actin mRNA were not affected significantly by PMI. Nevertheless, additional analyses of associations with age and diagnosis were carried out excluding five schizophrenic cases with the longest PMIs (above 32 hours). These analyses re-vealed essentially the same associations as the analyses of the total group of schizophrenic patients. In this smaller subset of schizophrenic cases (n 5 9), correlations with age were significant for synaptotagmin 1, Rab3a, SNAP-25, and syntaxin 1A (2.756 , r, 2.606, .009, p, .042) but did not reach significance for synaptobrevin 1 mRNA (r 5 2.395, p 5 .146). Two-way ANOVAs revealed significant diagnosis by age– group interactions
for synaptotagmin 1, rab3a, synaptobrevin 1, SNAP-25, and syntaxin 1A [6.491,F(1,14),11.861, .004,p, .023] but not for synaptobrevin 2 mRNA [F(1,14) 5 0.193, p5.667]. Levels of all six mRNAs were nominally higher in young (58 –79 years) schizophrenic patients than in young (55–79 years) control subjects (data not shown).
BRAIN PH AND SAMPLE STORAGE TIME. Brain pH
(a measure that is believed may reflect acidosis at agonal state) in the subject groups studied here (Table 1) was similar to that reported in other studies (for example, mean 5 6.5 [Harrison et al 1995] and mean 5 5.9 [Johnston et al 1997]). Although brain pH in the control subjects was lower than in schizophrenic patients (6.0 and 6.4, respectively, Table 1), these variations in pH are unlikely to account for the age-associated differences in synaptic protein mRNA levels observed between schizo-phrenic patients and control subjects. First, no significant correlations between brain pH and synaptic protein mRNA levels measured as ratios tob-actin mRNA were found in control subjects (2.189,r,.559, .118,p,.681, n5 9), schizophrenic patients (2.083,r,.358, .209,p, .778, n5 14), and the combined subject groups (.098,
r, .340, .119 , p, .657, n5 23). Second, elevated levels of synaptic protein mRNAs in the younger (58 –79 years) subgroup of schizophrenic patients compared to the older (80 –95 years) subgroup of schizophrenic patients could not be associated with pH, because these two schizophrenic subgroups are not different in brain pH (6.460.2 and 6.360.1, respectively). Of note, analysis of the overall quality of RNA, isolated in two different laboratories from the same sample of control subjects and schizophrenic patients and rated blindly based on agarose gel electrophoresis, revealed no significant correlation with pH (data not shown). In addition, similar lack of a relationship between tissue pH and mRNA levels was observed in previously published in situ hybridization and RT-PCR studies of dopamine, serotonin, and glutamate receptor transcripts in an overlapping cohort of subjects (Hernandez and Sokolov 2000; Meador-Woodruff et al 1999; Sokolov 1998).
Similarly, no significant effect of sample storage time on synaptic protein mRNA measurements was found using correlational analysis (control subjects, .010,r, .575, .105,p,.980, n59; schizophrenic patients,2.506,
r, .147, .065 , p , .616, n 5 14; combined groups, 2.234, r, 2.156, .247,p,.476, n 523).
were nonsignificant trends toward negative correlations between neuroleptic-free intervals and synaptotagmin 1 (r 5 2.461, p 5 .113), rab3a (r 5 2.383, p 5 .196), SNAP-25 (r5 2.490, p5.159), syntaxin 1A (r5 2.414,
p5.159), and synaptobrevin 1 (r5 2.227, p5.456), but not synaptobrevin 2 (r 5.242, p 5.425).
Despite the fact that the analyses described above showed that neuroleptic treatment did not exert a statisti-cally significant influence on synaptic protein mRNAs, we re-examined correlations between synaptic protein mR-NAs and age using partial correlation analysis controlling for neuroleptic-free intervals. When controlled for neuro-leptic-free intervals, correlations with age remained sig-nificant for synaptotagmin 1 (r 5 2.584, p 5 .018), SNAP-25 (r 5 2.506, p5 .039), and syntaxin 1A (r5 2.558, p5.024), but not for rab3a (r5 2.362, p5.114) and synaptobrevin 1 (r 5 2.400, p 5 .088). These data indicate that the negative correlations between synaptic protein mRNA levels and age of schizophrenic patients are unlikely to have been due to treatment with neuroleptics.
