www.elsevier.com/locate/ibmb
The influence of smoke volatiles on sexual maturation and juvenile
hormone biosynthesis in the black army cutworm, Actebia fennica
(Lepidoptera: Noctuidae)
Claude Everaerts
1,a, Michel Cusson
b, Jeremy N. McNeil
a,*aDe´partement de biologie, Universite´ Laval, Sainte-Foy, Quebec, Canada G1K 7P4
bLaurentian Forestry Centre, Canadian Forestry Service, Natural Resources, Canada, Sainte-Foy, Quebec, Canada G1V 4C7
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
Outbreaks of the black army cutworm, Actebia fennica, are associated with recently burned sites, where larvae feed on early successional plants. In the present paper we show that smoke volatiles stimulate juvenile hormone biosynthesis in virgin females, resulting in a more rapid rate of oocyte maturation and a significant advance in the age of first calling (the release of the sex pheromone) compared to control females. The ecological implications of this physiological effect are discussed. 2000 Elsevier Science Ltd. All rights reserved.
1. Introduction
Migration is one strategy that insects have evolved to cope with predictable or unpredictable changes in habitat quality and, in migratory species, adults usually leave unsuitable habitats in search of ones where continued reproduction is possible. In such situations abiotic (e.g. temperature, photoperiod) or biotic (e.g host plant availability) factors serve as cues to induce the required morphological, behavioral and/or physiological changes for extended flight (Tauber et al., 1986).
The true armyworm, Pseudaletia unipuncta, annually establishes summer populations in Canada and north-eastern United States, but cannot survive the winter in these areas (Fields and McNeil, 1984). Under short-day, cool-temperature conditions there is a significant delay in sexual maturation, as measured by oocyte growth (Cusson and McNeil, 1989), the synthesis and release of the sex pheromone by females (Turgeon and McNeil, 1983; Delisle and McNeil, 1987a,b) and the responsive-ness of males to the female sex pheromone (Turgeon et al., 1983; Dumont and McNeil, 1992). McNeil (1987)
* Corresponding author.
1 Current address: CNRS, UMR SS48, Universite´ de Bourgogne, 6 blvd Gabriel, F-21000 Dijon, France.
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 5 8 - 8
postulated that such delays, in response to cues signaling predictable habitat deterioration, would facilitate north-ward and southnorth-ward annual migrations between suitable summer and winter sites. There is now clear evidence that the observed differences in the rates of sexual matu-ration are associated with differences in juvenile hor-mone (JH) biosynthesis (Cusson et al. 1990, 1994; McNeil et al., 1996; McNeil et al., 2000), a hormone known to influence both sexual maturation and flight in insects.
The absence of sunflower pollen, an important ovi-position stimulant for the sunflower moth, Homoeosoma
ellectellum (Delisle et al., 1989), induced similar delays
in the onset of female reproduction in this species (McNeil and Delisle, 1989). It has been hypothesized that pollen availability within a habitat, which is unpre-dictable in both time and space, is a major factor influ-encing migration in this species (McNeil and Delisle, 1989; McNeil et al., 1994). However, it is not known if the physiological processes regulating sexual maturation in species that migrate in response to unpredictable habi-tat deterioration are similar to those of species responding to predictable habitat change.
recently burned sites (Phipps, 1927; Wood and Nelson, 1956; Ross and Ilnytzky, 1977; Humble et al., 1989). Virgin black army cutworm females exhibit oriented, upwind flight in a smoke plume, and in the presence of volatiles from the ashes of recently burned vegetation they call (a behavior associated with the emission of the sex pheromone) at a significantly younger age than con-trols (McNeil et al., unpublished). It was postulated that these modifications permit females to effectively exploit suitable habitats that are unpredictable both in time and space. In this paper we specifically examined the effect of smoke volatiles on the age of first calling, as well as the age-related changes in the volume of the corpora allata (CA), JH biosynthesis and oocyte maturation in virgin black army cutworm.
2. Materials and methods
A laboratory colony was established with material from the Great Lakes Forestry Centre, Canadian Forestry Service, Saute Ste. Marie, and subsequently maintained at 20±1°C and 65±5% relative humidity under a 16L:8D photoperiodic regime. Larvae were reared individually on an artificial diet (modified from Shorey and Hale, 1965), while adults were held in cages containing a source of 8% sucrose in water, with cheese-cloth as an oviposition substrate. All subsequent experiments were carried out under identical rearing conditions, using females that had been isolated as pupae.
