www.elsevier.com / locate / bres
Research report
The role of intrinsic and agonist-activated conductances in determining
the firing patterns of preoptic area neurons in the guinea pig
*
Edward J. Wagner , Cruz Reyes-Vazquez, Oline K. Rønnekleiv, Martin J. Kelly
Department of Physiology and Pharmacology, L334, Oregon Health Sciences University, 3181 S.W. Sam Jackson Park Road, Portland, OR 97201,
USA
Accepted 11 July 2000
Abstract
Whole-cell and intracellular recordings were made in coronal hypothalamic slices prepared from ovariectomized female guinea pigs. 62% of preoptic area (POA) neurons fired action potentials in a bursting manner, and exhibited a significantly greater afterhyperpolariza-tion (AHP) than did non-bursting POA neurons. The majority (70%) of POA neurons (n576) displayed a time-dependent inward rectification (I ) that was blocked by CsCl (3 mM) or by ZD 7288 (30h mM). In addition, 51% of the cells expressed a low-threshold spike (LTS) associated with a transient inward current (I ) that was blocked by NiCl (200T 2 mM). A smaller percentage of POA neurons (29%)
1
expressed a transient outward, A-type K current that was antagonized by a high concentration of 4-aminopyridine (3 mM). Moreover, POA neurons responded to bath application of them-opioid receptor agonist DAMGO (93%) or the GABAB receptor agonist baclofen (83%) with a membrane hyperpolarization or an outward current. These responses were accompanied by a decrease in input resistance or an increase in conductance, respectively, and were attenuated by BaCl (1002 mM). In addition, the reversal potential for these responses
1
closely approximated the Nernst equilibrium potential for K . These results suggest that POA neurons endogenously express to varying
1
degrees an AHP, an I , an I and an A-type Kh T current. The vast majority of these neurons also are inhibited uponm-opioid or GABAB
1
receptor stimulation via the activation of an inwardly-rectifying K conductance. Such intrinsic and transmitter-activated conductances likely serve as important determinants of the firing patterns of POA neurons. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Postsynaptic mechanisms
Keywords: Preoptic area; Firing; Afterhyperpolarization; Inward rectification; Calcium current
1. Introduction thermoregulation, stress, reproduction and maternal
be-havior [3,20,34,36].
The preoptic area (POA) of the hypothalamus is an Thermoregulatory neurons are found in the POA that integrative center for many important physiological func- respond to changes in body temperature [2–4,50]. Warm-tions. It receives input from many different brain regions, sensitive neurons increase their firing rate in response to including hypothalamic and other limbic areas, cortex, increases in body temperature, thereby promoting heat midbrain and brain stem [34,35,43]. Likewise, the POA dissipation from the body. In contrast, cold-sensitive sends neuronal projections to many of these same regions neurons become activated upon a decrease in body tem-[34,44,49]. Some of the inputs to and neuronal projections perature, which facilitates heat production and retention. from the POA appear to be reciprocal [49]. It is therefore While intrinsic conductances are undoubtedly important not surprising that the POA plays an important role in determinants of the firing patterns of thermosensitive many physiological processes including, but not limited to, neurons, these cells are also modulated by pyrogens (i.e., interleukin-6), opioids and g-aminobutyric acid (GABA)B
receptor agonists, all of which elicit hyperthermia [8,50,51]. The increase in body temperature caused by
*Corresponding author. Tel.: 11-503-494-5830; fax: 1
1-503-494-interleukin-6 arises from an inhibition of warm-sensitive
4352.
E-mail address: [email protected] (E.J. Wagner). neurons and an activation of cold-sensitive neurons, both
of which are blocked by the opioid receptor antagonist 2.2. Drugs naloxone [50]. Thus, the hyperthermia evoked by these
factors is the result of a decreased capacity to dissipate All drugs were initially dissolved in Milli-Q H 0 unless2
heat, and an enhanced ability to produce and retain heat. otherwise stated. TTX (Sigma Chemical Co., St. Louis, The POA also contains gonadotropin hormone-releasing MO, USA) was dissolved in Milli-Q H O and diluted to2
hormone (GnRH) neurons that project to the median the appropriate volume with 0.1% acetic acid (final eminence, and the neuropeptide released from these neu- concentration 1 mM; pH 4–5). Cesium chloride (CsCl; rons acts as the primary secretagogue in the release of J.T. Baker Inc., Phillipsburg, NJ, USA) was prepared as a gonadotropins (i.e., follicle stimulating hormone, luteiniz- stock solution of 0.2 M. N-ethyl-N-phenylamino)-1,2-di-ing hormone (LH)) from the anterior pituitary [15,42]. methyl-6-(methylamino) pyrimidinium chloride (ZD 7288; POA neurons receive inhibitory GABAergic input [28], as Tocris Cookson Inc., Ballwin, MO, USA) was prepared as well as synaptic input from b-endorphin neurons emanat- a stock solution of 10 mM. Nickel chloride hexahydrate ing from the arcuate nucleus [9,18]. Activation of GABAB (NiCl ; Sigma) was prepared as a stock solution of 0.1 M.2
receptors inhibits naloxone-stimulated LH release in a 4-Aminopyridine (4-AP; Aldrich Chemical Co., Mil-variety of experimental models [7,11,30], thereby indicat- waukee, WI, USA) was prepared as a stock solution of 0.1 ing an indirect opioid modulation of LH secretion through M. Tetraethylammonium (TEA; Sigma) was prepared as a an interaction with GABAergic neurons. There is, how- stock solution of 10 mM. DAMGO (Peninsula Laborator-ever, ample evidence to suggest that opioid peptides and ies Inc., Belmont, CA, USA) and met-enkephalin (Penin-GABAergic ligands affect changes in reproductive status sula) were prepared as stock solutions of 1 and 5 mM, by acting at the level of the POA to directly inhibit GnRH respectively. (6)-Baclofen ((6)-b -(aminomethyl)-4-chlo-and thereby LH secretion [25,28,45]. robenzenepropanoic acid; Sigma) was dissolved in 0.1 N The purpose of the present study was to characterize the HCl to a stock concentration of 40 mM. Barium chloride prominent intrinsic and inhibitory, agonist-activated con- dihydrate (BaCl ; Sigma) was prepared as a stock solution2