Nonneuronal Expression of Synaptobrevin 2 mRNA
Unlike other synaptic protein mRNAs studied, levels of synaptobrevin 2 mRNA did not correlate with age of schizophrenic patients (Figure 2). In addition, levels of five synaptic protein mRNAs were highly correlated with each other, with r ranging from .761 to .943 (all ps , .001), whereas the levels of synaptobrevin 2 mRNA did not correlate with the other synaptic protein mRNA levels (.020,r,.308, .153,p,.928). These differences in synaptobrevin 2 mRNA behavior might be in part related to its nonneuronal expression. To test the hypothesis that synaptobrevin 2 mRNA may be expressed in nonneuronal cells, we examined the expression of all six synaptic protein mRNAs in cultured fibroblasts isolated from hu-man kidney. Fibroblasts were chosen as a readily available extreme example of nonneuronal cells that are very un-likely to be contaminated by cells of neuronal origin. Reverse transcription polymerase chain reaction amplifi-cation of total RNA isolated from fibroblasts produced DNA bands with electrophoretic mobilities corresponding to synaptobrevin 2 and b-actin mRNAs (Figure 1). Se-quencing of these DNA bands confirmed that they derive from synaptobrevin 2 andb-actin mRNAs, respectively. No bands corresponding to other synaptic protein mRNAs were amplified from fibroblasts (Figure 1). These data indicate that synaptobrevin 2 mRNA, as well as house-keeping b-actin mRNA, are expressed in fibroblast at significant levels, whereas no detectable expression of other five synaptic protein mRNA occurs. Thus, differ-ences in synaptobrevin 2 mRNA from other synaptic protein mRNAs may be related to its abundant nonneuro-nal expression.
Discussion
This study demonstrates that levels of mRNAs encoding three synaptic vesicle proteins (synaptotagmin 1, rab3a, and synaptobrevin 1) and two synaptic plasma membrane proteins (SNAP-25 and syntaxin 1A) decline significantly with age in the left superior temporal gyrus (BA22) of elderly schizophrenic patients. By contrast, no significant decreases with age were found in age-matched elderly control subjects. The levels of synaptic protein mRNAs in schizophrenic patients younger than 79.5 years were significantly higher than in control subjects and schizo-phrenic patients older than 79.5 years.
The results for mRNAs encoding three synaptic vesicle associated proteins (synaptotagmin 1, rab3a, and synapto-brevin 1) are consistent with previous analysis of three other mRNAs encoding synaptic vesicle proteins (synap-tophysin, synapsin 1A, and synapsin 1B; Tcherepanov and Sokolov 1997). Similar age-related correlations in schizo-phrenia were revealed for mRNAs encoding synaptic plasma membrane proteins—SNAP-25 and syntaxin 1A. The synaptic proteins examined here and previously have different functions and are involved in different steps of synaptic vesicle cycle. Furthermore, they have different subcellular localization and are differentially expressed in different neurons (Jahn and Su¨dhof 1994; Oyler et al 1989; Su¨dhof 1995; Ullrich and Su¨dhof 1995). Synaptotagmin 1 is localized to synaptic vesicles where it functions in the calcium-triggered release of neurotransmitters, possibly serving as a Ca21sensor for exocytosis (Shao et al 1997). Rab3a, a small GTP-binding protein, is concentrated on the surface of synaptic vesicles, unlike integral membrane proteins, such as synaptophysin, synaptobrevin, or synap-totagmin. Rab3a is not permanently associated with the synaptic vesicle membrane and dissociates from synaptic vesicles after exocytosis (Stahl et al 1996). Rab3a is thought to guide membrane fusion between a transport vesicle and the target membrane, and to determine the specificity of docking (Geppert et al 1997). Synaptobrevin is a synaptic vesicle protein that binds tightly to the complex of plasma membrane proteins syntaxin and SNAP-25 (Hayashi et al 1994; Pevsner et al 1994). The synaptobrevin–syntaxin–SNAP-25 complex bridges syn-aptic vesicle and plasma membranes and may function in the docking and/or fusion of synaptic vesicles (McMahon and Su¨dhof 1995). That similar abnormalities were found for multiple mRNAs encoding these different synaptic proteins with different functions and localizations suggests an overall abnormality in synaptic organization in the left superior temporal gyrus of schizophrenic patients rather than abnormalities in specific synaptic functions. The finding that levels of synaptic vesicle and synaptic plasma membrane protein mRNAs correlate highly with each other and reveal similar changes in brains of schizophrenic patients studied here is more consistent with a suggestion of an abnormal synaptic density or functional activity than with alterations in the number of synaptic vesicles per synapse. Future direct studies of synaptic density and synaptic vesicle per synapse ratios in brains from the same group of schizophrenic patients and control subjects would help to test this hypothesis.