To determine the effect of volatiles on calling behavior newly-emerged females were isolated in trans-parent 150 cm3plastic cages and provided with a source of 8% sucrose solution. One-half of the cages also con-tained a piece of Whatman No. 1 filter paper treated with 10µl of liquid smoke (Baldwin and Morse, 1994; Bald-win et al., 1994), which was changed every 2 days, while the controls had water-treated paper. Moths could not directly contact the filter paper, so only volatile cues would be detected. Both treatment and control groups had a minimum of 23 individuals, and were held in dif-ferent incubators to avoid potential problems of volatile contamination. Females were observed every 15 min during the first 5 h of the scotophase, using a flashlight covered with two layers of tissue paper and a Kodak red Wratten filter. The age at which females first exhibited calling behavior, as well as the mean onset time of call-ing on the first night of callcall-ing, was recorded and the differences between treatments were compared using Mann–Whitney U-tests.
To determine the effect of volatiles on CA growth and in vitro JH biosynthesis, as well as oocyte growth, newly-emerged females were held in individual cages in an incubator with a vial containing 250 µl of liquid smoke. This liquid was replaced daily. Controls were held in another incubator with no source of liquid smoke.
In both cases an ad libitum supply of 8% sucrose sol-ution was provided to all individuals. A minimum of six individuals was taken from the treated and control groups 5, 10, and 15 days after the initiation of the experiment. The corpora allata–corpora cardiaca (CA– CC) complexes of control and test females were dis-sected during the first 2 h of scotophase. The length (L) and width (W) of the left CA was measured using an ocular micrometer, and the volume subsequently com-puted as for an ellipsoid (4/3×[π×W2×
L]). The complex was then transferred to a 5 ml conical culture tube con-taining a few drops of medium 199 (Gibco) devoid of
l-methionine, and supplemented with Ficoll, calcium
chloride and sodium propionate (Cusson et al., 1990). Rates of in vitro JH biosynthesis were measured using a radiochemical assay modified for the true armyworm (see Cusson et al., 1990) in 50µl of the same medium that had been supplemented withl-[methyl-3
H]methion-ine (200 mCi/mmol; NEN) at a final concentration of 300µM. Each pair of glands was individually incubated for 3 h at 27°C, and the JH was extracted from the medium with 400µl isooctane. After 10 min centrifug-ation (3000 rpm, 4°C), 350µl were taken from the isooc-tane phase to which JH III in isoocisooc-tane was added as a cold marker (1 µg/µl; Sigma Chemical Company). The sample was dried under N2 and the residue resuspended in 150 µl diethyl ether before loading on aluminum-backed TLC plates (silica gel 60F254 20 cm×20 cm; 0.2 mm thickness; Merck). TLC was performed with xylene/ethyl acetate (75:25) as the solvent system. Fol-lowing development, the JH band was marked and the appropriate zones were cut and analyzed by liquid scin-tillation spectrometry (LSS).
The level of oocyte growth in individuals used for the JH biosynthesis analyses was also determined. The ovaries were removed and stained in Grenacher borax carmine (Cusson and McNeil, 1989), then washed twice in 70% ethyl alcohol. If chorionated eggs were not present then the width of the penultimate basal oocyte in each of the four ovarioles of the right ovary was meas-ured, using an ocular micrometer. When mature eggs were present the number was recorded and each was assigned a width of 0.78 mm, as the measurement of 40 randomly chosen eggs found they were all of very simi-lar size, with an SEM of 0.01 mm.
Differences in the rates of in vitro JH biosynthesis, CA volume and numbers of eggs as a function of age and treatment were compared using a Kruskal–Wallis ANOVA, while Mann–Whitney U-tests were used for pairwise comparisons.
The nature of the products released by A. fennica CA in vitro and the ratios of each compound were determ-ined in 15-day-old control virgins, an age at which
described above. The sample was dried under N2 and then dissolved in 10µl hexane before HPLC analysis on a 5 µm Micro Pack Silica column (30 cm×4 mm). The mobile phase consisted of 5% redistilled ethyl ether (50% of which was water saturated) in HPLC-grade hex-ane. Chromatographic conditions were as follows: flow rate, 1 ml/min; detector wavelength, 219 nm. Fractions were collected at 12 s intervals, and their radioactivity determined by LSS. Synthetic JHs (JH I and JH II from SciTech, Prague, Czech Republic; JH III from Sigma) were used as standards.
