Hippocampal Pathophysiology May Mirror Global
Brain Alterations
Masahiro Fujita, Dennis S. Charney, and Robert B. Innis
The recent development of [carbonyl-11C]WAY-100635 for serotonin (5-HT)1Aand [
18
F]setoperone and [18 F]al-tanserin for 5-HT2Apositron emission tomography recep-tor imaging has allowed studies of 5-HT neurotransmis-sion in depressive disorders. The hippocampus is likely to be an important brain structure in the pathophysiology of depression because it may mediate both cognitive deficits and hypercortisolemia found in this disorder. Decreased 5-HT1A binding was reported in the medial temporal cortex, which receives dense 5-HT innervation, and also throughout neocortical regions. Because the 5-HT1A an-tagonist pindolol may hasten antidepressant effects of selective serotonin reuptake inhibitor medications, its receptor occupancy has been measured in both presynap-tic and postsynappresynap-tic sites. The results are controversial but suggest that pindolol has preferential occupancy of soma-todendritic autoreceptors in the raphe. The results of 5-HT2A receptors are mixed, with one showing a signifi-cant decrease in the right orbitoinsular cortex and three not detecting a significant change. The disparate findings in patients with depression almost certainly reflect the heterogeneity of the disorder, and we highlight the utility of the hippocampus as a useful target region not only to compare depressed subjects with healthy subjects but also to correlate findings with cognitive function and activity of the limbic– hypothalamic–pituitary axis system. Biol Psychiatry 2000;48:801– 812 ©2000 Society of Biologi-cal Psychiatry
Key Words: Stress, corticosteroids, pindolol, emission tomography, neurogenesis, drug effect
Introduction
A
bnormalities in several neurotransmission systems, including dopamine, serotonin (5-hydroxytrypta-mine; 5-HT), norepinephrine, acetylcholine, and substanceP, may be relevant to the pathophysiology of depression. The 5-HT system has been the most extensively studied, in part because of the therapeutic efficacy of selective sero-tonin reuptake inhibitor (SSRI) medications. The hypoth-esis that depression is caused by low 5-HT transmission was derived from the findings of largely peripheral mea-sures, such as low plasma L-tryptophan, reduced 5-HT content and serotonin transporters in blood platelets, and low CSF levels of the 5-HT metabolite 5-hydroxyin-doleacetic acid (5-HIAA; reviewed in Maes and Meltzer 1995). These results have been replicated with sufficient consistency to justify direct measurements in brain tissue. With the development of tracers for 5-HT receptors and transporters, several postmortem studies have been per-formed in brain tissue from suicide victims, a portion of which had depressive disorders. As reviewed previously (Staley et al 1998), the results of these studies vary significantly. One potential source of this variability was that both suicidal attempt and depression may be relevant to the alterations in 5-HT system, and, in some studies, retrospective case histories of depression were missing or inaccurate.
Brain imaging studies of the 5-HT system in well-characterized living patients will be useful both to assess abnormalities and to delineate the potential roles of 5-HT neurotransmission in specific pathophysiologic features of the disorder (e.g., patients with and without suicidal features or those with and without cognitive deficits). Among more than a dozen 5-HT receptor subtypes, tracers suitable for PET imaging have been developed for 5-HT1A
([carbonyl-11C]WAY-100635 (N-[2-[4-(2-methoxyphe-nyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexane car-boxamide trihydrochloride)) and 5-HT2A([
18
F]setoperone and [18F]altanserin) receptors. This article will review the ongoing PET studies of 5-HT1Aand 5-HT2Areceptors as
well as preliminary single photon emission computed tomography (SPECT) investigations of serotonin trans-porter (SERT). Because the hippocampus is likely to play an important role in the pathophysiology of depression (including cognitive dysfunction and hypercortisolemia), these studies will be reviewed with the orientation that this
From the Departments of Psychiatry (MF, RBI) and Pharmacology, Yale University School of Medicine and VA Connecticut, West Haven (RBI), and Program on Mood and Anxiety Disorders, National Institute of Mental Health, Bethesda, Maryland (DSC).
Address reprint requests to Masahiro Fujita, M.D., Ph.D., VA Connecticut Healthcare System/116A2, 950 Campbell Avenue, West Haven CT 06516. Received March 9, 2000; revised May 30, 2000; accepted June 23, 2000.
brain structure will be a useful target to examine abnor-malities and covariance of symptomatology in this disorder.
Stress, Corticosteroids, and the
Hippocampal 5-HT
1AReceptor
Serotonin 1A receptors are densely distributed in the hippocampus. Increased activity of the hypothalamic– pituitary–adrenal (HPA) axis has been consistently re-ported in severe depression, and recent animal studies suggest that interactions among stress, corticosteroids, and hippocampal 5-HT1A receptor play a critical role in
depression.
Limbic–Hypothalamic–Pituitary–Adrenal Axis Function in Depression
Because mood disorders are frequently associated with high levels of the stress hormone cortisol, abnormalities in the system regulating its secretion may be pathogenic in the disorder. The secretion of cortisol is regulated by the limbic– hypothalamic–pituitary–adrenal (LHPA) axis. The neurons in the medial parvocellular division of the para-ventricular nucleus of the hypothalamus (mpPVN) synthe-size corticotropin-releasing hormone (CRH), which stim-ulates the secretion of adrenocorticotropin hormone (ACTH) from the anterior pituitary corticotropes; ACTH activates synthesis and release of glucocorticoids such as corticosterone and cortisol from the adrenal cortex. The hippocampus provides negative feedback to this HPA axis. The removal of the hippocampus reduces but does not eliminate the efficacy of cortisol inhibition (Sapolsky et al 1990). The hippocampus is distinguished from other feedback sites, including the hypothalamus and pituitary, by the high expression of both mineralocorticoid (MR) and glucocorticoid (GR) receptors (Jacobson and Sapolsky 1991), enabling it to modulate the HPA axis over a wide range of corticosteroid levels. The increased response of the LHPA axis to stress after hippocampal damage or antagonism of hippocampal GR (Feldman and Conforti 1980; Sapolsky et al 1984) suggests these receptors also contribute significantly to HPA axis regulation.
Decades of research have led to the conclusion that a subgroup of depressed patients exhibit hypercortisolemia due to LHPA dysregulation. Evidence supporting this assertion includes, but is not limited to, dexamethasone nonsuppression (Carroll et al 1981; Rush et al 1996), abnormal 24-hour secretion of plasma cortisol (Murphy 1991; Sachar et al 1973), and elevated secretion of 24-hour urinary free cortisol (Anton 1987; Rothschild et al 1993). Hypercortisolemia may be associated with more severe, recurrent illness with specific neuropsychologic
impair-ments (Force 1987; Nemeroff and Evans 1984; Rothschild et al 1993).
The mechanism underlying the hypercortisolemia of depression is multifactoral. Possible causes include 1) increased secretion of CRH from mpPVN, 2) enhanced pituitary secretion of ACTH, 3) enhanced adrenal secre-tion of cortisol, 4) decreased sensitivity to negative feed-back at the level of the hypothalamus, pituitary, or hippocampus (Krishnan et al 1991; McAllister-Williams et al 1998; Nemeroff 1996; Nemeroff et al 1992).
