www.elsevier.com / locate / bres
Research report
Improgan, a cimetidine analog, induces morphine-like antinociception
in opioid receptor-knockout mice
a ,
*
a a b b bL.B. Hough
, J.W. Nalwalk , Y. Chen , A. Schuller , Y. Zhu , J. Zhang ,
c c c b
W.M.P.B. Menge , R. Leurs , H. Timmerman , J.E. Pintar
a
Center for Neuropharmacology and Neuroscience, Albany Medical College MC-136, Albany, NY 12208, USA
b
Department of Neuroscience and Cell Biology, UMDNJ, R.W. Johnson Medical School, Piscataway, NJ, USA
c
Leiden /Amsterdam Center for Drug Research, Department of Pharmacochemistry, Vrije University, Amsterdam, The Netherlands Accepted 2 August 2000
Abstract
Improgan is an analog of the H antagonist cimetidine that does not act on known histamine receptors, but induces highly effective2 analgesia in rodents following intracerebroventricular (icv) administration. Since the mechanism of action of this compound remains unknown, improgan analgesia was characterized presently with the tail immersion nociceptive test in mutant mice lacking either them
(exon 1 of MOR-1),d(exon 2 of DOR-1) ork(exon 3 of KOR-1) opioid receptor. Improgan (30mg, icv) induced reversible, maximal analgesia in both sexes of all three genotypes (1/1, 1/2 and 2/2) of MOR-1 mutant mice 10 and 20 min after administration, whereas morphine analgesia was reduced (1/2) or abolished (2/2) in these subjects. In DOR-1 mutant mice, improgan was equally
3 2 5
effective in all three genotypes, despite the reduction (1/2) or complete loss (2/2) ofdopioid receptor ( H-[D-Pen ,D-Pen ]enkephalin, DPDPE) binding. Similarly, improgan analgesia was equivalent in all three genotypes of KOR-1 mutant mice, whereas k-mediated
3
analgesia (U50,488) andkopioid ( H-U69,593) binding were abolished in the homozygous (2/2) mice. These studies demonstrate that improgan analgesia does not require intact MOR-1, DOR-1, or KOR-1 genes, and support the hypothesis that improgan-like analgesics act in the CNS by non-opioid mechanisms. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Pain modulation: pharmacology
Keywords: Antinociception; Analgesia; Improgan; Cimetidine; H -receptor; Histamine; Brain; MOR-1; DOR-1; KOR-1; Opioid receptor2
1. Introduction effects, both of which result from stimulation of opioid receptors. Improgan (N-cyano-N9 -[3-(imidazole-4-Pain continues to be a worldwide health problem, and yl)propyl]-N99-methylguanidine, formerly known as there is an urgent need to discover new kinds of pain- SKF92374) is an analog of the histamine H2 receptor relieving medications [6]. Despite progress, the opioid antagonist cimetidine, that has been shown to inhibit morphine remains the standard by which new medications thermal and mechanical nociception in rodents following are compared. Thus, an ideal analgesic would show icv administration [13,14]. Of particular note is the effica-morphine-like activity in pain tests, but lack opioid side- cy of this compound in attenuating high-temperature tail flick responses in rats, an activity shared with morphine, but with few other classes of analgesic agents [13,14]. At maximal antinociceptive doses in rats, improgan does not
2
Abbreviations: CNS, central nervous system; DPDPE, [D-Pen , D- alter locomotor or rotorod performance, suggesting a
5
Pen ]enkephalin; icv, intracerebroventricular; M6G, morphine-6-glucuro- selective analgesic activity [14]. Furthermore, daily in-nide
jections of morphine and improgan showed tolerance to the
*Corresponding author. Tel.: 11-518-262-5785; fax: 1
1-518-262-analgesic activity of the former, but not to the latter,
5799.
E-mail address: [email protected] (L.B. Hough). suggesting a favorable clinical profile [1]. Other
pounds structurally related to H and H antagonists also2 3 2.3. KOR-1 knockout mice induce analgesia, and a novel structure–activity
relation-ship for this effect has been established [7,9]. Exon 3 of the murine KOR-1 gene was replaced with In vitro screens at more than 50 receptors [6] have not the neomycin resistance cassette [27]. Homozygous knock-yet identified a site of improgan action, and little is known out mice were born viable, grossly normal and fertile, and
3
about the mechanism of this drug. Several studies have contained no detectable H-U69,593 binding in brain shown that improgan analgesia is not mediated by known homogenates.