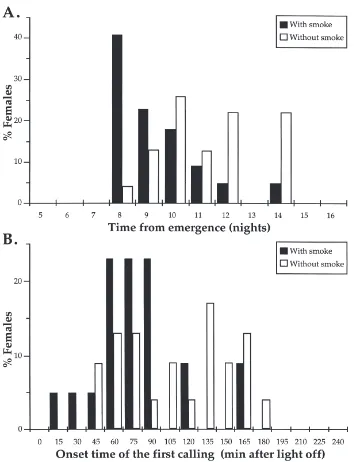
Fig. 1. The age of first calling following emergence (A) and the mean onset time of calling following the lights-out signal (B) when black army cutworm virgin females were held in the presence (black) or absence (white) of liquid smoke volatiles.
3. Results
Both the age (Fig. 1A) and the onset time of the first calling (Fig. 1B) were significantly affected by exposure to liquid smoke. Treated females called at a significantly younger age after emergence (9.3±0.3 vs. 11.2±0.4 days;
P=0.001; U-test) and started calling sooner after the lights-off signal (78.4±8.1 vs. 115±9.8 min; P=0.02; U-test) than control females.
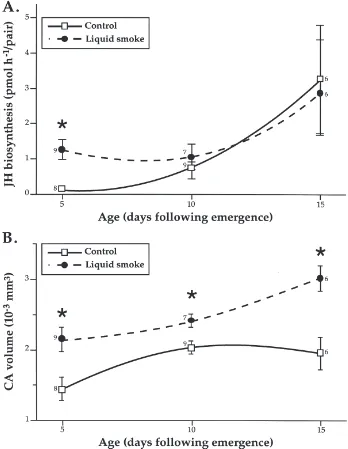
Fig. 2. Temporal changes in total JH biosynthesis (A) and the size of the corpora allata (B) in black army cutworm virgin females when held in the presence (broken line) or absence (solid line) of liquid smoke volatiles, at 20°C, 8L:16D. For any given age, significant differences between control and treated females are indicated by an asterisk (Mann–Whitney U-test, p=0.05); the numbers indicate the sample sizes.
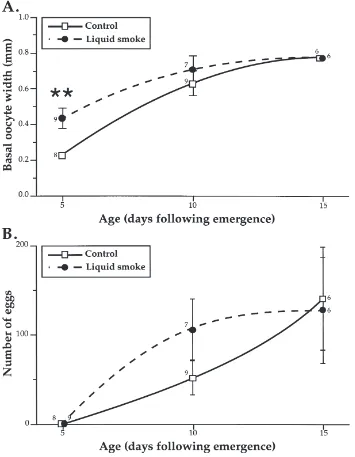
and CA volume (Fig. 2B), as well as basal oocyte width (Fig. 3A) and the number of mature eggs (Fig. 3B). The CA volumes were significantly larger in treated females, regardless of age, while their basal oocyte width and the rate of JH biosynthesis were only significantly greater than controls in 5-day-old individuals. Neither group contained mature eggs on day 5 but by day 10 the mean number of chorionated eggs in the ovaries of treated females was twice that of controls (106.5±34.5 vs. 52.4±19.7 eggs/female). However, due to high individual variability (86 and 67% of treated and control females contained some mature eggs) this difference was not significant (p=0.09). On day 15, all individuals contained chorionated
eggs, and the means for treated and control females were similar (127.7±58.7 vs. 141.2±57.2, respectively).
HPLC analysis revealed three peaks with retention times, similar to those of synthetic JH I, II and III (Fig. 4). The total rate of JH biosynthesis was 0.83 pmol h21/CA pair, with JH II accounting for 58% while JH III and JH I, respectively, represented 29% and 13% of the total JH produced.
4. Discussion
Fig. 3. The temporal evolution of basal oocyte width (A) and the number of mature eggs present in the ovaries of black army cutworm virgin females when held in the presence (broken line) or absence (solid line) of liquid smoke volatiles, at 20°C, 8L:16D. For any given age significant differences between control and treated females are indicated by an asterisk (Mann–Whitney U-test, p=0.05); the numbers indicate the sample sizes.