5-HT, Corticosteroids, and Hippocampal Neurogenesis
Studies in laboratory animals have shown that glucocorti-coids, which are released during stress, are associated with damage to neurons of the hippocampus, (McEwen et al 1992; Sapolsky 1996; Sapolsky et al 1990). Monkeys who died spontaneously following exposure to severe stress were found on autopsy to have multiple gastric ulcers, consistent with exposure to chronic stress, and hyperplas-tic adrenal corhyperplas-tices, consistent with sustained cortisol release. These monkeys also had damage to the CA3 subfield of the hippocampus (Uno et al 1989). Studies in a variety of animal species indicate that stress and in-creased glucocorticoid exposure results in dein-creased hip-pocampal dendritic branching (Watanabe et al 1992; Woolley et al 1990), alterations in hippocampal synaptic terminal structure (Magarinos et al 1997), and a loss of hippocampal neurons (Uno et al 1990).
Contrary to prior beliefs, neurogenesis (i.e., cell divi-sion with the production of new neurons) is known to occur in the adult as well as the developing brain. Gould and colleagues demonstrated neurogenesis initially in rodent hippocampus (Cameron et al 1993). Neurogenesis was subsequently demonstrated in nonhuman primate (Gould et al 1998) and even human hippocampus (Eriks-son et al 1998). Stress has been shown to reduce neuro-genesis in the CA3 hippocampal region of adult rodents and primates (Gould et al 1998). Furthermore, reduction of corticosteroids by adrenalectomy restored neurogenesis in the hippocampus of aged rats (Cameron and McKay 1999). The implication of these findings for both depres-sion and stress disorders is that the associated elevation of cortisol may not only damage existing neurons, but also decrease the production of new neurons. In addition, a vicious cycle could be initiated in which cortisol-induced damage of the hippocampus further reduces the ability of this structure to inhibit additional release of cortisol.
Both postsynaptic 5-HT1Areceptor in the hippocampus
corticosteroids and the 5-HT1A receptor are robust and
may have clinical relevance (Lopez et al 1998; Meijer and de Kloet 1998). Several groups have reported that adre-nalectomy causes increases in 5-HT1A receptor binding
and gene expression (Chalmers et al 1994; Mendelson and McEwen 1990). Acute and chronic uncontrollable stress increased plasma corticosterone and decreased hippocam-pal 5-HT1A receptor binding and 5-HT1A mRNA levels
(Lopez et al 1998; Lopez et al 1999; Maines et al 1999). Furthermore, the elimination of the stress-induced increase in corticosteroids by adrenalectomy prevented the de-crease in hippocampal 5-HT1Areceptor levels (Lopez et al
1998). These results suggest that the 5-HT1A
downregu-lation observed after chronic stress is mediated, at least in part, by increases in plasma corticosteroids; however, there is also a report showing that long-term exposure to high corticosterone decreased 5-HT induced hyperpolar-ization but did not decrease 5-HT1AmRNA levels (Karten
et al 1999).
The mechanisms underlying the effect of stress-induced corticosteroid downregulation of 5-HT1A receptors may
relate to the localization of MR, GR, and 5-HT1Areceptors in the hippocampus. The highest concentration of 5-HT1A mRNA expression occurs in the granular layer of the dentate gyrus, which also contains high densities of MR and GR mRNA. In fact, areas positive for 5-HT1A
recep-tors overlap with 95% of those positive for MR mRNA (Lopez et al 1998). Although the close association be-tween hippocampal 5-HT1A, GR, and MR mRNA does not absolutely confirm the colocalization of these receptors in the same neurons, the data are consistent with the electro-physiologic studies indicating that these receptors are colocalized in the pyramidal cells of the CA1 subfield (Joels et al 1991). Corticosteroids probably decrease 5-HT1Areceptors via interactions with MR. Under
phys-iologic conditions, the association of corticosteroids with MR tonically suppresses the responsiveness of postsynap-tic 5-HT1Areceptors, probably mediated by both receptor
downregulation and suppression of 5-HT1A receptor
me-diated hyperpolarization of CA1 pyramidal neurons (Mei-jer et al 1997). Further, Gould and colleagues have recently presented preliminary data that 5-HT stimulates the production of new hippocampal granule cell neurons through 5-HT1Areceptors (Jacobs et al 1998; Radley et al 1998) and that chronic antidepressant administration in-creases neurogenesis in adult rats (Malberg et al 1999).
The animal studies reviewed above suggest that stress or elevated cortisol associated with depression would decrease neuronal density and neurogenesis. In fact, MRI studies of the hippocampus have reported decreased size in patients with depression and that the decrease was correlated with the lifetime duration of depressive symp-toms (Sheline et al 1999). Furthermore, patients with
hippocampal atrophy may tend to be those who are treatment resistant (Shah et al 1998). Therefore, all of these studies can be combined into a single hypothesis that a subgroup of patients with depression would be charac-terized by hypercortisolemia, reduced hippocampal vol-ume, reduced hippocampal 5-HT1Areceptor density, and treatment resistance. Receptor imaging studies in depres-sion will be reviewed within this hypothetical framework.
The Postsynaptic 5-HT1A Receptor in Depression:
Postmortem Studies
Based on the animal studies and the hypothesis described above, 5-HT1A receptor density in hippocampus is
ex-pected to be decreased in depression, particularly in patients with high cortisol levels. The results of postmor-tem studies have not been consistent, however. Two groups did not find a change in 5-HT1A density in
hippocampus in suicide victims with major depression using [3H]8-hydroxy-2-[di-n-propyl-amino]tetralin (8-OH-DPAT) autoradiography (Lowther et al 1997; Stock-meier et al 1997). One group reported a significantly decreased hippocampal 5-HT1A mRNA, decreased
hip-pocampal MR mRNA, and a decreased hiphip-pocampal MR/GR ratio in suicide victims with a history of major depression, changes similar to those found in animals subjected to chronic unpredictable stress (Lopez et al 1998).
A few groups also studied the density of 5-HT1A
receptors in frontal cortex of suicide victims using [3
H]8-OH-DPAT. Two did not find a change in receptor density (Lowther et al 1997; Stockmeier et al 1997), whereas two found an increased receptor density (Matsubara et al 1991; Arango et al 1995), one of which found an increase only in nonviolent suicide (Matsubara et al 1991). Only one of these studies confirmed an ongoing episode of depression at the time of suicide, however (Stockmeier et al 1997). The increased 5-HT1A receptor densities reported by the
two groups may not necessarily contradict the hypothesis regarding hippocampal pathology in depression. Regula-tion of 5-HT1A receptors in brain may be regionally selective. For example, hippocampal 5-HT1A receptors,
because their colocalization with MR and GR (Joels et al 1991), may be more sensitive to circulating corticoste-roids, whereas receptors in the prefrontal cortex may be less responsive to corticosteroids and more responsive to local 5-HT concentrations.
which is distinct from, but interactive with, that of depres-sion. For example, lower 5-HIAA levels were reported in more medically damaging suicide attempts (Mann et al 1996). Because of the difficulty of retrospectively deter-mining diagnoses and symptomatology in suicide victims, PET studies of living patients more easily provide com-plete characterizations, which may be assessed relative to in vivo neurochemical measurements.