Normal cortical development is accompanied by sub-stantial (30 – 40%) decline in synaptic density during adolescence (synaptic pruning; Huttenlocher 1979) with further, though less dramatic (;20 –25%), decrease be-tween ages 16 and 60 years (Masliah et al 1993). No
further decrease with normal aging (but rather a nonsig-nificant trend toward an increase in synaptic density) was found between ages 60 and 98 years (Masliah et al 1993). This finding is consistent with no decrease, (but rather a nonsignificant trend toward an increase) in levels of multiple synaptic protein mRNAs with aging in our cohort of 55–96-year-old normal control subjects (here and pre-viously [Tcherepanov and Sokolov 1997]), which provides an additional support to the view that levels of synaptic protein mRNAs may be valuable markers of synaptic density (Eastwood et al 1995). As such, increased levels of multiple synaptic protein mRNAs found in the temporal cortex of relatively young schizophrenic patients are consistent with the hypothesis that irregularities in pro-grammed synaptic elimination may be involved in schizo-phrenia (Feinberg 1982). In contrast to elderly control subjects, the levels of synaptic protein mRNAs in elderly schizophrenic patients declined significantly with increas-ing age, suggestincreas-ing that synaptic prunincreas-ing may possibly be delayed in schizophrenia. Interestingly, computer simula-tion analysis of a speech percepsimula-tion neural network indi-cates that eliminating up to 65% of working memory connections improves perceptual ability (Hoffman and McGlashan 1997). Thus, a delay in synaptic elimination in Brodmann’s area 22 (an area involved in auditory percep-tion and interpretapercep-tion of auditory stimuli and implicated in thought disorders and auditory hallucinations (Barta et al 1997; Hirayasu et al 1998; Levitan et al 1999; Menon et al 1995; Nestor et al 1993; Pearlson 1997; Pearlson et al 1996; Penfield and Perot 1963; Ross and Pearlson 1986; Shenton et al 1992) may be among the mechanisms involved in impaired speech perception, auditory halluci-nations, and thought disorder in schizophrenia.
associations between synaptic protein mRNA levels and clinical dementia rating was found (data not shown).
A previous immunochemical study of an overlapping cohort of elderly (52–95 years) chronically institutional-ized schizophrenic and control cases revealed significant increase in the levels of synaptophysin, syntaxin and SNAP-25 in the cingulate cortex of schizophrenic patients with similar trends in Brodmann’s areas 20 and 8 but not in Brodmann’s area 7 (Gabriel et al 1997). Analysis of synaptic mRNAs in the temporal cortex (here and previ-ously [Tcherepanov and Sokolov 1997]) provides addi-tional evidence that the expression of synaptic proteins may be increased in some areas of the brains of elderly schizophrenic patients and suggests that this increased expression is present mostly in schizophrenic patients between the ages of 52 and 75 years. Studies of other brain regions in different groups of schizophrenic patients sim-ilar to our cohort of younger schizophrenic patients (mean ages 54.5615.0 and 57.065.0 years) revealed decreased synaptophysin expression in CA4, CA3, subiculum, para-hippocampal gyrus (Eastwood and Harrison 1995; Eas-wood et al 1995) and prefrontal cortex (Brodmann’s areas 9 and 46) but not in the primarily visual cortex (Brodma-nn’s area 17; Glantz and Lewis 1997). Another study reported a decrease of synapsin 1 but not synaptophysin immunoreactivity in hippocampi of schizophrenic patients with mean age of 61.3611.2 years (Browning et al 1993). Significant decrease in synaptophysin and SNAP-25 but not their encoding mRNAs was reported in Brodmann’s area 10 of schizophrenic patients (Karson et al 1999). Increased levels of SNAP-25 in Brodmann’s area 9, decreased in Brodmann’s areas 10 and 20, and normal levels in Brodmann’s area 17 were reported in schizophre-nia (Thompson et al 1998). These data indicate that synaptic abnormalities in schizophrenia may be region specific (increased synaptic density or function in some brain regions, decreased in others and not changed in still other regions) and may derive from differences in patterns of synaptic density development in the normal and dis-eased states.
