www.elsevier.com/locate/ibmb
The juvenile hormones: historical facts and speculations on future
research directions
Lawrence I. Gilbert
a,*, Noelle A. Granger
b, R. Michael Roe
caDepartment of Biology, Campus Box #3280 Coker Hall, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3280, USA bDepartment of Cell Biology and Anatomy, Campus Box #7090, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7090,
USA
cDepartment of Entomology, Campus Box 7647, North Carolina State University, Raleigh, NC 27695-7647, USA
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
1. Historical aspects
In all of endocrinology there is no more wondrous name for a hormone than the insect juvenile hormone (JH). Could V.B. Wigglesworth have predicted some six decades ago that his term “juvenile hormone” would offer promise of immortal youth to the aged, the expec-tation of a bloom of dollars to agrochemical concerns, and the hope of solutions to basic problems by develop-mental biologists and entomologists. The aged have been disappointed and the high expectations of commercial firms have not been met, but hope remains that JH can be used as a probe to ultimately solve basic questions in development.
It has been more than two centuries since Lyonet (1762) described granulated vessels in the thorax of lepi-dopteran larvae that proved to be the prothoracic glands. By contrast, the corpora allata were not mentioned in the literature until Mu¨ller (1828) described organs in the cockroach that he called pharyngeal bodies and which he thought innervated the dorsal vessel and esophagus. During the remainder of the 19th century, the corpora allata were described as sympathetic ganglia or other components of the nervous system, as indicated by the various descriptive terms given them, e.g. accessory gan-glia, tracheal gangan-glia, lateral ganglion, lateral head gang-lion, appendage of the pharyngeal ganggang-lion, etc. In 1899, Heymons dubbed these organs the corpora allata and correctly described their embryological origin, but also believed that they were a pair of sympathetic ganglia concerned with the innervation of the digestive system.
* Corresponding author. Tel.:+1-919-966-2055; fax:+ 1-919-962-1344.
E-mail address: [email protected] (L.I. Gilbert).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 3 4 - 5
In 1910, Police suggested that the corpora allata were endocrine organs in phasmids, but that they probably had a nervous function in other insects.
It was Nabert in 1913 who, on the basis of studying a variety of insects representing several orders, finally stated that the corpora allata were glandular and exhib-ited periodic internal secretions. This was confirmed by the work of Ito (1918), who concluded that the corpora allata were indeed organs of internal secretion and that they functioned in the adult moth as well. All of this work was anatomical and histological with a bit of microsurgery, with the corpora allata being observed under the light microscope using a variety of stains. Ito was probably the first to use planimetrics to measure the diameter of the corpora allata and to demonstrate variations in size during various stages of metamor-phosis.
molting hormone. Further surgical studies suggested that the insect also contained an “inhibitory factor” which prevented the first four larval stages from molting directly into adults. In 1935 he suggested that both the molting and inhibitory hormones were produced by the corpus allatum, and in 1936 his classic paper on the function of the corpus allatum in the growth of Rhodnius was published. He showed that the corpus allatum was the source of the inhibitory hormone that prevents meta-morphosis in young larvae and that corpora allata from these young larvae when implanted into fifth instars caused them to undergo a supernumerary molt. He per-fected the surgery of allatectomy and showed that such animals could molt, indicating that the corpus allatum was not the source of molting hormone. Wigglesworth concluded that each cell has the potential for larval or adult differentiation and that hormone titers determine which potential is realized. It is the concentration of the corpus allatum hormone that determines the extent of metamorphosis at the next molt, wrote Wigglesworth (1948), and of course, analogous experiments were done on other insects over the next twenty-five years showing that Professor Wigglesworth was correct. It is of interest that some of his work on this subject probably would not be accepted for publication today because of the small sample size used, i.e. only two of the five operated fifth instars molted into intermediates after receiving cor-pus allatum implants from fourth instars. Our own per-sonal bias is that Wigglesworth had the best intuition of any scientist we have met or read about.