ductances in POA neurons of the ovariectomized female of 10 mM. Aliquots of the various stock solutions were guinea pig. To this end, whole-cell and intracellular stored at 2808C (TTX, DAMGO, met-enkephalin, ba-recordings were made from coronal hypothalamic slices. clofen), 2208C (ZD 7288)or 48C (CsCl , NiCl , 4-AP,2 2
2 4
Methionine (met)-enkephalin and [D-Ala , N-Me-Phe , TEA, BaCl ) until used for experimentation.2 5
Gly-ol ]-enkephalin (DAMGO), opioid peptides selective
for them-receptor [17], were used to assess the effects of 2.3. Hypothalamic slice preparation
m-opioid receptor activation. Likewise, the selective
GABA receptor agonist baclofen [6] was used to evaluateB On the day of experimentation, the animal was decapi-the effects of GABAB receptor activation. The results are tated, and its brain rapidly removed from the skull. The discussed in a context pertinent to the important role of the brain was rinsed with ice-cold artificial cerebrospinal fluid POA in physiological processes such as thermoregulation (aCSF; NaCl, 124; KCl, 5; NaH PO , 2.6; dextrose, 10;2 4
and reproduction. HEPES, 10; MgSO , 2; CaCl , 2; in mM) and the4 2
hypothalamus immediately dissected. Four coronal slices (450 mM) through the rostro-caudal extent of the POA were cut with the aid of a vibratome. The slices ranged
2. Materials and methods from the organum vasculosum laminae terminalis at the
rostral end (slice one) to the anterior hypothalamic border 2.1. Animals at the caudal end (slice four). We then transferred the slices to a multi-well auxiliary chamber containing oxygenated Female Topeka guinea pigs (440–670 g) were obtained (95% O , 5% CO ) aCSF, where they were kept until2 2
from our institutional breeding facility, and maintained electrophysiological recording. under conditions of constant temperature (72.460.18F) and
light (lights on between 06:30 and 20:30 h). Animals were 2.4. Electrophysiology housed individually, with food and water provided ad
Novato, CA, USA) pulled on either a P-87 or a P-97 the application of further suction or a transient voltage Flaming Brown puller (Sutter Instrument Co.). They were pulse via the preamplifier. Membrane currents were re-filled with a 3% biocytin solution in 1.75 M KCl and 0.025 corded in voltage clamp with access resistances of 25–40 M Tris (pH 7.4). Electrode resistances varied from 100 to MV, and underwent analog–digital conversion via a 225 MV. The membrane potential of POA neurons was Digidata 1200 interface coupled to either pClamp 6.0 or measured in current clamp. Potentials were amplified and 7.0 software. Low-pass filtering of the currents was current was passed through the electrode using an Axo- conducted at a frequency of 2 KHz. Series resistance was clamp 2A preamplifier (Axon Instruments, Foster City, compensated up to 80%. The liquid junction potential was CA, USA). Current and voltage traces were stored on a 211 mV, and was corrected in subsequent data analysis. digital oscilloscope (Tektronix 2230, Tektronix, Beaverton, Voltage command protocols used to characterize the OR, USA), and were recorded on a chart recorder (Gould current / voltage (I /V ) relationships of the prominent intrin-2200, Gould Inc., Glen Burnie, MD, USA). They also sic conductances (I , Ih T and I ) are described in theA
underwent analog–digital conversion with a CyberAmp legends to their respective figures. Pharmacological 320 signal conditioner (for amplification) connected to a characterization of these conductances was ascertained DigiData 1200 A / D converter (sampling frequency: 62 Hz following the application of CsCl (3 mM)or ZD 7288 (30 for the gap-free tape mode, 10–50 KHz for the oscillos- mM; I ), NiCl (200h 2 mM; I ) or 4-AP (3 mM; I ) 15 minT A
cope mode) and subsequent storage on a computer con- before and during a second protocol run for the appropriate taining Axoscope software (Axon Instruments). Following conductance. Responses elicited by m-opioid or GABAB
successful impalement, action potentials were collected for receptor activation were evaluated by applying met-en-the subsequent determination of firing pattern, frequency kephalin (300 nM), DAMGO (1 mM) or baclofen (100 and the interspike interval (ISI). Slices then were perfused mM), respectively, until a new steady-state membrane with 2 mM TTX for at least 6 min to block spontaneous potential or holding current was obtained (4–7 min). I /V firing, and all subsequent drug solutions were sup- relationships were generated before and near the end of plemented with 1mM TTX. Pre-drug voltage-current (V/ I) agonist application according to the voltage command relationships were established by giving hyperpolarizing protocol described in the legends for the appropriate and depolarizing current pulses (0.2 Hz; 1 s duration) of figures. Pharmacological verification that these agonist varying magnitudes, and monitoring the resultant voltage effects were mediated via an IKir was determined by deflections. These were performed in order to determine applying BaCl (1002 mM) in the presence of agonist 15 both the input resistance (R , measured by linear regres-in min prior to and during the generation of a final I /V sion between260 and280 mV) and the magnitude of the relationship.