(1927) to postulate that, in late summer and early fall, females preferentially selected recently burned areas as oviposition sites. Fire-induced changes in soil chemistry (e.g. Dryness et al., 1989) affect the growth of early suc-cessional plants (Baldwin and Morse, 1994; Baldwin et al., 1994; Keeley and Fotheringham, 1998) and species exhibiting post-fire regeneration (Stein et al., 1992). Therefore, in keeping with the plant vigor hypothesis (Price, 1991), such an oviposition strategy by black army cutworm females would ensure their progeny access to preferred host plants (Humble et al., 1989), such as fireweed, dandelion, and coltsfoot (Ross and Ilnytzky, 1977) the following year. An alternate, but not mutually
exclusive, explanation is that recently burned sites afford enemy-free space, so that the temporary decline in natu-ral enemies would ensure that larval survival is high. The ability of females to locate recently burned areas, which are unpredictable in both time and space, is facili-tated by their oriented, upwind flight in a smoke plume (McNeil et al., unpublished).
It is clear from the present study that the younger age of first calling and the increased rate of oocyte growth seen in black army cutworm females, when held in the presence of smoke volatiles, are associated with changes in JH biosynthesis. Thus, the reproductive biology of A.
Fig. 4. HPLC radiochromatogram of products released from in vitro corpora allata of 10-day-old black army cutworm virgin females when the CA were incubated for 3 h at 27°C in 50µl of medium 199 supplemented withl-[methyl-3H]methionine (200 mCi/mmol; final concentration, 300
µM). Arrows indicate the retention times of synthetic JH homologues.
unpredictable changes in habitat quality, has evolved physiological mechanisms similar to those of P.
unipuncta, a species responding to more predictable
changes in habitat quality (Cusson et al. 1990, 1994). The accelerated rate of sexual maturation induced by volatiles from recently burned vegetation would reduce the time to mating and oviposition, once suitable sites are located.
Changes in size of CA have already been reported in various cockroaches, increasing and decreasing along with JH biosynthesis during successive ovarian cycles (Engelmann, 1959; Scharrer and Von Harnack, 1959; Wilhelm and Lu¨scher, 1974). These changes in volume associated with levels of JH synthesis may involve an ovarian factor (Stay and Woodhead, 1990) and/or a feed-back loop, with the released JH directly affecting the CA (Kikukawa et al., 1988; Chiang et al., 1995). Some form of ovarian factor and/or autocrine control may also occur in A. fennica, as there was no additional increase in CA volume of control females once JH biosynthesis and oocyte maturation had started. However, in the presence of smoke volatiles female CA continued to increase in size over the 15-day period following emergence, despite measurable JH biosynthesis and oocyte growth. Thus, while the association between JH biosynthesis and oocyte growth remains similar to that of controls, there is a dissociation between CA growth and JH biosynthesis when females are exposed to smoke cues. Changes in CA size and JH biosynthesis may be regulated indepen-dently in cockroaches (Stay and Tobe, 1977; Szibbo and Tobe, 1981) and different neural pathways may be
involved in the control of these two processes (Chaing et al., 1999). It is clear that further physiological, histo-logical and neurohisto-logical studies, similar to those carried out in cockroaches, are required in order to gain a clearer understanding of the relationships between CA growth and JH biosynthesis in A. fennica when females are reared under different ecological conditions.
In the true armyworm, rearing adults under summer (25°C; 16L:8D) and spring/fall (10°C; 12L:12D) con-ditions not only resulted in differences in JH biosynthesis but also in the relative proportions of the three JH homol-ogues, raising the possibility that varying ratios may play a role in the trade-off between migration and reproduction (Cusson et al., 1993). We have shown that black army cutworm females, like other Lepidoptera, produce all three major JH homologues, and that JH II is the most abundant (e.g. Judy et al., 1973; Jennings et al., 1975; Kramer and Law, 1980; Cusson et al. 1990, 1993). However, we do not know whether the homologue ratios change with age and rearing conditions. Therefore, further studies are required to determine whether the ratio of JH homologues changes in the presence or absence of smoke volatiles as a function of age and the size of the CA, and whether the homologues play a role in the expression of long-distance flight in A. fennica.
Acknowledgements
NSERC (J.N.M.), FCAR (J.N.M. and M.C.) and NATO (C.E.) grants.
References
Baldwin, I.T., Morse, L., 1994. Up in smoke: II. Germination of
Nicoti-ana attenuata in response to smoke derived cues and nutrients in
burned and unburned soils. J. Chem. Ecol. 20, 2373–2391. Baldwin, I.T., Staszak-Kosinski, L., Davidson, R., 1994. Up in smoke:
I. Smoke derived germination cues for postfire annual, Nicotiana
attenuata Torr Ex. Watson. J. Chem. Ecol. 20, 2345–2371.