In the first of many studies of JH function, Piepho (1938a,b, 1950) showed via histological studies that the formation and nature of the cuticle of Galleria was under hormonal control. In the initial experiments, the integu-ment from one larva was grafted to another, and the graft integument molted with the host. This led to a series of experiments in which a fragment of integument from one larva was implanted into the abdominal cavity of another. The epidermis of the fragment regenerated around the cuticle of the implant to give rise to an epi-dermal vesicle with cuticle at its interior. Piepho’s lab-oratory showed that when the host molted, the vesicle molted in concert. His studies very beautifully demon-strated that molting was under hormonal control, that the epidermis reacts to JH by laying down larval structures (cuticle) and that the epidermis does not “count the molts”. The classic study of Piepho (1942, 1950) revealed important phenomena concerning tissue com-petence and tissue responsiveness. First, it appeared that the epidermal cells remained responsive to JH until very late in the last stadium. When implants were done very late in the stadium, there was a patch of larval cuticle at the wound site in the resulting pupa. Second, the results showed that wounded epidermis is much more sensitive to the action of the JH than intact regions. This was a vital finding for the development of the Galleria wax
test that was used successfully for some twenty years before analytical analyses of JH came to the fore (Gilbert and Schneiderman, 1960; Schneiderman and Gilbert, 1964), and allowed the first semiquantitative titer of JH (Gilbert and Schneiderman, 1961b; Table 1).
During that same era, the classic studies of Bounhiol (1936–1938) showed the effects of corpora allata extir-pation in Lepidoptera (Bombyx). Bounhiol’s results were confirmed and extended by those of Fukuda (1942); Fukuda (1944). It is of interest that despite the use of HPLC, mass spectrometry, etc., to measure JH, our con-cept of how the changing titer affects developmental pro-cesses has not advanced a great deal over the past fifty years.
The “modern” era of JH research began with the criti-cal finding by Carroll Williams (1956) that the male
Hyalophora cecropia moth contained a store of a
lip-oidal “golden oil” with JH activity when assayed on lepi-dopteran pupae. The original intent of the experiment was to determine if the life of the male saturniid moth could be extended if it were parabiosed to a pupa. As is so common in biology, serendipity ruled, and the pupa molted into a second pupa, indicating that the moth had furnished the pupa with JH. Furthermore, Williams’ dis-covery elicited a frenzy of studies of JH in a variety of insects — for the first time, an active extract of this amazing hormone was available and it worked on most insect species. Indeed, from one male H. cecropia abdo-men enough JH could be extracted and diluted in peanut oil or mineral oil to conduct hundreds or even thousands of experiments. If you could find one male moth, you were in business for life! If Williams had used the other saturniid studied in his laboratory in his original experi-ments, the parabiotic pupal partner would have molted into an adult since the adult Antheraea polyphemus is almost devoid of JH. In retrospect, it is clear from these early experiments that Williams, Wigglesworth, Piepho, etc. all had an almost uncanny ability to interpret cor-rectly from unexpected, incomplete and seemingly bizarre results.
It was the patience and persistence of a young German scientist, Herbert Ro¨ller, that allowed his group to finally deduce the structure of JH I (Ro¨ller et al., 1967). At the time, there was competition between the Ro¨ller labora-tory and that of Howard Schneiderman, the latter con-firming the structure of JH I and identifying JH II several years later (Meyer et al., 1970). The difference between the approach of the two laboratories was that Ro¨ller et al. used glass columns in their gas chromatographic analyses while the Schneiderman group used all metal columns, which enhanced the lability of the JH mol-ecule. In any event, pure JHs were available about thirty years ago.
Table 1
Juvenile hormone content during the life history of the Cecropia silkworma
Stage Approximate juvenile Approximate juvenile Approximate juvenile
hormone concentration of hormone concentraion per hormone activity per gram extract: (Cecropia insect or fragment: fresh weight: (Cecropia units/gram extract) (Cecropia units/animal or units/gram animal or
fragment) fragment fresh weight)
Unfertilized eggs Minimum of: 165 0.042 8.151
Unfertilized eggs from alatectomized “0” “0” “0”
7-day embryos Minimum of: 165 0.031 7.095
7-day embryos from allatectomized Minimum of: 70 0.012 3.108
1st instar larvae (newly hatched) Minimum of: 165 0.031 7.475
5th instar larvae (mixed sex) 35 4.162 0.571
Diapausing pupae (1 month old) 20 5.780 1.468
Diapausing pupae (1 month old) 20 6.300 1.114
Chilled pupae (6 months old) “0” “0” “0”
Chilled pupae (6 months old) “0” “0” “0”
2-day old developing adults “0” “0” “0”
8-day old developing adults “0” “0” “0”
11-day old developing adults “0” “0” “0”
14-day old developing adults “0” “0” “0”
17-day old developing adults “0” “0” “0”
20-day old developing adults 125 25 13.70
22-day old developing adults 400 122.68 201.64
Adult (4 days) 675 151.88 298.75
Adult (7 days) 1000 178.30 418.00
Adult (7 days) 125 17.50 11.63
aActivity of extracts is expressed in Cecropia units. One Cecropia unit is equivalent to the juvenile hormone activity found in one milligram
of extract obtained from the abdomens of seven-day old male Cecropia moths. Extractions of developing adults and adult moths were conducted on the abdomens only and the activity noted is for the abdominal extract. From Gilbert and Schneiderman (1961b).