Recent morphometric studies have revealed abnormally high neuronal density in the cortexes of schizophrenic patients with no changes in glial density (Rajkowska et al 1998; Selemon et al 1995). These findings may suggest that increased expression of synaptic protein genes re-ported here and previously (Gabriel et al 1997; Tcherepanov and Sokolov 1997) may reflect an increased neuronal or neuropil density in schizophrenia.
In conclusion, this study describes age specific abnor-malities in the abundance of mRNAs encoding six synap-tic proteins in the temporal cortex of postmortem brains obtained from patients with schizophrenia. The data re-vealed that in schizophrenic patients, age-related changes
occur in the abundance of mRNAs that encode both for synaptic vesicle and synaptic plasma membrane associated proteins. These findings are consistent with the hypothesis that overall changes in synaptic function, rather than changes in a specific synaptic function or in the number of synaptic vesicles per synapse, may be present in the temporal cortex of schizophrenic patients. These results replicate and extend our previous findings on age-related abnormalities in abundance of various multiple synaptic protein mRNAs in the temporal cortex of elderly schizo-phrenic patients and provide additional support for the hypothesis that developmental synaptic abnormalities may contribute to the pathophysiology of schizophrenia. Future studies using independent methods are necessary to con-firm the findings in this group of elderly chronic schizo-phrenic patients.
This work was supported in part by MHCRC (NIH R2NH56083). The authors gratefully acknowledge Drs. D. Perl and D. Purohit for their neuropathological characterization of the cases studied, and Drs. M. Davidson and P. Powchik for antemortem assessment of schizophrenic cases. We thank Dr. J. Schmeidler for his helpful comments on the statistical analysis.
A preliminary report on the work described here was presented at the Society of Biological Psychiatry Meeting in San Diego, CA, May 1997.
References
Archer BT III, Ozcelik T, Jahn R, Francke U, Su¨dhof TC (1990): Structures and chromosomal localizations of two human genes encoding synaptobrevins 1 and 2. J Biol Chem 265: 17267–17273.
Barta PE, Pearlson GD, Brill LB II, Royall R, McGilchrist IK, Pulver AE, et al (1997): Planum temporale asymmetry reversal in schizophrenia: Replication and relationship to gray matter abnormalities. Am J Psychiatry 154:661– 667. Barton AJ, Pearson RC, Najlerahim A, Harrison PJ (1993):
Pre-and postmortem influences on brain RNA. J Neurochem 61:1–11.
Begun J, Gabriel KR (1981): Closure of the Newman-Keuls multiple comparison procedure. J Am Stat Assoc 76:241–245. Browning MD, Dudek EM, Rapier JL, Leonard S, Freedman R(1993): Significant reductions in synapsin but not synapto-physin specific activity in the brains of some schizophrenics.
Biol Psychiatry 34:529 –535.
Chomczynsky P, Sacchi N (1987): Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 172:156 –159.
Damasio H, Damasio AR (1989): Appendix. In: Damasio H, Damasio AR, editors. Lesion Analysis in Neuropsychology. New York: Oxford University Press, 184 –221.
Davidson M, Harvey P, Powchik P, Parella M, White L, Knobler H, et al (1995): Severity of symptoms in chronically institu-tionalized geriatric schizophrenic patients. Am J
Psychia-try152:197–206.
synap-tophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience 66:309 –319.
Eastwood SL, Harrison PJ (1995): Decreased synaptophysin in the medial temporal lobe in schizophrenia demonstrated using immunoautoradiography. Neuroscience 69:339 –343. Feinberg I (1982): Schizophrenia: Caused by a fault in
pro-grammed synaptic elimination during adolescence? J
Psychi-atr Res 17:319 –334.
Gabriel SM, Haroutunian V, Powchik P, Honer WG, Davidson M, Davies P, et al (1997): Increased concentrations of presynaptic proteins in the cingulate cortex of schizophrenics.
Arch Gen Psychiatry 54:559 –566.
Geppert M, Goda Y, Stevens CF, Sudhof TC (1997): The small GTP-binding protein Rab3a regulates a late step in synaptic vesicle fusion. Nature 387:810 – 814.
Glantz LA, Lewis DA (1997): Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry 45:943–952.
Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC (1995): The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: Selective mRNA vulnerability and com-parison with their encoded proteins. Neurosci Lett 200:151– 154.