The Postsynaptic 5-HT1A Receptor in Depression:
PET Studies
Positron emission tomography studies focusing on 5-HT1A
receptor have been hampered by the lack of an appropriate tracer. A major advance was the development of the highly selective antagonist WAY-100635 (Fletcher et al 1994). This tracer was subsequently labeled with [11C] at either theO-methyl or carbonyl positions. Carbonyl labeling is preferable, although more difficult, because it does not produce radiolabeled lipophilic metabolites which enter the brain and increase nondisplaceable activity (Osman et al 1998). On the other hand, theO-methyl labeled tracer does produce a radiolabeled lipophilic metabolite that enters the brain and binds to 5-HT1Aanda1
-adrenorecep-tors (Osman et al 1996).
One potential problem of [carbonyl-11C]WAY-100635
is the generation of an active metabolite, [carbonyl
-11
C]desmethyl-WAY-100635, which also binds to 5-HT1A
receptors (Osman et al 1998; Pike et al 1998). However, the production of the desmethyl metabolite appears to be negligibly small in human (Osman et al 1998; Pike et al 1998). In fact, the desmethyl compound itself (i.e., [ car-bonyl-11C]desmethyl-WAY-100635) can be used as a
5-HT1Areceptor probe (Pike et al 1998). Although [
car-bonyl-11C]WAY-100635 seems well suited as a PET tracer, improved ligands are under development, including [18F]FCWAY (fluoro-cyclohexyl WAY analog; Lang et al
1999), which showed high specific-to-nondisplaceable ratio and suitable pharmacokinetic characteristics for PET studies in nonhuman primates (Carson et al 2000) and has technical advantages of the longer lived18F nuclide (T
1/2
110 min) compared with11C (T1/220 min). Because of the
short half-life of [carbonyl-11C]WAY-100635, the reli-ability of the late time point data (even 60 min after injection) may be poor, particularly the measurement of the radiotracer’s concentration in plasma. The use of the longer physical half-life of 18F should ameliorate these
difficulties.
Quantitation of PET receptor imaging often requires concurrent analysis of the parent tracer in arterial plasma. Errors in these plasma measurements confound the recep-tor outcome values and may be particularly problematic for short-lived isotopes such as 11C. Lammertsma has
developed a reference tissue model for the analysis of neuroreceptor levels that does not require plasma measure-ments (Lammertsma and Hume 1996). This method uses a brain region devoid of receptors (e.g., cerebellum) as a substitute for arterial plasma levels of the radiotracer. An extension of this method by Gunn et al (1998) provides an image in which individual pixel values correlate with receptor densities. Images of groups (e.g., depressed pa-tients vs. healthy subjects) can then be analyzed by a pixel-based method, such as statistical parametric mapping (SPM), to survey all regions and pixels of the brain. Both the tissue reference model and its extension to pixelwise measurements were developed in part to analyze 5-HT1A
receptor images and are certainly significant advances in PET image analysis. Nevertheless, controversy still exists as to whether these methods can be accurately applied to [carbonyl-11C]WAY-100635 images because the cerebel-lum may not reflect nondisplaceable uptake or may show significant differences between subjects or between groups. The report from a recent meeting on PET imaging of the 5-HT1A receptor provides a discussion of these technical issues (http://www.ki.se/org/way/).
The first 5-HT1Areceptor imaging studies in depressed patients were performed at Hammersmith Hospital, where the tracer [carbonyl-11C]WAY-100635 was developed (Sargent et al 1999). To date, two complete studies have been published using a reference tissue analytic method: one from Hammersmith Hospital (Sargent et al 2000) and one from University of Pittsburgh (Drevets et al 1999). Sargent et al (2000) found moderate reductions (about 10%) of 5-HT1Areceptor levels throughout brain
includ-ing medial temporal cortex (hippocampus and amygdala) in unmedicated depressed patients. Drevets et al (1999) found a greater 27% decrease of binding potential in medial temporal cortex and a 42% decrease in the dorsal raphe of seven unmedicated depressed patients (Table 1). The decrease in mesiotemporal cortex was not correlated with plasma cortisol nor with 24-hour urinary free cortisol levels. There was a difference in the magnitude of de-creases between these two studies, and there was also a difference in subject population: Drevets et al included patients with a history of bipolar disorder, whereas Sargent et al did not. Sargent et al (2000) also studied the effects of SSRI treatment. The reduced levels of 5-HT1Areceptors were not altered in 10 of the subjects, who were scanned again during SSRI treatment. Half of these 10 patients were considered SSRI responders, but the resulting sample size was probably inadequate to assess potential normal-ization of receptor levels with remission of depression.
predom-inantly binds to the high-affinity state of the receptor, whereas the PET studies used an antagonist [carbonyl
-11
C]WAY-100635, which binds to both high- and low-affinity states. The low-affinity state is regulated by coupling and uncoupling to a G-protein. The extent of G-protein coupling in vivo is unknown under normal conditions, and the level of one G-protein subunit was reported to be decreased in prefrontal cortex of depressed suicide vic-tims, with the hippocampus not examined (Pacheco et al 1996). Therefore, changes in G-protein coupling to the 5-HT1A receptor may be a source of the discrepancy
between some postmortem studies and these PET studies. Furthermore, in vivo extracellular levels of 5-HT may modify radioligand binding by either blocking access of the tracer to the receptor or modifying its cellular traffick-ing, which is known to exist for most G-protein coupled receptors (Bloch et al 1999). One study showed a lower level of total tissue 5-HT levels in the right hippocampus of suicide victims with depression (Cheetham et al 1989), whereas another did not (Owen et al 1986). The influence of extracellular 5-HT levels on the PET measurement of [carbonyl-11C]WAY-100635 binding are currently under investigation and not fully understood (Parsey et al 1999); however, if depression is associated with low 5-HT neurotransmission (and low synaptic 5-HT concentra-tions), then the decreased uptake of [carbonyl-11 C]WAY-100635 in depressed patients could not be explained by 5-HT blockade of the receptor and, thereby reduced binding access of the radiotracer. The effects of agonists and antagonists on cellular trafficking are complex and may well modify the ability of the tracer to bind to an internalized and potentially modified receptor. Neverthe-less, with these caveats in mind, the first two 5-HT1A
receptor imaging studies in depression both reported the predicted decrease in receptor binding, but in a global and not regionally selective manner.
The Presynaptic 5-HT
1AReceptor
The presynaptic 5-HT1A receptors in the raphe nuclei
regulate the release of 5-HT through a negative feedback mechanism. Blier and de Montigny (1994) have
hypothe-sized that abnormally increased function of the somato-dendritic 5-HT1Areceptor has an etiologic role in
depres-sion and that a common mechanism of action of several antidepressant treatments is the downregulation of presyn-aptic 5-HT1Areceptor function. That is, the inhibition of
5-HT reuptake by SSRIs does not lead to increased 5-HT levels in terminal synapses until an adaptive desensitiza-tion of inhibitory 5-HT1Aautoreceptors has occurred. In a
series of studies, De Montigny, Blier, and colleagues have demonstrated that the antidepressant mechanism of action of several classes of antidepressant medications are related to downregulation of dorsal raphe 5-HT1A receptors,
resulting in a net increase in forebrain 5-HT transmission (Blier and de Montigny 1998).