(receptors). We simply could not summarize or do jus-tice to all the presentations made during the symposium and chose topics we felt were important and with which we were personally involved. We apologize to those individuals we did not cite since it is possible that their contributions may be even more important than those summarized here.
2. Control of the corpora allata
Changes in the JH titer, which regulate the growth and development of immature insects and reproduction in adults, are controlled precisely by various physiological and biochemical processes i.e. synthesis, degradation, sequestration and secretion. Of these, the regulation of synthesis has generally been considered the most important, and a large body of evidence for both stimu-latory (allatotropic) and inhibitory (allatostatic) control of JH synthesis by the corpus allatum has accumulated from many years of studies in vivo.
2.1. Allatotropins (ATs.)
Since the corpora allata are innervated by the axons of both peptidergic neurosecretory cells and typical neurons located within the brain, the mechanisms stimulating and
inhibiting corpora allata function promised to be com-plex from the outset. Research in this area in the last decade has confirmed this prediction. The primary focus of this work has been on regulatory peptides, termed allatotropins and allatostatins. With regard to allatotrop-ins, despite a wealth of indirect evidence for the stimu-lation of JH production by neural factors, particularly during larval development, only one had been isolated and sequenced prior to this conference, an allatotropin from adult Manduca (Mas-AT) (Kataoka et al., 1989). This amidated tridecapeptide stimulates JH biosynthesis only by the corpora allata of adult Manduca. The gene
Mas-AT is expressed as three different mRNAs which
the corpora cardiaca and corpora allata. Thus, although Mas-AT has no apparent effect on the larval corpora allata of Manduca, it must have some other unknown function at this stage. Interestingly, while Mas-AT does stimulate adult corpora allata, its mRNAs were not detected in the prepupal, pharate adult or adult brain, but were present in the pterothoracic and unfused abdominal ganglia of the pupa (Bhatt and Horodyski, 1999). A recent study by Lee et al. (1998) demonstrated the inhi-bition of midgut ion transport in Manduca fifth instars by Mass-AT, an effect which appears to be species-spe-cific. It has another effect as well, exhibiting cardioacce-leratory activity in the pharate adult, but not the larva (Veenstra et al., 1994). These observations are illustra-tive of a phenomenon observed with a number of other insect neuropeptides i.e. different effects which are both stage- and tissue-specific. As was discovered with allato-statins, these effects can be species-specific as well (see below).
In the decade since the identification of Mas-AT, no allatotropin sequences were published for any other species, and until recently, there was a dearth of work on other putative allatotropins. The exceptions are the demonstration of allatotropic activity in the subeso-phageal ganglion of crickets (Lorenz and Hoffmann, 1995), and some recent work with Galleria (Bogus and Scheller, 1996). In the latter study, monoclonal anti-bodies raised to a protein fraction of larval brains that stimulated JH II synthesis by corpora allata in vivo and in vitro, were used to identify a single 20 kDa peptide by immunoblotting. Immunoreactivity was also observed in two pairs of cerebral neurosecretory cells and in cells of the corpus cardiacum. Although the size of this puta-tive allatotropin makes it an unlikely homolog of Mas-AT, a pair of immunoreactive median cerebral neuro-secretory cells were observed with antibodies to Mas-AT. This leads to the suggestion that Mas-AT and
Gal-leria AT have common epitopes due to splicing from
the same preprohormone.