AHP. The membrane time constant (t) was calculated as
the time necessary for a voltage deflection (|10 mV) to 2.5. Histology
reach 63% of its maximum. For some experiments, single
electrode voltage clamp was performed using the dis- Following recording with biocytin-filled electrodes, continuous mode with a switching frequency of 1–2 KHz slices were fixed with 4% paraformaldehyde in Sorensen’s and 30% duty cycle. The optimization of clamp parameters phosphate buffer (pH 7.4) for 90–180 min [39]. They were and the monitoring of the headstage output was done on a immersed overnight in 20% sucrose dissolved in Soren-separate oscilloscope. sen’s buffer, and then frozen in Tissue-Tek embedding For whole-cell recordings, electrodes were fabricated medium (Miles, Inc., Elkhart, IN, USA). Coronal sections from thin wall borosilicate glass (A-M Systems, Carlsborg, (16 mm) were cut on a cryostat, and were mounted on WA; 1.5 mm O.D.) pulled on a Model 720 vertical pipette slides coated with poly-L-lysine. These sections were puller (David Kopf Instruments, Tujunga, CA). Resultant washed with 0.1 M sodium phosphate buffer (pH 7.4), and electrodes were then filled with an internal solution then processed with streptavidin–fluorescein isothio-containing 0.5% biocytin and consisting of the following: cyanate (FITC) as described previously [39]. After
localiz-1
K gluconate, 128; NaCl, 10; MgCl , 1; EGTA, 11;2 ing the biocytin-filled neuron, the slides containing the HEPES, 10; ATP, 1; GTP, 0.25; in mM; pH adjusted to 7.3 appropriate sections were processed with a monoclonal with 1 N KOH; 292 mOsm. Voltage pulses were amplified tyrosine hydroxylase (TH) antibody (DiaSorin, Stillwater, and passed through the electrode using an Axopatch 1D MN, USA) at a 1:3000 dilution using fluorescence im-preamplifier (Axon Instruments). munohistochemistry [39].
The resultant current deflections were monitored using a
digital oscilloscope (Tektronix TDS 340A). Upon the 2.6. Statistical analyses reduction of the current deflection, negative pressure was
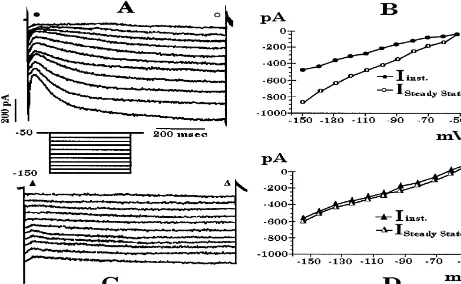
were not homogeneous, then the comparisons between two instantaneous current, and reached steady-state within 1 s. groups were performed using the Mann–Whitney U-test. The threshold for activation was #50 mV, and the mag-Evaluation of the frequency of occurrence was accom- nitude of this current increased with progressively hy-plished using the Chi-square test in conjunction with a two perpolarizing voltage commands. Bath application of CsCl by two contingency table. Differences were considered (3 mM; Fig. 4C and D) or ZD 7288 (30mM; not shown) statistically significant if the probability of error was less completely blocked the expression of this current. The Ih
than 5%. was associated with a time-dependent rectification (TDR) in current clamp that was manifested by a depolarizing relaxation of the membrane potential in response to
3. Results hyperpolarizing current pulses (Fig. 5). In addition, the Ih
and / or TDR was observed in 87% of phasically firing Electrophysiological recording followed by fluorescent POA neurons.
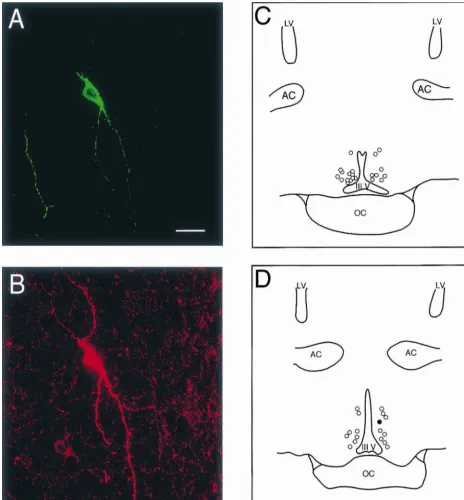
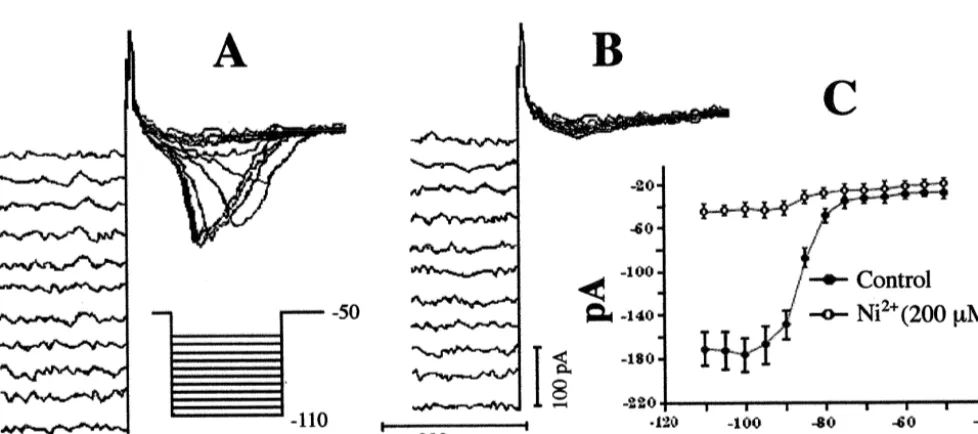
histochemistry was performed in cells from any one of 51% of POA neurons expressed a low threshold spike four slices through the POA (see Methods). The dis- (LTS) in current clamp on the off-step of a hyperpolarizing tribution of cells from which electrophysiological record- current pulse (Fig. 5). The LTS was associated with a ings were acquired ranged from the anteroventral region of transient inward current like that shown in Fig. 6, which the POA to the medial, periventricular region of the more appeared immediately following the off-step of a hy-caudal POA. This latter region contains the somata of A14 perpolarizing voltage command (Fig. 6A) and was marked-dopamine neurons [32], an example of which is illustrated ly attenuated by NiCl2 (200 mM; Fig. 6B and C). The by the dual biocytin / TH immunohistofluoresence shown in transient inward currents elicited immediately following Fig. 1A and B. The majority of the recordings were the onset of depolarizing voltage commands from a performed in the intermediate slices (two and three), and holding potential of 2100 mV reached maximum am-the documented cell locations within am-these slices, including plitude at |245 mV, and a resultant fit of the normalized
the TH-positive neuron mentioned above, are shown in currents to the Boltzmann equation revealed a half-maxi-Fig. 1C and D. mal activation voltage (V1 / 2) of |270 mV (Fig. 7).