Consistent with this hypothesis, the concomitant use of a 5-HT1A receptor antagonist with antidepressants may
hasten medication response. Recent therapeutic studies have evaluated whether coadministration of pindolol (an antagonist at both b-adrenoreceptors and 5-HT1A recep-tors) and an SSRI will hasten antidepressant drug sponse. Some studies showed accelerated therapeutic re-sponse by concurrent use of pindolol (Bordet et al 1998; Perez et al 1997; Zanardi et al 1997), but others did not (Berman et al 1997; Perez et al 1999). This discrepancy may indicate that concurrent use of pindolol is effective in only a subgroup of patients, which has not yet been clearly identified.
Positron Emission Tomography Studies of Presynaptic 5-HT1AReceptors
As mentioned above, two studies with [carbonyl
-11C]WAY-100635 have reported decreased presynaptic
5-HT1A receptor levels in the raphe region of depressed patients. Sargent et al (2000) reported a 14% decrease; Drevets et al (1999) reported a 42% decrease (Table 1). Both of these in vivo results are contrary to the 5% to 30% increase (the increase was dependent on the rostral-to-caudal level) of postmortem 5-HT1Areceptor binding in
dorsal raphe reported by Stockmeier et al (1998). As described in the section on postsynaptic receptor imaging, difference of tracers (agonist vs. antagonist) and the
Table 1. Binding at 5-HT Receptors Measured in Positron Emission Tomography
Receptor Author Brain region Findings
1A Drevets et al 1999 Mesiotemporal cortex 27% decrease
Raphe 42% decrease
Sargent et al 1999, 2000 Cortices including limbic areas 11% decrease
Raphe 14% decrease
influence of extracellular 5-HT must be considered as sources of this discrepancy. In fact, tissue 5-HT levels in midbrain are more than twice as that in terminal regions (Dewar et al 1992). Furthermore, in vivo imaging methods such as PET have intrinsic limitations in their abilities to measure structures as small as the raphe nuclei. Decreased 5-HT1Areceptor binding measured with PET may actually
reflect a decrease in the number of 5-HT neurons and shrinkage of raphe without change in the density of the receptors per remaining neuron. In the opposite direction, a postmortem study reported a 32% significant increase in neuronal density without change in the volume of dorsal raphe in suicide victims primarily with major depression (Underwood et al 1999). If substantiated, this increased neuronal density may help explain the increased number of 5-HT1A receptors in the postmortem study of
Stock-meier et al (1998).
Positron emission tomography receptor imaging would seem particularly well suited to measure 5-HT1A
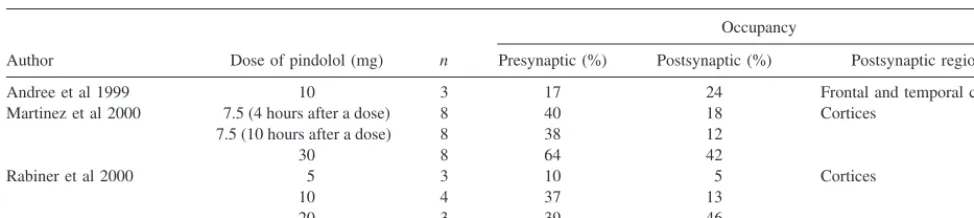
occu-pancy by pindolol and the hypothesis that pindolol has higher affinity/occupancy of presynaptic (i.e., raphe) than postsynaptic (i.e., hippocampal) sites. 5-HT1A receptor
occupancy by pindolol in human subjects has been mea-sured by three groups. Following oral administration, receptor occupancy was dose related. Occupancies in the presynaptic site were as follows: 17 to 40% for 7.5 to 10 mg and 39 to 64% for 20 to 30 mg; and in postsynaptic regions 12 to 24% for 7.5 to 10 mg and 42 to 46% for 20 to 30 mg (Table 2). Using [carbonyl-11C]WAY-100635, two of the three studies reported that pindolol had higher occupancy of presynaptic than postsynaptic sites (Andree et al 1999; Martinez et al 2000; Rabiner et al 2000). Rabiner et al showed a higher occupancy of pindolol at presynaptic receptors at a dose of 10 mg (37% vs. 13%) but not at the doses of 5 and 20 mg given orally 2 hours before the PET study (Table 2; Rabiner et al 2000) indicating that the appropriate dose range of pindolol is small. Using a larger sample size, Martinez et al showed higher occupancy at the presynaptic site by both 7.5 and 30 mg given after 1 week of treatment with 7.5 mg q.d. The difference in the occupancy in pre- and postsynaptic
sites was greater after 7.5 mg. The results by these two groups may indicate that differential occupancies are achieved by a relatively low dose.
The 5-HT
2AReceptor
5-HT2A Receptor Density
As reviewed previously (Staley et al 1998), more than 10 studies have reported the densities of 5-HT2Areceptors in
prefrontal cortex of suicide victims. Many studies used the antagonist [3H]ketanserin, which has only moderate (2- to 140-fold, most findings are close to the lower end) selectivity for the 5-HT2Avs. 5-HT2Creceptor. About half
of the studies did not detect a significant change; the other half showed significantly increased binding; and only one detected a significant decrease. As in other 5-HT markers, however, the potentially differential effects of suicide and depression were difficult to discriminate in these postmor-tem studies.
Preliminary SPECT and PET 5-HT2Areceptor imaging
studies were performed in patients with depression using [2-123I]ketanserin and [11C]-N-methyl spiperone. These tracers suffer from major limitations of high nonspecific uptake or no selectivity relative to dopamine D2receptors.
Recently, several PET studies of depression have been performed using a new generation of tracers, [18 F]setoper-one and [18F]altanserin. The former has 100 times greater affinity for 5-HT2Athan 5-HT2Creceptors but only 10- to
50-fold higher affinity for 5-HT2 compared with D2
receptors. Even this 10- to 50-fold selectivity may be inadequate in the striatum, where the density of D2
receptors is so much higher than that of 5-HT2receptors.