Hayashi T, McMahon HT, Yamasaki S, Binz T, Hata Y, Su¨dhof TC, et al (1994): Synaptic vesicle membrane fusion complex: Action of clostridial neurotoxins on assembly. EMBO J 13:5051–5061.
Hernandez I, Sokolov BP (1997a): Abnormal expression of serotonin transporter mRNA in the frontal and temporal cortex of schizophrenics. Mol Psychiatry 2:57– 64.
Hernandez I, Sokolov BP (1997b): Differential changes in gene expression of six serotonin receptors in schizoprenia. Biol
Psychiatry 41(7S):40.
Hernandez I, Sokolov BP (2000): Abnormalities in 5-HT2A receptor mRNA expression in frontal cortex of chronic elderly schizophrenics with varying histories of neuroleptic treatment. J Neurosci Res 59:218 –225.
Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, et al (1998): Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia com-pared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 155:1384 – 1391.
Hoffman RE, McGlashan TH (1997): Synaptic elimination, neurodevelopment, and the mechanism of hallucinated “voices” in schizophrenia. Am J Psychiatry 154:1683–1689. Huttenlocher PR(1979): Synaptic density in the human frontal cortex— developmental changes and effects of aging. Brain
Res 163:195–205.
Jahn R, Su¨dhof TC (1994): Synaptic vesicles and exocytosis.
Annu Rev Neurosci 17:219 –246.
Johnston NL, Cervenak J, Shore AD, Torrey EF, Yolken RH, Cerevnak J (1997): Multivariate analysis of RNA levels from postmortem human brains as measured by three different methods of RT-PCR. J Neurosci Methods 77:83–92. Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JC,
Griffin WSR (1999): Alterations in synaptic proteins and their encoding mRNAs in the prefrontal cortex in
schizophre-nia: A possible neurochemical basis for “hypofrontality”. Mol
Psychiatry 4:39 – 45.
Khachaturian ZS (1985): Diagnosis of Alzheimers’s disease.
Arch Neurol 42:1097–1105.
Levitan C, Ward PB, Catts SV (1999): Sutperior temporal gyral volumes and laterality correlates of auditory hallucinations in schizophrenia. Biol Psychiatry 46:955–962.
Ma YJ, Costa ME, Ojeda SR (1994a): Developmental expression of the genes encoding transforming growth factor alpha and its receptor in the hypothalamus of female rhesus macaques.
Neuroendocrinology 60:346 –359.
Ma YJ, Hill DF, Junier MP, Costa ME, Fedler SE, Ojeda SR (1994b): Expression of epidermal growth factor receptor changes in the hypothalamus during the onset of female puberty. Mol Cell Neurosci 5:246 –262.
Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD (1993): Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43:192–197.
McMahon HY, Sudhof C (1995): Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity a-SNAP binding site. J Biol Chem 270:2213–2217. Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M,
Davis KL, Watson SJ (1999). Dopamine receptor transcript expression in the striatum, prefrontal and occipital cortex: Focal abnormalities in area 11 in schizophrenia. Arch Gen
Psychiatry 54:1089 –1095.
Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, et al (1995): Posterior superior temporal gyrus in schizophrenia: Grey matter changes and clinical correlates.
Schizophr Res 16:127–135.
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al (1991): The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part ii. Standardization of the neuropathological assessment of Alzheimer’s disease.
Neu-rology 41:479 – 486.
Miyakawa T, Sumiyoshi S, Deshimura M, Suzuki T, Tomonari H, Yasouaka F, et al (1972): Electron microscopy study on schizophrenia: Mechanism of pathological changes. Acta
Neuropathol (Berl) 20:67–77.
Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, et al (1993): Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J
Psychiatry 150:1849 –1855.
Ong WY, Garey LJ (1993): Ultrastructural features of biopsied temporal cortex (area 38) in a case of schizophrenia.
Schizo-phr Res 10:15–27.
Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingley M, Bloom FE, et al (1989): The identification of a novel synaptosomal-associated protein, SNAP-25, differentially ex-pressed by neuronal subpopulations. J Cell Biol 109:3039 – 3052.
Pearlson GD (1997): Superior temporal gyrus and planum temporale in schizophrenia: A selective review. Prog
Neuro-psychopharmacol Biol Psychiatry 21:1203–1229.
Pearlson GD, Petty RG, Ross CA, Tien AY (1996): Schizophre-nia: A disease of heteromodal association cortex?
Neuropsy-chopharmacology 14:1–17.