(H , H , or H ) histamine receptors [8,13,15]. Further-1 2 3
more, improgan antinociception was unaffected by the 2.4. Animals opioid antagonist naltrexone [15], suggesting an activity
that is independent of opioid receptors. Since multiple Mice were labeled with coded genotypes and shipped to types of opioid receptors can contribute to pain-relieving Albany Medical College, where antinociceptive testing and mechanisms [11], and, because improgan analgesia is radioligand binding were performed. They were housed in poorly understood, the activity of this compound was groups of 4 to 6, separated by sex and genotype, and presently studied in three types of mutant mice: those maintained on a normal 12 h light / dark cycle with food lacking them (MOR-1),d(DOR-1) ork (KOR-1) opioid and water freely available. All procedures were approved
receptors. by the Albany Medical College Institutional Animal Care
and Use Committee.
2.5. Drugs and solutions
2. Materials and methods
DPDPE (Sigma, St Louis, MO), morphine sulfate 2.1. MOR-1 knockout mice
(Mallinckrodt, Paris, KY) and U50,488 (6-trans-U-50,488 methanesulfonate, RBI) were dissolved in saline. Improgan Mice deficient in exon 1 of the MOR-1 gene were
base was synthesized according to example 12 of Durant et generated by use of a targeting vector in which exon 1 was
al. [3] starting from 4-(3-aminopropyl) imidazole replaced by a neomycin resistance cassette, as
character-dihydrobromide, which was prepared according to [25]. ized in detail [20]. Offspring derived from heterozygous
The compound (mp5152–1538C) was dissolved in 1.0 M matings showed a Mendelian inheritance pattern,
produc-HCl at a concentration of 150 mg / ml, titrated to pH 7.0 ing the three expected genotypes studied presently.
and diluted with saline. Doses of all drugs refer to the 3
Radioligand binding assays with the m agonist
H-chemical form administered. DAMGO found approximately one-half and no specific
binding in the brains of heterozygous (1/2) and
homo-2.6. Tail-immersion nociceptive testing zygous (2/2) mice, respectively [20]. Hybridization
studies found no changes in mRNA levels representative of
Animals were loosely restrained in a conical
poly-d(DOR-1) receptors,k (KOR-1) receptors, proenkephalin
propylene tube. The tail (2–3 cm) was immersed into a or prodynorphin in MOR-1 mutant mice as compared with
558C water bath and the latency to sudden movement wild-type controls [20].
(flick) or removal of the tail was recorded with an 8-s cutoff [14].
2.2. DOR-1 knockout mice
2.7. Microinjection and testing procedure in mice Exon 2 of the murine DOR-1 gene was replaced with a
neomycin resistance cassette, as described in detail [28]. Following base-line testing, animals were lightly anes-Heterozygote matings of the mutants showed Mendelian thetized with ether. A microliter syringe was connected to inheritance of the three genotypes studied presently. Im- a 26-gauge needle with PE20 tubing. The needle was munostaining [28] and radioligand binding studies ([28] inserted into the lateral ventricle through a stereotaxically and present) showed no detectable DOR-1 protein product drilled Plexiglas plate as described previously in detail [4]. and either trace amounts or zero levels of pharmaco- A total of 2ml of drug solution was injected over a 1-min logically-definedd1ord2receptors [28] in the homozygote period. The animals were conscious within 3 min after the mutant mouse brain. Additional characterization of these injection and were tested several times up to 2 h later. One