The present study included a total of 79 cells, 29 of Coupled with its transient nature, this current has the
21
which were recorded from intracellularly using sharp characteristics of the T-type Ca current (I ). As with theT
electrodes and 50 of which were recorded from using the I , the I and / or LTS was detected in a high percentageh T
whole-cell configuration. POA neurons exhibit a resting (87%) of phasically firing POA neurons. Most importantly, membrane potential of 250.060.7 mV (from sharp elec- all phasically firing POA neurons displayed either an trode and whole-cell recordings), as well as a Rin of I / TDR or an I / LTS, and many of these neurons (73%)h T
445664 MV and a t of 15.661.6 ms (from sharp expressed both conductances (Fig. 5).
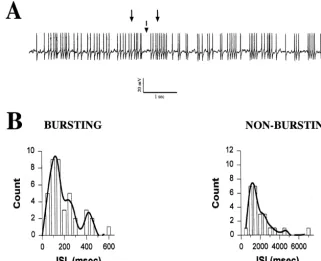
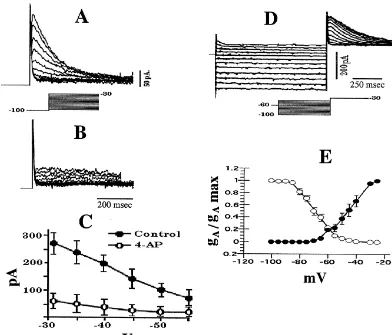
electrode recordings). We also observed that 62% of POA 29% of POA neurons exhibited a transient outward neurons spontaneously fire in a bursting pattern. This was current similar to that shown in Fig. 8. This current characterized empirically by the intermittent clustering of appeared immediately following the onset of depolarizing action potentials (.3 Hz) interspersed among periods of voltage commands from a holding potential of 2100 mV quiescence ($200 ms) as shown in the intracellular, sharp (Fig. 8A). This transient outward current also was sensitive electrode recording in Fig. 2A. to a high dose of 4-AP (3 mM; Fig. 8B and C), indicative The remaining 38% of POA neurons either fired action of the expression of the I . Alternatively, it could beA
potentials at uniform, regular intervals or were silent at observed upon a depolarizing voltage step to 230 mV rest. Evaluation of the ISI histograms revealed a bimodal given subsequently to a series of pre-pulses ranging from distribution for burst firing POA neurons and a unimodal 2100 to240 mV (Fig. 8D). We used these two protocols distribution for nonbursting POA neurons (Fig. 2B). The to construct the inactivation and activation curves for the mean firing frequency of burst firing POA neurons was I , which are shown in Fig. 8E. It should be noted that theA
significantly greater than that observed in nonbursting expression of this current and that of the I were, in mostT 2
POA neurons (4.761.2 Hz vs. 1.060.7 Hz; P,0.05). In cases, mutually exclusive (x 56.4; P,0.05).
Fig. 1. (A) Confocal image of the biocytin–streptavidin–FITC labeling of a neuron in the periventricular zone of the POA. (B) Confocal image of TH immunoreactivity in the soma of the cell in A as visualized with CY-3. Note the multiple spines that exist along the processes radiating from the cell body. The scale bar equals 25mm (for both A and B). (C) Camera lucida drawing through slice two showing the distribution of documented recording sites in this slice. (D) Camera lucida drawing illustrating the distribution of documented cell locations in slice three. The location of the double-labeled neuron in
A and B is denoted by the filled circle. The documented cell locations in these two slices were determined primarily from sharp electrode, intracellular
recordings and, in some cases, from whole-cell patch recordings. LV5lateral ventricle, AC5anterior commissure, III V5third ventricle, OC5optic chiasm.
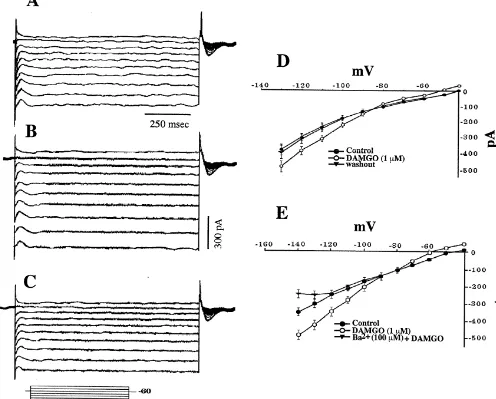
conductance (Fig. 9A–C) that reversed polarity near the together, these data demonstrate that m-opioid peptides
1
equilibrium potential for K (EK1; Fig. 9D). The I /V plot inhibit POA neurons via an increase in IKir.
Fig. 2. (A) A representative example of a POA neuron firing action potentials in a bursting manner. Its resting membrane potential was251 mV, and its mean firing frequency was 5.8 Hz. The action potentials were not fully reproduced by the chart recorder. (B) Histograms illustrating the distribution of the ISI for bursting and non-bursting POA neurons. The histogram of the burst firing POA neuron was obtained from the cell in A. ISI distributions were derived from digitally captured segments of spontaneous firing. The curves were generated from a user-defined transform that shaded the ISI bin-width (50 ms) / count pairs to their respective axes. The curve for the bursting POA neuron revealed a bimodal distribution. The first mode occurred at 138.5 ms, which reflected the ISI determined during a burst (denoted by the solid arrows in A). The other mode occurred at 415.4 ms, which represented the interburst interval (denoted by the dashed arrow in A). By contrast, the distribution of the non-bursting POA neuron (mean firing frequency50.5 Hz) is unimodal in nature, with the mode occurring at 1429 ms.