Perin MS, Johnston PA, Ozcelik T, Jahn B, Francke U, Su¨dhof TC (1991): Structural and functional conservation of synap-totagmin (p65) in Drosophila and humans. J Biol Chem 266:615– 622.
Pevsner J, Hsu SC, Braun JEA, Calacos N, Ting AE, Bennett MK, et al (1994): Specificity and regulation of a synaptic vesicle docking complex. Neuron 13:353–361.
Ponte P, Ng SY, Engel J, Gunning P, Kedes L (1984): Evolu-tionary conservation in the untranslated regions of actin mRNAs: DNA sequence of human beta-actin cDNA. Nucleic
Acids Res 12:1687–1996.
Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson D, Davis KL (1998): Alzheimer disease and related neurodegen-erative diseases in elderly patients with schizophrenia. A postmortem neuropathologic study of 100 cases. Arch Gen
Psychiatry 55:205–211.
Rajkowska G, Selemon LD, Goldman-Rakic PS (1998): Neuro-nal and glial somal size in the prefrontal cortex. A postmor-tem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 55:215–224.
Ross CA, Pearlson GD (1986): Schizophrenia, the heteromodal association neocortex and development: Potential for a neu-rogenetic approach. Trends Neurosci 19:171–176.
Ryan TA (1960): Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol Bull 57:318 –328.
Schramm M, Falkai P, Tepest R, Schneider-Axmann T, Przkora R, Waha A, et al (1999): Stability of RNA transcripts in post-mortem psychiatric brains. J Neural Transm 106:329 –335. Selemon LD, Rajkowska G, Goldman-Rakic PS (1995): Abnor-mally high neuronal density in the schizophrenic cortex: A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 52:805– 818.
Shao X, Li C, Fernandez I, Zhang X, Sudhof TC, Rizo J (1997): Synaptotagmin-syntaxin interaction: The C2 domain as a Ca21-dependent electrostatic switch. Neuron 18:133–142. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible
CG, et al (1992): Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med 327:604 – 612. Sokolov BP (1998): Expression of NMDA, GluR1, GluR7 and
KA1 glutamate receptor mRNAs is decreased in frontal cortex of “neuroleptic-free” schizophrenics. Evidence on
reversible up-regulation by typical neuroleptics. J Neurochem 71:2454 –2464.
Sokolov BP, Ala-Kokko L, Dhulipala R, Arita M, Khillan JS, Prockop DJ (1995): Tissue-specific expression of the gene for type I procollagen (COL1A1) in transgenic mice. Only 476 base pairs of the promoter are required if collagen genes are used as promoters. J Biol Chem 270:9622–9629.
Soustek Z (1989): Ultrastructure of cortical synapses in the brain of schizophrenics. Zentralbl Allg Pathol Anat 135:25–32. Stahl B, Chou JH, Li C, Sudhof TC, Jahn R (1996): Rab3
reversibly recruits rabphilin to synaptic vesicles by a mech-anism analogous to raf recruitment by ras. EMBO J15:1799 – 1809.
Stanta G, Bonin S (1998): RNA quantitative analysis from fixed and paraffin-embedded tissues: Membrane hybridization and capillary electrophoresis. Biotechniques 24:271–276. Su¨dhof TC (1995): The synaptic vesicle cycle: A cascade of
protein–protein interactions. Nature 375:645– 653.
Tcherepanov AA, Sokolov BP (1997): Age-related abnormalities in expression of mRNAs encoding synapsin 1A, synapsin 1B, and synaptophysin in the temporal cortex of schizophrenics.
J Neurosci Res 49:639 – 644.
Thompson PM, Sower AC, Perrone-Bizzozero NI (1998): Al-tered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry 43:239 –243.
Ullrich B, Su¨dhof TC (1995): Differential distributions of novel synaptotagmins: Comparison to synapsins.
Neuropharmacol-ogy 34:1371–1377.
Zahraoui A, Toushot N, Chardin P. Tavitian A (1989): The human rab genes encode a family of gtp-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem 264:12394 –12401.
Zamorano PL, Mahesh VB, Brann DW (1996): Quantitative RT-PCR for neuroendocrine studies. Neuroendocrinology 63:397– 404.
Zhang RD, Maksymowych AB, Simpson LL (1995): Cloning and sequence analysis of a cDNA encoding human syntaxin 1A, a polypeptide essential for exocytosis. Gene 159:293– 294.