3
mice found normal levels of m ( H-DAMGO) and k to two weeks later, mice received systemic or icv opioid 3
( H-U69593) receptor binding; in situ hybridization found treatments, and were re-tested. Following all testing, similar levels of mRNA for proenkephalin, prodynorphin, animals received pentobarbital, and an icv injection of and pro-opiomelanocortin in mutant and wild-type brains India Ink (1 ml) to verify the accuracy of injections into
2.8. Data analysis in all three DOR-1 genotypes. Subsequent challenge of these mice with DPDPE (8mg, 20 min, icv) unexpectedly Antinociceptive responses for each animal were calcu- showed equivalent analgesia in all three genotypes (mean lated as percent of maximum possible effect (% MPE), % MPE6S.E.M., were 87.166.2, 66.968.5 and 86.467.2
where: for 1/1 [n512], 1/2 [n511] and 2/2 [n510]
3 groups, respectively). As expected however, brain H-drug latency 2baseline latency
DPDPE binding was either reduced (1/2) or abolished
]]]]]]]]]
% MPE5 3100
cutoff latency 2baseline latency
(2/2) in the DOR-1 mutants, as compared with 1/1
controls (Fig. 2). Results are expressed as % MPE (mean6S.E.M.), which
were subjected to multi-factor analysis of variance
3.3. KOR-1-deficient mice (ANOVA) with repeated-measures (time). Newman–Keuls
comparisons were used for post-hoc testing (Statistica,
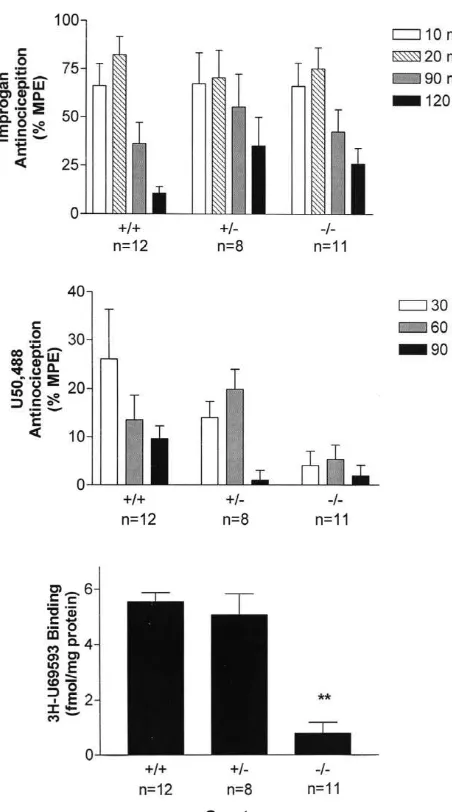
As found in the other mutant mice (above), improgan CSS, Inc., Tulsa, OK).
caused analgesia in all KOR-1 genotypes (Fig. 3). The time-course of action was similar to that seen in other 2.9. Radioligand binding
mutant mice (Fig. 3). A 20mg dose of improgan was used in the KOR-1 mice because pilot studies in 1/1 mice In some experiments, whole brains were harvested and
found that a higher dose (30 mg) caused maximal re-homogenized following nociceptive testing. Membrane
sponses that did not return to baseline within 2 h (data not fractions were assayed ford or k opioid receptor binding
shown). ANOVA found significant effects (P,0.01) across by radioligand methods [19]. The former was studied with
time, but not across genotype or gender. Subsequent 3
H-DPDPE (2 nM, 33 Ci / mmol) incubated for 4 h at 258C
analgesic studies with U50,488, a selective k opioid with naloxone (10mM) for non-specific binding; the latter
agonist (Fig. 3), found mild antinociceptive responses in 3
was performed with H-U69,593 (2 nM, 44 Ci / mmol)
1/1 control mice, but not in2/2 mutant mice. ANOVA incubated for 30 min at 378C with naloxone (10mM) for
showed this effect to be significantly (P,0.05) different non-specific binding.
across genotype, although the lack of a significant time by genotype interaction term prevented post-hoc comparisons. However, a gender by genotype interaction term was
3. Results significant from the ANOVA (P,0.05), with males
show-ing significantly more U50,488 analgesia (not shown). As 3
3.1. MOR-1-deficient mice expected, H-U69,593 binding to k opioid sites was
virtually abolished in the 2/2 KOR-1 mice (Fig. 3). Improgan induced maximal antinociception in male and
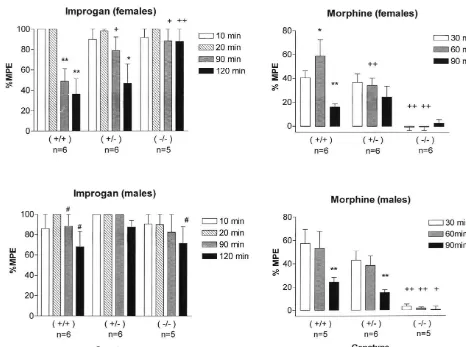
female mice of all three genotypes 10 and 20 min after
administration (Fig. 1). This activity was reversible, with 4. Discussion responses declining over the subsequent 120 min period.