Therefore, [18F]setoperone is useful to study cortex where the density of D2 receptors is low. On the other hand,
[18F]altanserin has more than 100-fold selectivity relative to dopamine D2 receptors but only 20-fold selectivity
relative to 5-HT2C receptors (Tan et al 1999). Even this
modest selectivity relative to 5-HT2C receptors may be
adequate for quantitative studies, when combined with the relatively low density of these sites in neocortex. One
Table 2. Receptor Occupancy by Pindolol Measured in Positron Emission Tomography
Author Dose of pindolol (mg) n
Occupancy
Presynaptic (%) Postsynaptic (%) Postsynaptic regions
Andree et al 1999 10 3 17 24 Frontal and temporal cortices
Martinez et al 2000 7.5 (4 hours after a dose) 8 40 18 Cortices 7.5 (10 hours after a dose) 8 38 12
30 8 64 42
Rabiner et al 2000 5 3 10 5 Cortices
10 4 37 13
limitation of [18F]altanserin is that it produces radioactive lipophilic metabolites, which probably cross the blood– brain barrier. Intersubject variability in peripheral olism may introduce different levels of lipophilic metab-olites in brain and may affect measures of specific-to-nondisplaceable ratio, in which cerebellum is used as a receptor-poor region. These metabolites have been par-tially characterized (Baldwin et al 1998). Furthermore, a deuterated analog of [18F]altanserin has been synthesized to decrease production of these metabolites (Tan et al 1997, 1999), and a method using bolus plus constant infusion has been instituted to correct for the presence of any residual radiolabeled metabolites that have entered the brain (van Dyck et al, in press).
Using [18F]altanserin, a significant reduction in tracer uptake was detected in right posterolateral orbitofrontal and anterior insular cortices in medication-free depressed patients (Biver et al 1997); however, three studies using [18F]altanserin (Meltzer et al 1999) and [18F]setoperone (Attar-Levy et al 1999; Meyer et al 1999b) did not detect a significant change in medication-free depressed patients (Table 1). Unfortunately, some of these studies suffer from major methodologic limitations. By applying large vol-umes of interest (VOIs), Meyer et al (1999b) and Attar-Levy et al (1999) may have overlooked small regions with significant changes. In fact, postmortem studies indicated that changes in receptor density often are modest in magnitude and anatomically restricted to only one or two Brodmann areas (Arango et al 1997). Therefore, pixel-based analysis such as SPM may be necessary to detect these changes. Some readers may doubt the results by Biver et al because they initially analyzed relative changes in receptor density by applying global normalization and by using only brain data without correction for plasma tracer levels; however, they did post hoc VOI analyses for the region detected by SPM and corrected the data using the uptake in cerebellum. Therefore, the significant de-crease they detected may be true. Nevertheless, lipophilic metabolites of [18F]altanserin may enter the brain and contribute activity in nondisplaceable compartment. Therefore, the measurement using cerebellum as a refer-ence region is not justified if there is a systematic difference in the metabolism of [18F]altanserin between depressed patients and healthy subjects.
In summary, the results of 5-HT2Areceptor imaging in
depression report either decreased levels or no change, in
apparent contradiction to postmortem studies that have often found increased 5-HT2Areceptor levels. Limitations
in technical aspects of the 5-HT2Areceptor PET studies
justify caution in the generalizability of these findings and support the utility of studies with larger sample sizes to examine potential correlates of depressive symptomatol-ogy and subtypes with the imaging results.
The Effect of Antidepressants on 5-HT2AReceptors The 4- to 6-week time lag between initiating antidepressant treatment and clinical response suggest that alterations in the 5-HT system occurring during this period are relevant to the therapeutic mechanism of the medications. Animal studies have shown that administration of tricyclic antidepressants and monoamine oxidase inhibitors cause a downregulation in the number of 5-HT2 receptors (Cowen 1990; Eison and
Mullins 1996), whereas the results with SSRIs are mixed (Beasley et al 1992; Cadogan et al 1993; Eison and Mullins 1996). Two PET [18F]setoperone studies were conducted to
investigate changes in 5-HT2Areceptors before and after 3 to
4 weeks of desipramine (Yatham et al 1999) or clomipramine (Attar-Levy et al 1999) treatment in depressed patients. Both of these studies showed significant decreases in tracer bind-ing in almost all cortical regions (Table 3), which is consis-tent with prior animal experiments. The PET studies have significant methodologic limitations, however. The analysis by Yatham et al (1999) was based on the assumption of no substantial change in global binding, although the results showed such global alterations with desipramine treatment. In addition, because the analysis used global normalization in SPM, the results did not indicate a decrease in absolute quantity of binding potential but a decrease relative to the binding potential in the whole brain. Potential changes in the clearance of tracer were not taken into account in the measurement by Attar-Levy et al (1999). Because the affinity of 5-HT at 5-HT2Areceptors is low compared with that at
5-HT1A receptors, potential changes of extracellular 5-HT
levels induced by antidepressants probably did not affect the measurements. In fact, a single oral administration of 20 mg paroxetine did not change the binding of [18F]setoperone
(Meyer et al 1999a).
The 5-HT Transporter
Because the 5-HT transporter in platelet has been consid-ered a model of its counterpart in brain, many studies have
Table 3. The Effects of Antidepressant Treatment on 5-HT2AReceptors Measured in Positron
Emission Tomography
Author Medication Decrease of binding (%) Brain region
been conducted to compare [3H]imipramine and [3
H]par-oxetine binding between depressed patients and healthy subjects. Although the results were mixed, the majority (65–75%) of studies have confirmed reductions in platelet SERT levels in patients with major depression. A meta-analysis has shown this effect to be robustly significant across studies, including those examining high-affinity binding sites (Ellis and Salmond 1994). Consistent with findings of decreased platelet SERT binding, and as reviewed previously (Staley et al 1998), two postmortem studies have reported a significantly decreased 5-HT transporter levels in hypothalamus.
Using [123I]b-CIT and SPECT, the binding potential in
hypothalamus and midbrain was measured in patients. Medication-free depressed patients showed a significant decrease of 18% (Malison et al 1998). A significant decrease was also detected in midbrain in alcoholism, and the decrease was significantly correlated with depressive symptoms (Heinz et al 1998). Therefore, decreased SERT levels in the midbrain may be related to depressive symptomatology.
A significant limitation of [123I]b-CIT is its lack of
selectivity for serotonin versus dopamine transporters (i.e., SERT vs. DAT). Although studies in nonhuman primates suggest that the majority of activity detected in the “midbrain” region is associated with SERT (Laruelle et al 1993), a more selective tracer should be used in patients with depression. In addition, [123I]b-CIT does not show
measurable specific binding in cortical regions. Therefore, efforts have been focused on the development of improved PET and SPECT tracers selective for SERT and capable of measuring the relatively low cortical levels above the nonspecific uptake of the tracer. The following tracers are recently developed: [11C] and [18F]McN5652 (Suehiro et
al 1993, 1996), [11C]-labeled cocaine analogs with high
selectivity (Helfenbein et al 1999), [123I] and [76
Br]nitro-quipazine (Jagust et al 1996; Lundkvist et al 1999), [123I]IDAM (Kung et al 1999), and [123I]ODAM (Acton et
al 1999). Most of these tracers suffer from some limita-tions such as slow kinetics, insufficient specific-to-nondis-placeable ratio, or need for additional validation studies. Among them, [123I]IDAM is perhaps the most promising
at the moment. Nevertheless, none of these tracers have been shown to provide measurable specific binding in cortical regions.