Chiang, A.-S., Tsai, W.H., Schal, C., 1995. Neural and hormonal regu-lation of growth of corpora allata in the cockroach, Diploptera
punctata. Mol. Cell. Endocrinol. 115, 51–57.
Chaing, A.-S., Pszczolkowski, M.A., Lee, C.-M., Wei, T.-W., 1999. Protocerebral neurons inhibiting proliferation of corpus allatum cells in the cockroach, Diploptera punctata. J. Comp. Neurol. 413, 593–602.
Cusson, M., McNeil, J.N., 1989. Ovarian development in female army-worm moths, Pseudaletia unipuncta: its relationship with phero-mone release activity. Can. J. Zool. 67, 1380–1385.
Cusson, M., McNeil, J.N., Tobe, S.S., 1990. In vitro biosynthesis of juvenile hormone by corpora allata of Pseudaletia unipuncta virgin females as a function of age, environmental conditions, calling behaviour and ovarian development. J. Insect Physiol. 36, 139–146. Cusson, M., Yagi, K.J., Tobe, S.S., McNeil, J.N., 1993. Identification of release products of corpora allata of male and female armyworm moths, Pseudaletia unipuncta. J. Insect Physiol. 39, 775–783. Cusson, M., Tobe, S.S., McNeil, J.N., 1994. Juvenile hormones: their
role in the regulation of the pheromonal communication system of the armyworm moth, Pseudaletia unipuncta. Arch. Insect Biochem. Physiol. 25, 329–345.
Delisle, J., McNeil, J.N., 1987a. Calling behaviour and pheromone titre of the true armyworm, Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae), under different temperature and photoperiodic con-ditions. J. Insect Physiol. 33, 315–324.
Delisle, J., McNeil, J.N., 1987b. The combined effect of photoperiod and temperature on the calling behaviour the true armyworm,
Pseu-daletia unipuncta. Physiol. Entomol. 12, 127–164.
Delisle, J., McNeil, J.N., Underhill, E.W., Barton, D., 1989. Helianthus
anuus pollen, an oviposition stimulant for the sunflower moth, Homoesoma electellum. Entomol. Exp. Appl. 50, 53–60.
Dryness, C.T., Van Cleve, K., Levinson, J.D., 1989. The effect of wild-fire on soil chemistry in four forest types in interior Alaska. Can. J. Forest. Res. 19, 1389–1396.
Dumont, S., McNeil, J.N., 1992. Responsiveness of Pseudaletia
unipuncta (Lepidoptera: Noctuidae) males, maintained under
dif-ferent temperature and photoperiodic conditions, to female sex pheromone. J. Chem. Ecol. 18, 1797–1807.
Engelmann, F., 1959. The control of reproduction in Diploptera
punctata (Blattaria). Biol. Bull. Mar. Biol. Lab. Woods Hole 116,
406–419.
Fields, P.G., McNeil, J.N., 1984. The overwintering potential of the armyworm, Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae), populations in Quebec. Can. Entomol. 116, 1647– 1652.
Humble, L.M., Shepherd, R.F., Maher, T.F., 1989. Biology, outbreak characteristics and damage caused by the black army cutworm (Lepidoptera: Noctuidae). In: Alfaro, R.I., Glover, T.F. (Eds.), Insects Affecting Reforestation: Biology and Damage. Pacific For-estry Centre, Victoria, BC, pp. 82–88.
Jennings, R.C., Judy, K.J., Schooley, D.A., Hall, M.S., Siddal, J.B., 1975. The identification and biosynthesis of two juvenile hormones from the tobacco budworm moth (Heliothis virescens). Life Sci. 16, 1033–1040.
Judy, K.J., Schooley, D.A., Dunham, L.L., Hall, M.S., Bergot, B.J., Siddal, J.B., 1973. Isolation, structure and absolute configuration of a new natural insect juvenile hormone from Manduca sexta. Proc. Natl. Acad. Sci. U. S. A. 70, 1509–1513.
Keeley, J.E., Fotheringham, C.J., 1998. Mechanism of smoke-induced seed germination in a post-fire chaparral annual. J. Ecol. 86, 27–36. Kikukawa, S., Tobe, S.S., Stay, S., 1988. Modulation of juvenile hor-mone release and oocyte growth following (RS)-hydroprene treat-ment in virgin female Diploptera punctata. Physiol. Entomol. 13, 425–432.
Kramer, S.J., Law, J.H., 1980. Control of juvenile hormone production: the relationship between precursor supply and hormone synthesis in the tobacco hornworm, Manduca sexta. Insect Biochem. 10, 569–575.