Saline-treated mice (not shown) exhibited antinociceptive Improgan is a cimetidine analog, which shows charac-scores between 0 and 5%. A two-factor repeated-measures teristics of a selective, highly effective analgesic agent ANOVA found significant (P,0.05) interaction terms after CNS administration [13,14]. However, the mecha-(genotype–time, gender–time, genotype–gender–time). In nism of improgan analgesia has not yet been discovered. females, improgan analgesia was enhanced in heterozy- Bioassays [13], agonist and antagonist treatments [15] and gous (1/2) and homozygous (2/2) mutants, as com- in vivo structure–activity studies [9] have excluded roles pared with control (1/1) subjects; no such differences for known histamine (H , H and H ) receptors.
1 2 3
were found in males. As expected, morphine analgesia was Radioligand receptor screening for improgan is not yet attenuated (1/2) or abolished (2/2) in these mice as complete, but more than 40 G-protein-coupled receptors compared with 1/1 controls (Fig. 1). and ion channels have been excluded [6], suggesting the
possibility that the improgan target is novel.
Because improgan attenuates high-temperature nocicep-3.2. DOR-1-deficient mice
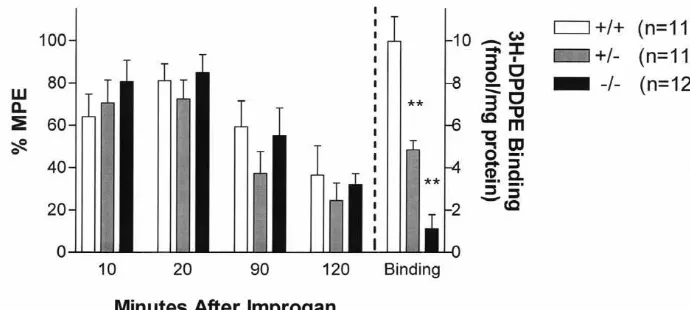
tive responses in rats (an effect shared with morphine), opioid receptors have been considered as potential sites of Improgan also induced antinociception in both sexes of
improgan action. However, binding studies with brain all DOR-1 genotypes 10 and 20 min after icv
administra-membranes and cloned cells found improgan (10 mM) to tion (Fig. 2). A time-related reversal of the analgesia was
have no affinity at m opioid receptors [6]. The compound observed 90 and 120 min later. ANOVA found significant
also showed very low affinity at d opioid receptors by effects (P,0.01) across time, but not across genotype or
Fig. 1. Effects of improgan (left) and morphine (right) on antinociceptive scores (% MPE, mean6S.E.M., ordinate) in three genotypes of MOR-1 knockout mice. (Left) Mice (sexes shown separately) were tested for baseline responses, received improgan (30mg, icv) and were re-tested at the times indicated. (Right) One to two weeks later, the same mice were tested for baseline responses, received morphine sulfate (10 mg / kg, s.c.) and were re-tested at the times shown. Following the last test, the animals were re-anesthetized, and injected with India Ink to verify icv injection sites. Baseline latencies (s, mean6S.E.M., females and males, respectively) were improgan: 1.0560.07, 1.2860.06; morphine: 1.0160.08, 1.1161.10. Data from one improgan-treated female (2/2) were omitted because the icv injection was not verified to be in the lateral ventricle. Morphine results from this subject were included. Another subject (1/1male) died several days after improgan treatment, and thus was not included in the morphine experiment. For all panels,
1,11
significant differences (P,0.05, 0.01, respectively) were: *,** time differences vs. first time period in the same group; genotype differences vs. [,[[
control (1/1) mice in the same gender at the same time; gender differences in the same groups.
also had low affinity at k1 and ORL sites by radioligand1 action of this drug. Results showing that the analgesia binding [6]. Thus, improgan does not appear to act directly evoked by morphine, but not by improgan (Fig. 1), is onm,d, k, or ORL-1 opioid receptors. abolished in MOR-1 2/2 mutant mice are in agreement Although direct opioid actions of improgan have been with several previous studies which have established the excluded, the drug could still be activating endogenous essential role of MOR-1 in morphine action opioid circuitry. For example, supraspinally-administered [12,16,17,20,22]. The slight, but statistically significant GABAA antagonists [2], excitatory amino acids [5] and gender difference in improgan analgesia in the MOR-1
b-endorphin [24] are all thought to work in part by knockout mice (Fig. 1) is interesting, but the explanation activation of spinal opioid mechanisms. Thus, further for this is unknown. Previous work has demonstrated analysis of the significance of opioid receptors as medi- sexual dimorphisms in both opioid [10] and non-opioid
ators of improgan analgesia was needed. [23] analgesic responses.