Conclusions
The development of suitable PET tracers for 5-HT1Aand
5-HT2Areceptors has recently allowed studies to further
elucidate probable abnormalities of serotonergic neuro-transmission in depression. These studies have consis-tently shown decreased 5-HT1A receptor binding in
pre-and postsynaptic brain areas in depressed patients, but much more mixed results in cortical 5-HT2A receptor
levels. The latter finding may well be related to the heterogeneity of depressive disorders and could reflect selected symptoms present in subsets of patients (e.g., suicidality). Preliminary studies have also reported de-creased SERT binding in the brain stem of depressed patients, but the tracer ([123I]b-CIT) also binds to
dopa-mine transporters and therefore is not selective for SERT. Although preliminary, these studies are clearly promising and justify further efforts. For example, study of larger sample sizes would help determine whether these brain markers are associated with subtypes of the disorder or with specific clinical symptoms. New tracers would also be useful, including probes selective for SERT or entirely new tracers (e.g., glucocorticoid, CRH or substance P receptors) of likely relevance to depressive pathophysiol-ogy. Finally, because of the documented abnormalities in the LHPA axis in depression (including hypercortisolemia and cognitive deficits), the hippocampus is likely to be a valuable target area for several of these studies. PET studies of molecular alterations in the hippocampus can be connected meaningfully to pathophysiologic features of the disorder. The changes in this region might mirror global changes and can be used either as a model for or an important contrast to changes in other brain regions.
This work was supported by funds from the National Institute of Mental Health (Grant Nos. MH58620 and MH30929) and the Department of Veterans Affairs (Research Enhancement Award Program in Depression).
The authors thank Drs. E.A. Rabiner (Hammersmith Hospital) and M. Laruelle (Columbia University) for helpful discussions.
Aspects of this work were presented at the conference “Depression in the Twenty-First Century: New Insights into Drug Development and Neurobiology,” February 21–22, 2000, Dana Point, California. The conference was sponsored by the Society of Biological Psychiatry through an unrestricted educational grant provided jointly by Pharmacia & Upjohn and Janssen Pharmaceutica.
References
Acton PD, Mu M, Plossl K, Hou C, Siciliano M, Zhuang ZP, et al (1999): Single-photon emission tomography imaging of serotonin transporters in the nonhuman primate brain with [123I]ODAM.
Eur J Nucl Med26:1359 –1362.
Andree B, Thorberg SO, Halldin C, Farde L (1999): Pindolol binding to 5-HT1A receptors in the human brain confirmed with positron emission tomography. Psychopharmacology (Berl)144:303–305.
Anton RF (1987): Urinary free cortisol in psychotic depression.
Biol Psychiatry22:24 –34.
Arango V, Underwood MD, Mann JJ (1997): Postmortem findings in suicide victims. Implications for in vivo imaging studies.Ann N Y Acad Sci836:269 –287.
Attar-Levy D, Martinot JL, Blin J, Dao-Castellana MH, Crouzel C, Mazoyer B, et al (1999): The cortical serotonin2 receptors studied with positron-emission tomography and [18
F]-se-toperone during depressive illness and antidepressant treat-ment with clomipramine.Biol Psychiatry45:180 –186. Baldwin RM, Tan P-Z, van Dyck CH, Al-Tikriti M, Amici L,
Roth B, et al (1998): Radiometabolites of [18F]altanserin in
rats and humans: 4-(p-[18F]fluorobenzoyl)piperidine and
[18F]altanserinol. J Labeled Compounds Radiopharm 41:
133–135.
Beasley CM, Masica DN, Potvin JH (1992): Fluoxetine: A review of receptor and functional effects and their clinical implications.Psychopharmacology (Berl)107:1–10. Berman RM, Darnell AM, Miller HL, Anand A, Charney DS
(1997): Effect of pindolol in hastening responses to fluoxetine in the treatment of major depression: a double-blind placebo-controlled trial.Am J Psychiatry154:37– 43.
Biver F, Wikler D, Lotstra F, Damhaut P, Goldman S, Men-dlewicz J (1997): Serotonin 5-HT2 receptor imaging in major depression: Focal changes in orbito-insular cortex. Br J Psychiatry171:444 – 448.
Blier P, de Montigny C (1994): Current advances and trends in the treatment of depression.Trends Pharmacol Sci15:220 – 226.
Blier P, de Montigny C (1998): Possible serotonergic mecha-nisms underlying the antidepressant and anti-obsessive-com-pulsive disorder responses.Biol Psychiatry44:313–323. Bloch B, Dumartin B, Bernard V (1999): In vivo regulation of
intraneuronal trafficking of G protein-coupled receptors for neurotransmitters.Trends Pharmacol Sci20:315–319. Bordet R, Thomas P, Dupuis B (1998): Effect of pindolol on
onset of action of paroxetine in the treatment of major depression: Intermediate analysis of a double-blind, placebo-controlled trial. Reseau de Recherche et d’Experimentation Psychopharmacologique.Am J Psychiatry155:1346 –1351. Cadogan AK, Marsden CA, Tulloch I, Kendall DA (1993):
Evidence that chronic administration of paroxetine or fluox-etine enhances 5-HT2 receptor function in the brain of the guinea pig.Neuropharmacology32:249 –256.
Cameron HA, McKay RD (1999): Restoring production of hippocampal neurons in old age.Nat Neurosci2:894 – 897. Cameron HA, Woolley CS, McEwen BS, Gould E (1993):
Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat.Neuroscience56:337–344.
Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, et al (1981): A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical util-ity.Arch Gen Psychiatry38:15–22.
Carson RE, Lang L, Watabe H, Der MG, Adams HR, Jagoda E, et al (2000): PET evaluation of [18F]FCWAY, an analog of
the 5-HT1Aantagonist WAY 100635.Nucl Med Biol27:493–
497.
Chalmers DT, Lopez JF, Vazquez DM, Akil H, Watson SJ (1994): Regulation of hippocampal 5-HT1A receptor gene expression by dexamethasone. Neuropsychopharmacology
10:215–222.
Cheetham SC, Crompton MR, Czudek C, Horton RW, Katona CL, Reynolds GP (1989): Serotonin concentrations and turn-over in brains of depressed suicides.Brain Res502:332–340. Cowen PJ (1990): A role for 5-HT in the action of antidepressant
drugs.Pharmacol Ther46:43–51.
Dewar KM, Grondin L, Carli M, Lima L, Reader TA (1992): [3H]paroxetine binding and serotonin content of rat cortical
areas, hippocampus, neostriatum, ventral mesencephalic teg-mentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment.
J Neurochem58:250 –257.
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al (1999): PET imaging of serotonin 1A receptor binding in depression.Biol Psychiatry46:1375–1387.
Eison AS, Mullins UL (1996): Regulation of central 5-HT2A receptors: A review of in vivo studies. Behav Brain Res
73:177–181.
Ellis PM, Salmond C (1994): Is platelet imipramine binding reduced in depression? A meta-analysis. Biol Psychiatry
36:292–299.
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nor-dborg C, Peterson DA, et al (1998): Neurogenesis in the adult human hippocampus.Nat Med4:1313–1317.
Feldman S, Conforti N (1980): Participation of the dorsal hippocampus in the glucocorticoid feedback effect on adre-nocortical activity.Neuroendocrinology30:52–55.