A significant outcome of this conference is direct evi-dence from several different studies showing that Mas-AT is a functional moiety in other Lepidoptera (see Stay, this symposium). A stimulatory peptide in methanol extracts of adult brains of Spodoptera frugiperida, the fall army worm, has been identified by Edman degra-dation and mass spectrometry as Mas-AT (Oeh et al., this symposium). Exposure of adult corpora allata to synthetic Mas-AT results in a strong, dose-dependent and reversible stimulation of JH biosynthesis. The most interesting observation of this study is that a synthetic
Manduca allatostatin (Mas-AS) did not affect the rate of
JH production by Spodoptera glands but inhibited
Mas-AT-stimulated synthesis. In the tomato moth, Lacanobia oleracea, larval corpora allata can be stimulated and
inhibited, respectively, by synthetic AT and Mas-AS, in contrast to Manduca, where only Mas-AS is
effective (Audsley et al., this symposium). Furthermore, a Mas-AT-like peptide has been identified by ELISA in extracts of larval brains of this species. Genes containing sequences identical to those for Mas-AT and Mas-AS have been cloned in Pseudaletia unipunctata, the true armyworm moth (Truesdell et al., this symposium), and immunocytochemical analysis with antibodies to Mas-AT have revealed immunoreactivity in the brain, abdominal ganglia, and corpora allata of Pseudaletia adults. Finally, Mas-AT stimulates the corpora allata of honey bee larvae in a dose-dependent, reversible, and stage-specific manner, suggesting that this peptide could be a regulatory factor in Hymenoptera as well (Rachinsky et al., this symposium).
2.2. Non-neural allatotropins
If Mas-AT does not stimulate larval glands in
Mand-uca, what does? Perhaps, as seen in Spodoptera, the
activity of one peptide is only detected in the presence of the other. Thus in Manduca, Mas-AT may only stimulate allatostatin-inhibited glands. Alternatively, stimulation may occur as a result of the absence of the allatostatin or the presence of an AT-like factor from another tissue source. It has been suggested for Diploptera that the absence of allatostatins results in the stimulation of JH biosynthesis, and that certainly could suggest the pres-ence of an allatotopin in certain experiments in vivo. Alternatively, the source of a stimulatory factor could be other than neural tissues (see Stay, this symposium). The ovaries have been indicated as a source of allatotro-pin in Diploptera (see Stay, this symposium), in Grillus
bimaculatus (Hoffmann et al., 1996), and the male
accessory sex gland in Drosophila (Moshitsky et al., 1996). As reported at this conference, the sex peptide of the medfly Ceratitis capitata stimulates the corpora allata to produce a lipoidal molecule related to JH (Moshitsky et al., this symposium). In Diploptera, the ovarian factor appears to be proteinaceous and water sol-uble, with a molecular weight of less than 10 kDa (Unnithan et al., 1998). Ovary-preconditioned medium stimulates corpora allata activity in vitro, but only when the glands’ neural connections to the brain are severed, further complicating the picture of gland control in this species.
2.3. Future research on allatotropins
stimu-lation of JH production mirrors the trend observed for the allatostatins (see below) and also deserves further investigation. Is the allatotropic effect the primary func-tion of the molecule? Perhaps most importantly, the interactions between allatostatins and allatotropins in the control of the corpora allata are fertile, but unexplored, territory, and should lead to studies of their receptors and the downstream events controlled via the ligand-receptor interaction. It is these paths that typify the most recent work on allatostatins.
2.4. Allatostatins (AS)
In contrast to the slowly developing field of research on allatotropins, there has been an explosion of research on allatostatins, which inhibit JH synthesis, beginning with the seminal work of Stay and Tobe on the allatostat-ins of Diploptera punctata (Stay et al., 1991). At the time of the Sixth Conference on the Juvenile Hormones, 13 allatostatins had been identified in Diploptera, each with a similar C terminus: Y/F-X-F-G-L/I-NH2, with one
terminating in isoleucine. All of the Diploptera allatosta-tins appear to be produced in the same cell, and all inhibit JH synthesis in vitro but with different potencies, ranging in concentration from an ED50 of 102
11 M to
1027 M (Stay et al., 1996). Localization of allatostatin
immunoreactivity in the nervous system of Diploptera revealed numerous immunoreactive neurons in the brain and also in all other ganglia (Stay et al., 1994; Stay, this symposium). The arborization of the axons of cerebral allatostatin immunoreactive cells within the corpora car-diaca suggested that allatostatins could be released from this neurohemal organ, and allatostatin I was found in the hemolymph. Thus the corpora allata of Diploptera appear to be regulated by allatostatins delivered directly to the gland via the axons of the lateral neurosecretory cells of the brain or indirectly via the hemolymph.