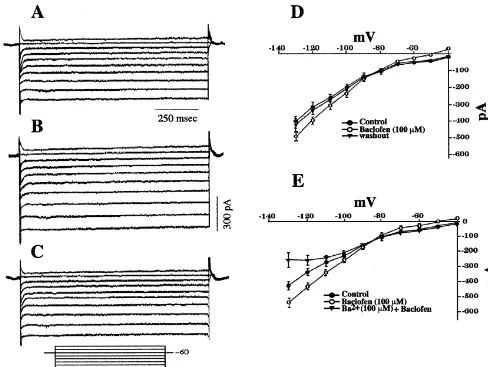
peptides, we observed qualitatively similar responses in in Fig. 10E, BaCl2 (100 mM) completely abolished the whole-cell patch recordings. The baclofen-induced out- increase in slope conductance produced by baclofen. ward current (19.261.9 pA; n532) was accompanied by a Coadministration of baclofen and DAMGO elicited no reversible increase in conductance (Fig. 10A–D), and this additional outward current or increase in slope conduct-current reversed polarity near EK1 (Fig. 10D). As depicted ance when compared to that obtained with either of the
Fig. 4. (A) Current record from a whole-cell recording of a POA neuron demonstrating the hyperpolarization-activated inward current (I ). This current hash
the appearance of a sag following the instantaneous current observed with the onstep of the hyperpolarizing voltage command. The cell was held at250 mV, and delivered a series of hyperpolarizing voltage commands (10 mV, 1 s) down to 2150 mV. (B) I /V plot of the instantaneous (inst.) vs. the steady-state membrane current of the cell in A showing the inward rectification of the I and an activation threshold ofh #250 mV. Instantaneous currents (solid circles) and steady-state currents (open circles) were measured from the points along the traces in A indicated by their respective symbols. C and D, current record and I /V plot of the instantaneous (inst.) vs. the steady-state membrane current showing the cesium (3 mM) block of the I observed in theh
cell in A. Instantaneous currents (solid triangles) and steady-state currents (open triangles) were measured from the traces in C at the points indicated by their respective symbols.
agonists alone (not shown). Thus, m-opioid and GABAB burst firing neurons show a more robust AHP than do receptors couple to a common pool of inwardly-rectifying non-bursting POA neurons. The AHP is mediated by a
1 21 1
K channels. small conductance (SK), Ca -dependent K current
[40,41], and three SK channel subtypes have been cloned [27]. In addition, apamin-sensitive SK2 and SK3 channel
4. Discussion subtypes are highly expressed throughout the
hypo-thalamus of the rat and guinea pig, including the supraoptic The present study provides the first characterization of nucleus and POA [27,38]. While the pharmacology or the prevalent intrinsic and agonist-activated conductances modulation of the AHP observed in the present study was displayed by POA neurons. For example, we observed a not evaluated, an apamin-sensitive AHP has been observed AHP in the majority of POA neurons. Similarly, 70% of in magnocellular neurons of both the rat and guinea pig these neurons expressed an I . Over 50% of POA neuronsh supraoptic nucleus [5,14]. Furthermore, it is generally exhibited an inward current that, based on its transient accepted that the AHP plays a pivotal role in spike nature, NiCl sensitivity and low threshold for activation,2 frequency adaptation [40,41], and the results of the present is indicative of the I . Nearly one-third of these neuronsT study would suggest that it also plays an important role in exhibited an IA that was largely mutually exclusive with regulating the interburst interval in burst firing POA the expression of the I . Finally,T m-opioid and GABAB neurons.
Fig. 7. Representative IT activation curve derived from a whole-cell recording of a POA neuron. The cell was held at2100 mV, and delivered a series of incremental, depolarizing voltage commands (5 mV, 120 ms) up to 240 mV. The resultant data points were then fit according to the Boltzmann equation. This cell had a V1 / 2of269.6 mV and a slope factor (k) of 9.12.
Fig. 5. Current clamp recording of a POA neuron that exhibited a LTS on
the off-step of a hyperpolarizing current pulse (200 pA, 1 s) delivered at a slowly developing, hyperpolarization-activated, CsCl-rest (245 mV). Note also the TDR (7 mV) that slowly activated during
and ZD 7288-sensitive inward current in 70% of POA
the current pulse, as well as the damped oscillation that followed the
neurons. Moreover, the I and / or the TDR was present toh
initial LTS.
an even greater extent (87%) in burst firing POA neurons. This is consistent with the proposed supportive role of the the TDR, and is thought to underlie the ‘pacemaker’ I in phasically firing magnocellular neurons of the guineah
potential described in the heart and in thalamocortical relay pig hypothalamus [13], and in rhythmically bursting neurons [21,24,31]. In the present study we observed such thalamocortical relay neurons of the cat [31].
Fig. 6. (A) Transient inward currents observed in a whole-cell recording from a POA neuron on the off-step of incremental hyperpolarizing voltage commands (5 mV) delivered from a holding potential of250 mV. The pulse duration (1 s) was long enough to ensure complete deinactivation of the current. (B) Lack of any transient inward current observed in the cell in A following the application of NiCl (2002 mM). (C) Composite I /V plot
21
Fig. 8. (A) Transient outward current generated in a whole-cell recording from a POA neuron. The cell was held at2100 mV and issued a series of depolarizing voltage commands (5 mV, 500 ms) up to230 mV. TEA (5 mM) was applied 15 min prior to and during the recording in order to block the delayed rectifier. (B) Antagonism of the transient outward current observed in the cell in A by 4-AP (3 mM). (C) Composite I /V plot showing the sensitivity of the transient outward current to 4-AP (3 mM). Symbols represent means and vertical lines 2 S.E.M. of the peak current (leak subtracted) obtained at a given membrane voltage according to the protocol described for the cell in A (n54). (D) Transient outward currents in a whole-cell recording from a POA neuron elicited by a depolarizing voltage command to230 mV. The cell was held at260 mV and given a pre-pulse ranging from2100 mV to
240 mV (5 mV increments, 1 s) followed by the depolarizing voltage step to230 mV. As with the cell in A, these transient outward currents were recorded in the presence of TEA (5 mM). (E) Composite inactivation (s) and activation (d) curves for I in POA neurons. The curves were fit by the Boltzman
A
equation to the experimental data points, which were generated according to the protocols described in D (inactivation) and A (activation). The V1 / 2for the inactivation and activation curves was266.0 and242.1 mV, respectively. Symbols represent means and vertical lines 2 S.E.M. of normalized conductance values observed at a given membrane voltage (n54).