Fletcher A, Cliffe IA, Forster EA, Bill DJ, Y R (1994): A pharmacological profile of WAY-100635, a potent and selec-tive 5-HT1Areceptor antagonist.Br J Pharmacol112:91P.
Force AT (1987): The dexamethasone suppression test: An overview of its current status in psychiatry. The APA Task Force on Laboratory Tests in Psychiatry. Am J Psychiatry
144:1253–1262.
Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E (1998): Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress.Proc Natl Acad Sci U S A95:3168 –3171.
Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, et al (1998): Tracer kinetic modeling of the 5-HT1A
receptor ligand [carbonyl-11C]WAY-100635 for PET. Neu-roimage8:426 – 440.
Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, et al (1998): Reduced central serotonin transporters in alcoholism.Am J Psychiatry155:1544 –1549.
Helfenbein J, Sandell J, Halldin C, Chalon S, Emond P, Okubo Y, et al (1999): PET examination of three potent cocaine derivatives as specific radioligands for the serotonin trans-porter.Nucl Med Biol26:491– 499.
Jacobs BL, Tanapat P, Reeves AJ, Gould E (1998): Serotonin stimulates the production of new hippocampal granule neu-rons via the 5HT1A receptor in the adult rat.Soc Neurosci Abstr24:1992.
Jacobson L, Sapolsky R (1991): The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocor-tical axis.Endocr Rev12:118 –134.
Joels M, Hesen W, de Kloet ER (1991): Mineralocorticoid hormones suppress serotonin-induced hyperpolarization of rat hippocampal CA1 neurons.J Neurosci11:2288 –2294. Karten YJ, Nair SM, van Essen L, Sibug R, Joels M (1999):
Long-term exposure to high corticosterone levels attenuates serotonin responses in rat hippocampal CA1 neurons.Proc Natl Acad Sci U S A96:13456 –13461.
Krishnan KR, Doraiswamy PM, Lurie SN, Figiel GS, Husain MM, Boyko OB, et al (1991): Pituitary size in depression.
J Clin Endocrinol Metab72:256 –259.
Kung MP, Hou C, Oya S, Mu M, Acton PD, Kung HF (1999): Characterization of [123I]IDAM as a novel single-photon
emission tomography tracer for serotonin transporters. Eur J Nucl Med26:844 – 853.
Lammertsma AA, Hume SP (1996): Simplified reference tissue model for PET receptor studies.Neuroimage4:153–158. Lang L, Jagoda E, Schmall B, Vuong BK, Adams HR, Nelson
DL, et al (1999): The development of F-18 labeled, reversible 5-HT1A silent antagonists with polar metabolites. J Med Chem42:1576 –1586.
Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, Al-Tikriti MS, et al (1993): SPECT imaging of dopamine and serotonin transporters with [123I]b-CIT: Pharmacological
characterization of brain uptake in nonhuman primates. Syn-apse13:295–309.
Lopez JF, Chalmers DT, Little KY, Watson SJ (1998): Regula-tion of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression.Biol Psychiatry43:547–573. Lopez JF, Liberzon I, Vazquez DM, Young EA, Watson SJ
(1999): Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress.Biol Psychiatry45:934 – 937.
Lowther S, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW (1997): 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls.J Affect Disord42:199 –207.
Lundkvist C, Loc’h C, Halldin C, Bottlaender M, Ottaviani M, Coulon C, et al (1999): Characterization of bromine-76-labelled 5-bromo-6-nitroquipazine for PET studies of the serotonin transporter.Nucl Med Biol26:501–507.
Maes M, Meltzer H (1995): The serotonin hypothesis of major depression. In: Bloom F, Kupfer D, editors. Psychopharma-cology: The Fourth Generation of Progress. New York: Raven, 933–944.
Magarinos AM, Verdugo JM, McEwen BS (1997): Chronic stress alters synaptic terminal structure in hippocampus.Proc Natl Acad Sci U S A94:14002–14008.
Maines LW, Keck BJ, Smith JE, Lakoski JM (1999): Cortico-sterone regulation of serotonin transporter and 5-HT1A re-ceptor expression in the aging brain.Synapse32:58 – 66. Malberg JE, Eisch AJ, Nestler EJ, Duman RS (1999): Chronic
antidepressant administration increases granule cell genesis in the hippocampus of the adult male rat. Soc Neurosci Abstr
25:1029.
Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, et al (1998): Reduced brain serotonin transporter availability in major depression as measured by [123I]-2
beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single
photon emission computed tomography.Biol Psychiatry44: 1090 –1098.
Mann JJ, Malone KM, Psych MR, Sweeney JA, Brown RP, Linnoila M, et al (1996): Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpa-tients.Neuropsychopharmacology15:576 –586.
Martinez D, Mawlawi O, Hwang D-R, Kent J, Simpson N, Parsey RV, et al (2000): Positron emission tomography study of pindolol occupancy of 5-HT1A receptors in humans:
Preliminary analyses.Nucl Med Biol27:523–527.
Matsubara S, Arora RC, Meltzer HY (1991): Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims.J Neural Transm85:181–194. McAllister-Williams RH, Ferrier IN, Young AH (1998): Mood
and neuropsychological function in depression: The role of corticosteroids and serotonin.Psychol Med28:573–584. McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D,
Gannon MN, et al (1992): Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry31:177–199.
Meijer OC, de Kloet ER (1998): Corticosterone and serotonergic neurotransmission in the hippocampus: Functional implica-tions of central corticosteroid receptor diversity. Crit Rev Neurobiol12:1–20.
Meijer OC, Van Oosten RV, De Kloet ER (1997): Elevated basal trough levels of corticosterone suppress hippocampal 5-hydroxytryptamine(1A) receptor expression in adrenally intact rats: Implication for the pathogenesis of depression.
Neuroscience80:419 – 426.
Meltzer CC, Price JC, Mathis CA, Greer PJ, Cantwell MN, Houck PR, et al (1999): PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders.Am J Psychi-atry156:1871–1878.
Mendelson SD, McEwen BS (1990): Testosterone increases the concentration of [3H]8-hydroxy-2-(di-n-propylamino)tetralin
binding at 5-HT1A receptors in the medial preoptic nucleus of the castrated male rat.Eur J Pharmacol181:329 –331. Meyer JH, Cho R, Kennedy S, Kapur S (1999a): The effects of
single dose nefazodone and paroxetine upon 5-HT2A binding potential in humans using [18F]-setoperone PET. Psycho-pharmacology (Berl)144:279 –281.
Meyer JH, Kapur S, Houle S, DaSilva J, Owczarek B, Brown GM, et al (1999b): Prefrontal cortex 5-HT2 receptors in depression: An [18F]setoperone PET imaging study.
Am J Psychiatry156:1029 –1034.
Murphy BE (1991): Steroids and depression.J Steroid Biochem Mol Biol38:537–559.
Nemeroff CB (1996): The corticotropin-releasing factor (CRF) hypothesis of depression: New findings and new directions.
Mol Psychiatry1:336 –342.
Nemeroff CB, Evans DL (1984): Correlation between the dexa-methasone suppression test in depressed patients and clinical response.Am J Psychiatry141:247–249.