A Manduca allatostatin (Mas-AS) was purified and sequenced from pharate adult heads in 1991 (Kramer et al., 1991). Its sequence bears no homology to the YXFGLamide family of allatostatins, but it inhibits JH biosynthesis reversibly in both adults and larvae, with an ED50value of 2–5 nM with corpora allata from day
0 fifth instars. An immunohistochemical demonstration of Mas-AS revealed two groups of lateral neurosecretory cells in brains of last instars, and arborization of the axons of one group (Ib) within the corpora allata (Zˇ itnˇan et al., 1995).
Another factor has been identified in Manduca which also inhibits the corpora allata, but which does so in a non-reversible fashion (Bhaskaran et al., 1990). This cer-ebral factor, termed an allatinhibin, provides stable inhi-bition when corpora allata are treated with the factor in vitro and then implanted into penultimate instar larvae (Unni et al., 1993). Its structure is unknown.
2.4.1. Allatostatins in other species
At the time of the last JH conference, 30 different peptides belonging to the allatostatin family had been identified in several different insect species. Since that time a considerable number have been added to the list (see Stay, this symposium), and members of the allatos-tatin family have been identified by immunoreactivity in Crustacea as well: crab, lobster, and crayfish (Skiebe, 1999). Allatostatins with the typical C terminus sequence and an inhibitory effect on JH synthesis have been ident-ified in two other cockroaches, Periplaneta americana (Weaver et al., 1994) and Blatella germanica (Belles et al., 1994) and two crickets, Gryllus bimaculatus and
Acheta domestica (Lorenz and Hoffmann, 1995). Three
structurally similar allatostatins for which a function(s) has not yet been determined, also have been isolated from the mosquito, Aedes aegypti (Veenstra et al., 1997). In addition, structurally identical peptides were isolated from the blowfly, Calliphora vomitoria, where they inhibited corpora allata activity (Duve and Thorpe, 1994; Duve et al., 1996) and from the honeybee, Apis
melli-fera, where they did not (Rachinsky and Feldlaufer,
2000). As reported at this conference, multiple allatos-tatic peptides have been isolated from the brains of the stick insect (Lorenz et al., this symposium) and are identical to those in Diploptera and Schistocerca. How-ever, while these peptides strongly inhibit JH synthesis in crickets, they have no effect on the corpora allata of the stick insect, suggesting a primary function other than the inhibition of JH biosynthesis.
2.5. Structural allatostatins with other activities
activity. However, many thoracic motor neurons were immunoreactive, indicating a myotropic or anti-myotropic function in the larva. Interestingly, immuno-reactivity disappeared during metamorphosis and did not reappear in the adult.
Ten peptides belonging to the YXFGLamide allatosta-tin family have been identified and sequenced in
Schisto-cerca (Schoofs et al., 1997). These allatostatins, termed
schistostatins, inhibit peristaltic movements of the ovi-ducts. In the earwig, where Dip-AS 7 immunoreactivity is found predominantly in the recurrent and esophageal nerves, the last abdominal ganglion and the proctodeal nerve, Dip-AS significantly and reversibly decreased hindgut motility but had no effect on JH biosynthesis (Rankin et al., 1998a). HPLC fractions of earwig brain extract were found to contain Dip AS-like material by radioimmunoassay using antibodies to the cockroach allatostatin, and these fractions also inhibited JH biosynthesis by cockroach corpora allata and earwig hindgut motility (Rankin et al., 1998b). The demon-strated presence of allatostatins in Diploptera hemocytes (Skinner et al., 1997) suggests that they may also play an unknown role(s) in hemolymph functions. In Blatella
germanica, allatostatins inhibit vitellogenin release by
the fat body, presumably by inhibiting the process of vitellogenin glycosylation (Martin et al., 1996).