On the other hand, the IT is a rapidly activating and [33]). A considerably greater incidence of expression
21
inactivating Ca current [23]. It can be distinguished both (87%) was encountered in burst firing POA neurons. This physiologically and pharmacologically from the other is also consistent with the pivotal role proposed for the IT
21
types of Ca currents (i.e., L, N, P/ Q, and R) based on its in promoting phasic firing and / or bursting in vasopressin kinetics of activation, its low threshold for activation and neurons of the guinea pig supraoptic nucleus [14], in the its susceptibility to antagonism by NiCl2 [14,16,24,46]. medial preoptic nucleus of the rat [46] and in thalamocorti-Presently, 51% of POA neurons exhibited an inward cal relay neurons of the cat [31].
current and / or a LTS that matched these characteristics. It The I is a rapidly activating and inactivating, voltage-A
1
may be argued that the variable latency to peak and rate of dependent current mediated by the outflow of K ions inactivation observed in Fig. 6 is due to less than optimal through 4-AP-sensitive channels [37]. It increases the voltage clamp. However, it is established that both the interspike interval between action potentials, thereby reg-activation and inreg-activation kinetics of the T-current are ulating neuronal firing rate [37,40]. We observed such a voltage dependent [24,33,46]. Furthermore, our estimated transient, 4-AP-sensitive outward current in 29% of POA
Fig. 9. (A) Current record from a whole-cell, voltage clamp recording of a POA neuron prior to application of 1mM DAMGO. This cell was held at260 mV and delivered a series voltage commands (10 mV, 1 s) over a range of240 to2130 mV. (B) Current record from the cell in A in the presence of 1mM DAMGO. (C) Current record from the cell in A after the clearance of 1mM DAMGO from the slice. (D) Composite I /V plot showing the reversible,
1
DAMGO-induced increase in an IKirthat reverses polarity near the equilibrium potential for K (|287 mV). Symbols represent means and vertical lines 2
21 S.E.M. of the steady-state membrane current measured at a given voltage (n535–42). (E) Composite I /V plot illustrating the antagonism by Ba (100
mM) of the DAMGO-induced increase in an IKir(n55).
firing in POA neurons although, in some cells, it may modulating the ISI of regularly firing (e.g., warm-sensitive) regulate the number of action potentials contained within a POA neurons.
burst. We also noted that in most cases, the expression of the On the other hand, the percentage of POA neurons I and the I was mutually exclusive. This could mean thatA T
1
expressing an IA closely approximates the percentage of POA neurons express either A-type K channels or T-type
21
thermosensitive neurons within this region [2–4]. In Ca channels. Alternatively, due to its comparatively particular, regularly firing, warm-sensitive neurons com- faster activation rate [24], the I could be either maskingA
prise roughly 30% of all POA neurons [3,4]. These warm- the I or, at the very least, dampening its magnitude.T
sensitive neurons increase their firing rate in response to Activation of the ligand-gated IKir results in an outward increases in brain or core temperature [2–4,50]. This current (or hyperpolarization) at rest due to the selective
1
increase in firing rate is thought to involve the inactivation outflow of K . This BaCl -sensitive current exhibits a2
Fig. 10. (A) Current record from a whole-cell recording of a POA neuron prior to application of 100mM baclofen. This cell was held at260 mV and delivered a series voltage commands (10 mV, 1 s) over a range of240 to2130 mV. (B) Current record from the cell in A in the presence of 100mM baclofen. (C) Current record from the cell in A after the clearance of 100mM baclofen from the slice. (D) Composite I /V plot showing the reversible,
1
baclofen-induced increase in an IKirthat reverses polarity near the equilibrium potential for K (|287 mV). Symbols represent means and vertical lines 2
21
S.E.M. of the steady-state membrane current measured at a given voltage (n532). (E) Composite I /V plot illustrating the antagonism by Ba (100mM) of the baclofen-induced increase in an IKir(n54).
membrane potentials, and plays a pivotal role in the been implicated in fostering phasic firing in magnocellular regulation of neuronal firing frequency [40]. In the present neurosecretory cells of the guinea pig supraoptic nucleus study, 92% and 84% of POA neurons responded to m- [13,14], and phasic bursting has been shown to enhance opioid and GABAB receptor activation, respectively, with vasopressin release from terminals in the neurohypophysis such a BaCl -sensitive outward current and / or hyperpo-2 [12]. In thalamocortical relay neurons, rhythmic burst larization. The extensive m-opioid and GABAB receptor- firing is thought to arise solely from the interplay between mediated inhibition of POA neurons via an increase in this the I and the I [31]. That is, a hyperpolarizing stimulush T
1
K conductance is similar to that observed in the hypo- sufficient to activate the Ih would result in an inward thalamic arcuate nucleus, as well as in extrahypothalamic current (and a depolarizing TDR) that would then bring the structures such as the locus coeruleus and hippocampus membrane potential towards the threshold of activation for [6,26,48]. Furthermore, m-opioid and GABAB receptor- the I . This would lead to a transient inward current (and aT
mediated activation of IKir and the resultant inhibition of LTS) that would further depolarize the membrane potential
1
presumptive, warm-sensitive POA neurons might explain towards the threshold of activation of voltage-gated Na the hyperthermia elicited by stimulation of these receptor channels, and ultimately lead to the generation of a burst of
systems [8,50,51]. action potentials.
thalamic neuronal thermosensitivity, Ann. NY Acad. Sci. 813 (1997)
activated by inhibitory synaptic input and the intrinsic
133–138.