3
Fig. 2. Improgan antinociception (% MPE, mean6S.E.M., left ordinate) and brain H-DPDPE binding (fmol / mg protein, mean6S.E.M., right ordinate) in three genotypes of DOR-1 knockout mice. Subjects (pooled sexes, not different from each other, n-values in parentheses) were tested for baseline responses, received improgan (30mg, icv), and were re-tested at the times indicated (abscissa, min). Baseline latencies (s, mean6S.E.M.) were 1.1460.06. Following the last test, the animals were re-anesthetized, injected with India Ink to verify icv injection sites, and decapitated. Whole brains were rapidly
3
removed, homogenized, and assayed for H-DPDPE binding. **Significantly different from control (1/1) binding (P,0.01).
that otherm-preferring opioids (e.g. fentanyl, morphine-6- [28], implying a functional role for this gene in this glucuronide [M6G]) may act at another receptor, which is response. However, analgesia resulting from supras-an alternate product of MOR-1. For example, M6G pinally-administered DPDPE was unexpectedly retained, analgesia was found to be abolished in exon 2 mutants of suggesting the existence of more than one d-opioid re-MOR-1, but not in exon 1 knockouts [20]. However, ceptor [28]. The present experiments, showing supraspinal another group reported that M6G analgesia was abolished DPDPE analgesia in homozygous DOR-1 mutants (see in mice having a different mutation of exon 1 [12], and Results), are an independent confirmation of the earlier further studies are in progress to understand this dis- findings. The findings that both icv improgan and icv crepancy. If somem opioids can indeed induce analgesia DPDPE induced analgesia in DOR-1 mutant mice could in exon 1 mutations of MOR-1, then it might be argued imply that improgan acts on this secondd-like receptor. from the present data that improgan could also act in this This is unlikely, however, because the analgesia resulting manner. This is not likely, however, since there is a from icv DPDPE [28], but not that from icv improgan [15], consensus that the analgesia elicited by all m opioids is completely antagonized by naltrexone.
(including M6G) is completely blocked by the opioid Our finding that improgan analgesia is unaffected by antagonist naltrexone. Since improgan analgesia is not mutations in KOR-1 (Fig. 3A) suggest that k opioid blocked by naltrexone [15], or by other m antagonists receptors also do not contribute to improgan responses. (unpublished studies), it seems clear that the MOR-1 gene The loss ofk (U50,488) analgesia (Fig. 3B) andk opioid does not participate in improgan analgesia. binding sites (Fig. 3C) in the homozygous KOR-1 mutants Our present results, showing that improgan analgesia is confirms the absence of functional k opioid receptors. unchanged in any of the DOR-1 genotypes (Fig. 2), Previous work with a KOR-1 mutant different from that suggest that thedopioid receptor also does not play a role studied presently also showed deficits in k binding and in the action of this drug. Although there is considerable k-mediated analgesic responses [21]. The moderate an-pharmacological evidence for the existence of sub-types of tinociceptive scores observed presently after U50,488 in thed opioid receptor [26], bothd1andd2binding sites were control mice (25% MPE, Fig. 3B) are lower than found in abolished in brains of homozygous DOR-1 mutant mice, the earlier study [21] because different water temperatures suggesting that both sites are encoded by this gene [28]. were used in the tail immersion tests. The apparent The present studies, performed with the same DOR-1 analgesic efficacy of kappa opioids is known to be related
3
mutants, independently confirm the loss ofd1( H-DPDPE) to the intensity of the thermal nociceptive stimuli used in binding (Fig. 2). Taken together, the two studies suggest the assay [18].
that neither the d1 nor the d2 receptor contributes to The present findings, showing that improgan analgesia is
improgan analgesia. not diminished in mice lacking functionalm,d ork opioid
References
[1] M.D. Bannoura, J.W. Nalwalk, Y. Tang, M. Carlile, R. Leurs, W.M.P.B. Menge, H. Timmerman, L.B. Hough, Absence of an-tinociceptive tolerance to improgan, a cimetidine analog, in rats, Brain Res. 814 (1998) 218–221.
[2] D. Budai, H.L. Fields, Endogenous opioid peptides acting atd -opioid receptors in the dorsal horn contribute to midbrain modula-tion of spinal nociceptive neurons, J. Neurophysiol. 79 (1998) 677–687.