Nemeroff CB, Krishnan KR, Reed D, Leder R, Beam C, Dunnick NR (1992): Adrenal gland enlargement in major depression. A computed tomographic study. Arch Gen Psychiatry 49: 384 –387.
metabolites of the 5-HT1A receptor radioligand, [O-methyl-11C]WAY-100635, in monkey and human plasma by HPLC: Comparison of the behaviour of an identified radioactive metabolite with parent radioligand in monkey using PET.
Nucl Med Biol23:627– 634.
Osman S, Lundkvist C, Pike VW, Halldin C, McCarron JA, Swahn CG, et al (1998): Characterisation of the appearance of radioactive metabolites in monkey and human plasma from the 5-HT1A receptor radioligand, [carbonyl-11
C]WAY-100635— explanation of high signal contrast in PET and an aid to biomathematical modeling.Nucl Med Biol25:215–223. Owen F, Chambers DR, Cooper SJ, Crow TJ, Johnson JA, Lofthouse R, et al (1986): Serotonergic mechanisms in brains of suicide victims.Brain Res362:185–188.
Pacheco MA, Stockmeier C, Meltzer HY, Overholser JC, Dilley GE, Jope RS (1996): Alterations in phosphoinositide signal-ing and G-protein levels in depressed suicide brain.Brain Res
723:37– 45.
Parsey RV, Hwang DR, Simpson N, Guo NN, Mawlawi O, Kegeles LS, et al (1999): PET studies of competition between [11C]carbonyl-WAY 100635 and endogenous serotonin. J Nucl Med40:29P.
Perez V, Gilaberte I, Faries D, Alvarez E, Artigas F (1997): Randomised, double-blind, placebo-controlled trial of pindo-lol in combination with fluoxetine antidepressant treatment.
Lancet349:1594 –1597.
Perez V, Soler J, Puigdemont D, Alvarez E, Artigas F (1999): A double-blind, randomized, placebo-controlled trial of pindo-lol augmentation in depressive patients resistant to serotonin reuptake inhibitors.Arch Gen Psychiatry56:375–379. Pike VW, Halldin C, McCarron JA, Lundkvist C, Hirani C,
Olsson H, et al (1998): [carbonyl-11
C]Desmethyl-WAY-100635 (DWAY) is a potent and selective radioligand for central 5-HT1Areceptors in vitro and in vivo.Eur J Nucl Med
25:338 –346.
Rabiner EA, Gunn RN, Castro ME, Sargent PA, Cowen PJ, Koepp MJ, et al (2000):b-Blocker binding to human 5-HT1A
receptorsin vivoandin vitro. Implications for antidepressant therapy.Neuropsychopharmacology23:285–293.
Radley JJ, Jacobs BL, Tanapat P, Gould E (1998): Blockade of 5HT1A receptors prevents hippocampal granule cell genesis during and after pilocarpine-induced status epileptics. Soc Neurosci Abstr24:1992.
Rothschild AJ, Samson JA, Bond TC, Luciana MM, Schildkraut JJ, Schatzberg AF (1993): Hypothalamic-pituitary-adrenal axis activity and 1-year outcome in depression.Biol Psychi-atry34:392– 400.
Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR Jr, Weissenburger JE, et al (1996): The dexamethasone suppres-sion test in patients with mood disorders. J Clin Psychiatry
57:470 – 484.
Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher TF (1973): Disrupted 24-hour patterns of cortisol secretion in psychotic depression.Arch Gen Psychi-atry28:19 –24.
Sapolsky RM (1996): Why stress is bad for your brain.Science
273:749 –750.
Sapolsky RM, Krey LC, McEwen BS (1984): Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response.Proc Natl Acad Sci U S A
81:6174 – 6177.
Sapolsky RM, Uno H, Rebert CS, Finch CE (1990): Hippocam-pal damage associated with prolonged glucocorticoid expo-sure in primates.J Neurosci10:2897–2902.
Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al (2000): Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635:
Effects of depression and antidepressant treatment.Arch Gen Psychiatry57:174 –180.
Sargent PA, Rabiner EA, Kjær KH, Messa C, Cowen PJ, Bench CJ, et al (1999): PET imaging of 5HT1A receptors with
[11C]-WAY100635 in depressed patients before and after
SSRI treatment.J Cereb Blood Flow Metab19:318. Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM (1998):
Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study.Br J Psychiatry172:527–532. Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999):
De-pression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depres-sion.J Neurosci19:5034 –5043.
Staley JK, Malison RT, Innis RB (1998): Imaging of the serotonergic system: interactions of neuroanatomical and functional abnormalities of depression. Biol Psychiatry 44: 534 –549.
Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thomp-son PA, Meltzer HY (1997): Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology
16:162–173.
Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G (1998): Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity.J Neu-rosci18:7394 –7401.
Suehiro M, Greenberg JH, Shiue CY, Gonzalez C, Dembowski B, Reivich M (1996): Radiosynthesis and biodistribution of the S-[18F]fluoroethyl analog of McN5652.Nucl Med Biol
23:407– 412.
Suehiro M, Scheffel U, Dannals RF, Ravert HT, Ricaurte GA, Wagner HN, Jr (1993): A PET radiotracer for studying serotonin uptake sites: Carbon-11-McN-5652Z. J Nucl Med
34:120 –127.
Tan PZ, Baldwin RM, Soufer R, van Dyck CH, Charney DS, Innis RB (1997): Application of the deuterium isotope effect to enhance PET signal by impeding tracer metabolism: Synthesis of deuterium and fluorine-18 dual-labeled altan-serin and its nitro precursor. J Labeled Compounds Radio-pharm40:130 –132.
Tan PZ, Baldwin RM, Van Dyck CH, Al-Tikriti M, Roth B, Khan N, et al (1999): Characterization of radioactive metab-olites of 5-HT2A receptor PET ligand [18F]altanserin in
human and rodent.Nucl Med Biol26:601– 608.
Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, et al (1999): Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims.Biol Psychi-atry46:473– 483.
Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, et al (1990): Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus.
Brain Res Dev Brain Res53:157–167.
Hippocampal damage associated with prolonged and fatal stress in primates.J Neurosci9:1705–1711.
van Dyck CH, Tan P-Z, Baldwin RM, Amici L, Garg PK, Ng CK, et al (2000): PET quantification of 5-HT2A receptors in the human brain: a constant infusion paradigm with [18
F]al-tanserin.J Nucl Med41:234 –241.
Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS (1992): Tianeptine attenuates stress-induced morphologi-cal changes in the hippocampus. Eur J Pharmacol 222: 157–162.
Woolley CS, Gould E, McEwen BS (1990): Exposure to excess
glucocorticoids alters dendritic morphology of adult hip-pocampal pyramidal neurons.Brain Res531:225–231. Yatham LN, Liddle PF, Dennie J, Shiah IS, Adam MJ, Lane CJ,
et al (1999): Decrease in brain serotonin 2 receptor binding in patients with major depression following desipramine treat-ment: A positron emission tomography study with fluorine-18-labeled setoperone.Arch Gen Psychiatry56:705–711. Zanardi R, Artigas F, Franchini L, Sforzini L, Gasperini M,