AHP. In the present study, the vast majority of POA
[5] C.W. Bourque, D.A. Brown, Apamin and d-tubocurarine block the
neurons are hyperpolarized by an IKir elicited by m-opioid afterhyperpolarization of rat supraoptic neurosecretory neurons, and GABAB receptor activation, and burst firing POA Neurosci. Lett. 82 (1987) 185–190.
neurons had a comparatively more robust AHP than did [6] N. Bowery, GABA(B) receptors and their significance in mam-malian pharmacology, Trends Pharmacol. Sci. 10 (1989) 401–407.
non-bursting POA neurons. Indeed, the TH-positive neuron
[7] D.W. Brann, P.L. Zamorano, C.D. Putnam-Roberts, V.B. Mahesh,
shown in Fig. 1 not only possessed all these requisite
Gamma-aminobutyric acid-opioid interactions in the regulation of
features for burst firing but also showed such a firing gonadotropin secretion in the immature female rat, Neuroendoc-pattern. This is similar to the intrinsic membrane properties rinology 56 (1992) 445–452.
reported for A12 dopamine neurons [29]. A14 dopamine [8] X.-H. Chen, E.B. Geller, J.K. De Riel, L.-Y. Liu-Chen, M.W. Adler, Antisense oligodeoxynucleotides against m- or kappa-opioid
re-neurons with cell bodies residing in the periventricular
ceptors block agonist-induced body temperature changes in rats,
zone of the POA [32] make synaptic contact with GnRH
Brain Res. 688 (1995) 237–241.
neurons in the medial POA [22]. These neurons are also
[9] B.M. Chronwall, Anatomy and physiology of the neuroendocrine
responsive to m-opioid receptor stimulation, which is arcuate nucleus, Peptides 6 (1985) 1–11.
consistent with the findings of the present study, they are [10] J.T. Clark, S.M. Gabriel, J.W. Simpkins, S.P. Kalra, P.S. Kalra, Chronic morphine and testosterone treatment: Effect on sexual
sensitive to modulation by gonadal steroid hormones and
behavior and dopamine metabolism, Neuroendocrinology 48 (1988)
have been implicated in regulating sexual behavior in male
97–104.
rodents [10]. In addition, dopamine is reported to decrease
´
[11] A.O. Donoso, F.J. Lopez, A. Negro-Vilar, Cross-talk between
GnRH content in nerve terminals located in the median excitatory and inhibitory amino acids in the regulation of luteinizing eminence and organum vasculosum laminae terminalis hormone-releasing hormone secretion, Endocrinology 131 (1992)
1559–1661.
[47]. Thus, burst firing A14dopamine neurons may play an
[12] A. Dutton, R.E.J. Dyball, Phasic firing enhances vasopressin release
important role in regulating the excitability of GnRH
from the rat neurohypophysis, J. Physiol. (Lond.) 290 (1979) 433–
neurosecretory cells, and thereby influence reproductive
440.
status. Future studies will examine the potential dopa- [13] K.R. Erickson, O.K. Ronnekleiv, M.J. Kelly, Electrophysiology of minergic regulation of GnRH neurosecretory activity. guinea-pig supraoptic neurones: Role of a
hyperpolarization-acti-In conclusion, we have characterized the intrinsic and vated cation current in phasic firing, J. Physiol. (Lond.) 460 (1993) 407–425.
agonist-activated conductances present in POA neurons of
[14] K.R. Erickson, O.K. Ronnekleiv, M.J. Kelly, Role of a T-type
the guinea pig. These conductances all have been
impli-calcium current in supporting a depolarizing potential, damped
cated as important regulators of firing patterns in neurons oscillations, and phasic firing in vasopressinergic guinea pig supra-of both the central and peripheral nervous systems. We optic neurons, Neuroendocrinology 57 (1993) 789–800.
propose that, in conjunction with the AHP, this collection [15] M. Ferin, D. Van Vugt, S. Wardlaw, The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides, Recent
of prominent conductances, notably the I , I and Ih T Kirserve
Prog. Horm. Res. 40 (1984) 441–485.
as critical determinants of burst firing in POA neurons.
[16] T.E. Fisher, C.W. Bourque, Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus, J. Physiol. (Lond.) 486 (3) (1995) 571–580.
[17] A. Goldstein, A. Naidu, Multiple opioid receptors: ligand selectivity
Acknowledgements profiles and binding site signatures, Mol. Pharmacol. 36 (1989)
265–272.
[18] R.P. Hammer, L. Zhou, S. Cheung, Gonadal steroid hormones and
The authors thank Jason T. Deignan, Martha A. Bosch
hypothalamic opioid circuitry, Horm. Behav. 28 (1994) 431–437.
and Barry Naylor for outstanding technical assistance. The
[19] N.C. Harris, A. Constanti, Mechanism of block by ZD 7288 of the
experiments described in this study were supported by hyperpolarization-activated inward rectifying current in guinea pig PHS Grants NS35944 and DA00192 (RSDA to Martin J. substantia nigra neurons in vitro, J. Neurophysiol. 74 (1995) 2366–
2378.
Kelly).
[20] J.P. Herman, W.E. Cullinan, Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis, Trends Neurosci. 20 (1997) 78–84.
[21] B. Hille, Potassium channels and chloride channels, in: B. Hille
References (Ed.), Ionic Channels of Excitable Membranes, Sinauer Associates,
Sunderland, MA, 1992, pp. 115–139.
[1] T. Akasu, S. Shoji, H. Hasuo, Inward rectifier and low-threshold [22] T.L. Horvath, F. Naftolin, C. Leranth, Luteinizing hormone-releas-calcium currents contribute to the spontaneous firing mechanism in ing hormone and gamma-aminobutyric acid neurons in the medial neurons of the rat suprachiasmatic nucleus, Eur. J. Physiol. 425 preoptic are synaptic targets of dopamine axons originating in (1993) 109–116. anterior periventricular areas, J. Neuroendocrinol. 5 (1993) 71–79. [2] F. Baldino, H.M. Geller, Electrophysiological analysis of neuronal [23] J.R. Huguenard, Low-threshold calcium currents in central nervous
thermosensitivity in rat preoptic and hypothalamic tissue cultures, J. system neurons, Annu. Rev. Physiol. 58 (1996) 329–348. Physiol. (Lond.) 327 (1982) 173–184. [24] J.R. Huguenard, D.A. McCormick, Simulation of the currents [3] J.A. Boulant, Hypothalamic neurons: Mechanisms of sensitivity to involved in rhythmic oscillations in thalamic relay neurons, J.
temperature, Ann. NY Acad. Sci. 856 (1998) 108–115. Neurophysiol. 68 (1992) 1373–1383.