[3] G.L. Durant, J.C. Emmett, C.R. Ganellin, N-Cyano-N9(imidazolylalkyl)guanidines and similar guanidines. German Pa-tent[2344779 (1974).
[4] S.D. Glick, A.M. Crane, L.A. Barker, T.W. Mittag, Effects of
N-hydroxyethyl-pyrrolidinium methiodide, a choline analogue, on
passive avoidance behaviour in mice, Neuropharmacology 14 (1975) 561–564.
[5] D.L. Hammond, B.B. Donahue, P.E. Stewart, Role of spinald1and
d2 opioid receptors in the antinociception produced by microinjec-tion of L-glutamate in the ventromedial medulla of the rat, Brain Res. 765 (1997) 177–181.
[6] L.B. Hough, J.W. Nalwalk, W.G. Barnes, L.M. Warner, R. Leurs, W.M.P.B. Menge, H. Timmerman, M. Wentland, A third life for burimamide: discovery and characterization of a novel class of non-opioid analgesics derived from histamine antagonists, in: S.D. Glick, I.M. Maisonneuve (Eds.), New Medications For Drug Abuse, Vol. 909, N. Y. Acad. Sci, New York, 2000, pp. 25–40.
[7] L.B. Hough, J.W. Nalwalk, R. Leurs, W.M.P.B. Menge, H. Timmer-man, Antinociceptive activity of derivatives of improgan and burimamide, Pharmacol. Biochem. Behav. 65 (2000) 61–66. [8] L.B. Hough, J.W. Nalwalk, R. Leurs, W.M.P.B. Menge, H.
Timmer-man, Antinociceptive activity of impentamine, a histamine con-gener, after CNS administration, Life Sci. 64 (1999) PL79–PL86. [9] L.B. Hough, J.W. Nalwalk, B.Y. Li, R. Leurs, W.M.P.B. Menge, H.
Timmerman, C. Cioffi, M. Wentland, Novel qualitative structure– activity relationships for the antinociceptive actions of H2 antago-nists, H3 antagonists and derivatives, J. Pharmacol. Exp. Ther. 283 (1997) 1534–1543.
[10] K.L. Kepler, K.M. Standifer, D. Paul, B. Kest, G.W. Pasternak, R.J. Bodnar, Gender effects and central opioid analgesia, Pain 45 (1991) Fig. 3. Improgan antinociception (% MPE, mean6S.E.M., top), U50,488 87–94.
3
antinociception (% MPE, mean6S.E.M., middle), and brain H-U69593 [11] B.L. Kieffer, Opioids: first lessons from knockout mice, TIPS 20 binding (fmol / mg protein, mean6S.E.M., bottom) in three genotypes of (1999) 19–25.
KOR-1 knockout mice. (Top) Subjects (pooled sexes, not different from [12] N. Kitanaka, I. Sora, S. Kinsey, Z.Z. Zeng, G.R. Uhl, No heroin or each other, n-values given) were tested for baseline responses, received morphine 6b-glucuronide analgesia in m-opioid receptor knockout improgan (20mg, icv), and were re-tested at the times indicated. (Middle) mice, Eur. J. Pharmacol. 355 (1998) R1–R3.
One to two weeks later, the subjects were re-tested for baseline responses, [13] B.Y. Li, J.W. Nalwalk, L.A. Barker, P. Cumming, M.E. Parsons, L.B. received U50,488 (25 mg / kg, s.c.) and were tested at the times shown. Hough, Characterization of the antinociceptive properties of Baseline latencies (s, mean6S.E.M.) were 1.0960.06 and 0.9360.06 for cimetidine and a structural analog, J. Pharmacol. Exp. Ther. 276 improgan (top) and U50,488 (middle) treatments, respectively. (Lower) (1996) 500–508.
Following the last test, the animals were re-anesthetized, injected with [14] B.Y. Li, J.W. Nalwalk, J.M. Finkel, S.D. Glick, L.B. Hough, India Ink to verify icv injection sites, and decapitated. Whole brains were SKF92374, a cimetidine analog, produces mechanical and thermal
3
rapidly removed, homogenized, and assayed for H-U69593 binding. antinociception in the absence of motor impairment, Analgesia 3 **Significantly different from control (1/1) binding (P,0.01). (1997) 15–20.