McNeil, J.N., 1987. The true armyworm: a victim of the Pied Piper or a seasonal migrant? Insect. Sci. Appl. 8, 591–597.
McNeil, J.N., Delisle, J., 1989. Host plant pollen influences calling behavior and ovarian development of the sunflower moth,
Homo-esoma electellum. Oecologia 80, 201–205.
McNeil, J.N., Cusson, M., Delisle, J., Tobe, S.S., 1994. Hormonal con-trol of the sexual behaviour of moths that migrate in response to predictable and unpredictable habitat deterioration. In: Davey, K.G., Peters, R.E., Tobe, S.S. (Eds.), Perspectives in Comparative Endocrinology. National Research Council of Canada, Canada, pp. 464–468.
McNeil, J.N., Laforge, M., Be´dard, C., Cusson, M., 1996. Juvenile hormone production and sexual maturation in the true armyworm,
Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae): a
com-parison of migratory and non-migratory populations. Arch. Insect Biochem. Physiol. 32, 575–584.
McNeil, J.N., Millar, D., Laforge, M., Cusson, M., 2000 Dynamics of juvenile hormone biosynthesis, degradation and titers in females of the true armyworm: a comparison of migratory and non-migratory populations. Physiol. Entomol. 25, 103–111.
Phipps, C.R., 1927. The black army cutworm. A blueberry pest. Maine Agr. Exp. Sta. Bull. 340, 201–217.
Price, P.W., 1991. The plant vigor hypothesis and herbivore attack. Oikos 62, 244–251.
Ross, D.A., Ilnytzky, S., 1977. The black army cutworm Actebia
fen-nica (Tauscher) in British Columbia. Canadian Forestry Service,
Pacific Forestry Centre, Victoria, 23pp.
Scharrer, B., Von Harnack, M., 1959. Histophysiological studies on the corpus allatum of Leucophaea maderae. I. Normal life cycle in male and female adults. Biol. Bull. Mar. Biol. Lab. Woods Hole 115, 508–520.
Shorey, H.H., Hale, R.C., 1965. Mass rearing of the larvae of nine Noctuid species on a simple artificial medium. J. Econ. Entomol. 58, 522–524.
Skinner, B., 1998. The Colour Identification Guide to the Moths of the British Isles, 2nd edn. Penguin Group, London.
Stay, B., Tobe, S.S., 1977. Control of juvenile hormone biosynthesis during the reproductive cycle of a viviparous cockroach. I. Acti-vation and inhibition of the copora allata. Gen. Comp. Endocrinol. 33, 531–540.
Stay, B., Woodhead, A.P., 1990. Response of male corpora allata to ovarian stimulation in the cockroach, Diploptera punctata. Gen. Comp. Endocrinol. 77, 127–135.
Stein, S.J., Price, P.W., Abrahamson, W.A., Sacchi, C.F., 1992. The effect of fire on stimulating willow regrowth and subsequent attack by grasshopper and elk. Oikos 65, 190–196.
Szibbo, C.M., Tobe, S.S., 1981. The mechanism of compensation in juvenile hormone synthesis following unilateral allatectomy in
Diploptera punctata. J. Insect. Physiol. 27, 609–613.
Tauber, M.J., Tauber, C.A., Masaki, S., 1986. Seasonal Adaptations of Insects. Oxford University Press, Oxford.
induced by temperature conditions during pupal and adult develop-ment. Can. Entomol. 115, 1015–1022.
Turgeon, J.J., McNeil, J.N., Roelofs, W.L., 1983. Responsiveness of
Pseudaletia unipuncta males to the female sex pheromone. Physiol.
Entomol. 8, 339–344.
Wilhelm, R., Lu¨scher, M., 1974. On the relative importance of juvenile
hormone and vitellogenin for oocyte growth in the cockroach
Nau-phoeta cinerea. J. Insect Physiol. 20, 1887–1894.
![Fig. 4.HPLC radiochromatogram of products released from in vitro corpora allata of 10-day-old black army cutworm virgin females when theCA were incubated for 3 h at 27°C in 50 µl of medium 199 supplemented with l-[methyl-3H]methionine (200 mCi/mmol; final c](https://thumb-ap.123doks.com/thumbv2/123dok/3120523.1379279/6.598.128.470.74.292/radiochromatogram-products-released-corpora-females-incubated-supplemented-methionine.webp)