-endorphin on LH release in ovariectomized rats does not involve the phase of the ovulatory cycle, The Physiologist 42 (1999) A-23 preoptic GABAergic system, Exp. Clin. Endocrinol. Diabetes 103 (Abstract).
(1995) 317–323. [39] O.K. Ronnekleiv, M.D. Loose, K.R. Erickson, M.J. Kelly, A method [26] M.J. Kelly, M.D. Loose, O.K. Ronnekleiv, Estrogen suppresses for immunocytochemical identification of biocytin-labeled neurons
m-opioid- and GABA -mediated hyperpolarization of hypothalamicB following intracellular recording, BioTechniques 9 (1990) 432–438. 1
arcuate neurons, J. Neurosci. 12 (1992) 2745–2750. [40] B. Rudy, Diversity and ubiquity of K channels, Neuroscience 25 ¨
[27] M. Kohler, B. Hirschberg, C.T. Bond, J.M. Kinzie, N.V. Marrion, J. (1988) 729–749.
21 1
Maylie, J.P. Adelman, Small-conductance, calcium-activated potas- [41] P. Sah, Ca -activated K currents in neurones: types, physiological sium channels from mammalian brain, Science 273 (1996) 1709– roles and modulation, Trends Neurosci. 19 (1996) 150–154. 1714. [42] A.J. Silverman, L.C. Krey, E.A. Zimmerman, A comparative study [28] C. Leranth, N.J. MacLusky, H. Sakamoto, M. Shanabrough, F. of the luteinizing hormone releasing hormone (LHRH) neuronal
Naftolin, Glutamic acid decarboxylase-containing axons synapse on networks in mammals, Biol. Reprod. 20 (1979) 98–110.
LHRH neurons in the rat medial preoptic area, Neuroendocrinology [43] R.B. Simerly, L.W. Swanson, The organization of neural inputs to 40 (1985) 536–539. the medial preoptic nucleus of the rat, J. Comp. Neurol. 246 (1986) [29] M.D. Loose, O.K. Ronnekleiv, M.J. Kelly, Membrane properties and 312–342.
response to opioids of identified dopamine neurons in the guinea pig [44] R.B. Simerly, L.W. Swanson, Projections of the medial preoptic hypothalamus, J. Neurosci. 10 (1990) 3627–3634. nucleus: A phaseolus vulgaris leucoagglutinin anterograde tract-[30] C. Masotto, G. Wisniewski, A. Negro-Vilar, Different gamma- tracing study in the rat, J. Comp. Neurol. 270 (1988) 209–242.
aminobutyric acid receptor subtypes are involved in the regulation [45] M.J. Smith, R.V. Gallo, Further studies on the suppression of of opiate-dependent and independent luteinizing hormone-releasing luteinizing hormone release due to activation of medial preoptic hormone secretion, Endocrinology 125 (1989) 548–553. anterior hypothalamic area m-opioid receptors, Brain Res. Bull. 42 [31] D.A. McCormick, J.R. Huguenard, A model of the electrophysio- (1997) 1–7.
logical properties of thalamocortical relay neurons, J. Neurophysiol. [46] A.K. Sundgren-Andersson, S. Johansson, Calcium spikes and cal-68 (1992) 1384–1400. cium currents in neurons from the medial preoptic nucleus of rat, [32] K.E. Moore, Hypothalamic dopaminergic neuronal systems, in: H.Y. Brain Res. 783 (1998) 194–209.
Meltzer (Ed.), Psychopharmacology: the Third Generation of Pro- [47] M.M. Wilkes, R.M. Kobayashi, S.S.C. Yen, R.Y. Moore, Monoamine gress, Raven Press, New York, 1987, pp. 127–139. neuron regulation of LRF neurons innervating the organum vas-[33] I. Niespodziany, P. Derambure, P. Poulain, Properties of T-type culosum laminae terminalis and median eminence, Neurosci. Lett.
calcium current in enkephalinergic neurones in guinea-pig hypo- 13 (1979) 41–46.
thalamic slices, Eur. J. Physiol. 437 (1999) 871–880. [48] J.T. Williams, R.A. North, T. Tokimasa, Inward rectification of [34] M. Numan, T.P. Sheehan, Neuroanatomical circuitry for mammalian resting and opiate-activated potassium currents in rat locus coeruleus
maternal behavior, Ann. NY Acad. Sci. 807 (1997) 101–125. neurons, J. Neurosci. 8 (1988) 4299–4306.
[35] M.N. Perkins, S.A. Whitehead, Neural connexions between the [49] F.G. Wouterlood, R.P.A. Gaykema, Innervation of histaminergic medial forebrain bundle, the preoptic area and the basal hypo- neurons in the posterior hypothalamus by medial preoptic neurons. thalamus in the rat: an electrophysiological study, J. Physiol. (Lond.) Anterograde tracing with Phaseolus vulgaris leucoagglutinin com-291 (1979) 443–456. bined with immunocytochemistry of histidine decarboxylase in the [36] J.G. Pfaus, A. Jakob, S.P. Kleopoulos, R.B. Gibbs, D.W. Pfaff, rat, Brain Res. 455 (1988) 170–176.
Sexual stimulation induces fos immunoreactivity within GnRH [50] L. Xin, C.M. Blatteis, Hypothalamic neuronal responses to neurons of the female rat preoptic area: interaction with steroid interleukin-6 in tissue slices: effects of indomethacin and naloxone, hormones, Neuroendocrinology 60 (1994) 283–290. Brain Res. Bull. 29 (1992) 27–35.
[37] M.A. Rogawski, The A-current: how ubiquitous a feature of [51] M.R. Zarrindast, Y. Oveissi, GABA(A) and GABA(B) receptor sites excitable cells is it?, Trends Neurosci. 8 (1985) 214–219. involvement in rat thermoregulation, Gen. Pharmacol. 19 (1988) [38] O.K. Ronnekleiv, M.A. Bosch, B.R. Naylor, Deignan, 17b-Estradiol 223–226.