[15] B.Y. Li, J.W. Nalwalk, L.B. Hough, Effects of naltrexone and histamine antagonists on the antinociceptive activity of the cimetidine analog SKF92374 in rats, Brain Res. 748 (1997) 168–
improgan-like compounds may lead to the development of 174.
brain-penetrating, clinically useful analgesics. [16] H.H. Loh, H.C. Liu, A. Cavalli, W.L. Yang, Y.F. Chen, L.N. Wei,m
opioid receptor knockout in mice: effects on ligand-induced analge-sia and morphine lethality, Mol. Brain Res. 54 (1998) 321–326. [17] H.W.D. Matthes, R. Maldonado, F. Simonin, O. Valverde, S. Slowe,
Acknowledgements
I. Kitchen, K. Befort, A. Dierich, M. Le Meur, P. Dolle, E. Tzavara, J. Hanoune, B.P. Roques, B.L. Kieffer, Loss of morphine-induced
We thank the National Institute on Drug Abuse for grant analgesia, reward effect and withdrawal symptoms in mice lacking
[18] M.J. Millan, A. Czlonkowski, A. Lipowski, A. Herz, Kappa-opioid [23] W.F. Sternberg, J.S. Mogil, B. Kest, G.G. Page, Y. Leong, V. Yam, receptor-mediated antinociception in the rat. II. Supraspinal in J.C. Liebeskind, Neonatal testosterone exposure influences neuro-addition to spinal sites of action, J. Pharmacol. Exp. Ther. 251 chemistry of non-opioid swim stress-induced analgesia in adult
(1989) 342–350. mice, Pain 63 (1995) 321–326.
[19] S.M. Pearl, K. Herrick-Davis, M. Teitler, S.D. Glick, Radioligand- [24] L.F. Tseng, H.Q. Wang, J.Y. Xu, Inhibition of spinal nitric oxide
omega
binding study of noribogaine, a likely metabolite of ibogaine, Brain synthase by N -nitro-L-arginine blocks the release of
Met-en-Res. 675 (1995) 342–344. kephalin and antinociception induced by supraspinally administered [20] G.P. Schuller, M.A. King, J. Zhang, E. Bolan, Y.-X. Pan, D.J. b-endorphin in the rat, Neuroscience 78 (1997) 461–467.
Morgan, A. Chang, M. Czick, E.M. Unterwald, G.W. Pasternak, J.E. [25] R.C. Vollinga, W.M.P.B. Menge, H. Timmerman, A new convenient Pintar, Retention of heroin and morphine-6b-glucuronide analgesia route for the synthesis of 4-(omega-aminoalkyl)-1H-imidazoles, in a new line of mice lacking exon 1 of MOR-1, Nature Neurosci. 2 Rec. Trav. Chim. 112 (1993) 123–125.
(1999) 151–156. [26] P.A. Zaki, E.J. Bilsky, T.W. Vanderah, J. Lai, C.J. Evans, F. Porreca, [21] F. Simonin, O. Valverde, C. Smadja, S. Slowe, I. Kitchen, A. Opioid receptor types and subtypes: the d receptor as a model,
Dierich, M. Le Meur, B.P. Roques, R. Maldonado, B.L. Kieffer, Annu. Rev. Pharmacol. Toxicol. 36 (1996) 379–401.
Disruption of thek-opioid receptor gene in mice enhances sensitivi- [27] J. Zhang, M. King, G.W. Pasternak, J.E. Pintar, Production and ty to chemical visceral pain, impairs pharmacological actions of the analgesic characterization of KOR-1 deficient mice, Soc. Neurosci. selective k-agonist U-50,488 and attenuates morphine withdrawal, Abstr. 24 (1) (1998) 525.
EMBO J. 17 (1998) 886–897. [28] Y. Zhu, A. King, G.P. Schuller, J.F. Nitsche, M. Reidl, R. Elde, E. [22] I. Sora, N. Takahashi, M. Funada, H. Ujike, R.S. Revay, D.M. Unterwald, G.W. Pasternak, J.E. Pintar, Retention of supraspinal Donovan, L.L. Miner, G.R. Uhl, Opiate receptor knockout mice delta-like analgesia and loss of morphine tolerance in delta opioid define m receptor roles in endogenous nociceptive responses and receptor (DOR-1) knockout mice, Neuron 24 (1999) 243–252. morphine-induced analgesia, Proc. Natl. Acad. Sci. USA 94 (1997)