The existence of Mas-AS in other insects has also been suggested by the work of Jansons et al. (1996) and by results presented at this meeting (W.G. Bendena, M. Cusson., P. Koladich, M.D. Price, I.S. Jansons, P. Truesdell, and S.S. Tobe, unpublished observations). Brain cDNAs have been found in Pseudalecia and
Dro-sophila which specify a neuropeptide that upon
cleav-age, yields a peptide that is similar to Mas-AS. Although numerous cells in the brains of these two species are immunoreactive to antibodies to Mas-AS, synthetic Mas-AS does not affect JH biosynthesis in vitro by Drosophila ring glands and has limited ability to inhibit Pseudalecia glands in vitro (Jansons et al., 1996). The Drosophila peptide has no affect on JH biosynthesis in vitro, when tested with ring glands from third instars and day 2 adults (W.G. Bendena, personal communication). Thus in both Drosophila and
Pseuda-lecia, this peptide probably has a functional role
differ-ent from that in Manduca. Nevertheless, there is evi-dence for a cerebral allatostatin in Drosophila, based on the work of Richard et al. (1990) and Altaratz et al. (1991). As previously noted, the larval corpora allata of Lacanobia can be inhibited effectively by synthetic Mas-AS, and Mas-AS-like immunoreactivity has been detected in the central nervous system, midgut and Mal-pighian tubules of this species (Audsley et al., 1998 Audsley et al., 1999, this symposium). Its presence in sites other than the central nervous system suggests that other roles may exist for this peptide.
2.6. Control of allatostatin titer
If allatostatins can exert their effects via the hemo-lymph, how are their hemolymph titers controlled? While research on the synthesis of allatostatins and its control has not yet begun, some recent research explores their degradation. It is now known that allatostatin con-centrations are affected by a susceptibility to degradation (Bendena et al., 1997). Incubation of As 7 or Dip-AS 9 with hemolymph for 30 min revealed two primary catabolic cleavage steps: cleavage by a putative endopeptidase, yielding a C terminal hexapeptide, and subsequent cleavage of this product by an amastatin-sensitive aminopeptidase, to yield the C-terminal penta-peptide. Nevertheless, these hemolymph enzymes do not inactivate allatostatins since the C-terminal pentapeptide core (the minimal sequence necessary for the inhibition of JH synthesis) remains (Garside et al., 1997a). On the other hand, membrane preparations of brain, gut and corpora allata cleave allatostatins at the C-terminus, also in a two-step process, completely inactivating the pep-tide (Garside et al., 1997b). On the basis of information provided by the prior two studies, Nachman et al. (1999) have successfully synthesized pseudopeptide mimetic analogs of Dip-AS resistant to degradation by hemo-lymph and tissue-bound peptidases, by increasing steric hindrance of the degradative enzymes. As reported at this meeting, these analogs show varying resistance to catabolism, and all inhibit JH biosynthesis in vitro (Nachman et al., 1999; Garside et al., this symposium). Injection of mated Diploptera females with some of the analogs inhibited JH biosynthesis significantly by sub-sequently explanted corpora allata, as well as basal oocyte growth in vivo. This approach should allow more critical studies of the physiological processes modulated by allatostatins, since allatostatic activity was retained in the analogs.
2.7. Allatostatin receptors
Recent work on allatostatins has focused on their receptors, since the kind and number of receptors present in the corpora allata or any other tissue with a specific response to an allatostatin would control the timing, dur-ation, and strength of the response. Early work by Cus-son et al. (1991a) demonstrated by photoaffinity labeling the presence of two different putative receptors in
Diploptera glands. A later study, which employed an in
vitro binding assay in addition to the photoaffinity assay, identified a 37 kDa receptor for both AS-5 and Dip-AS-7 in adult female brains (Yu et al., 1995). This recep-tor had a single Kd of 9 × 10210 M for Dip-AS-5, but
two for Dip-As-7 (1.5 ×1029 and 3.8 ×1029 M),
their potencies in vitro. In the corpora allata, a single Kd
was obtained for an AS-7 receptor (7.2 × 10210M). A
subsequent structure-activity study, using synthetic anal-ogs of Dip-ASB2 (Dip-AS-2), again revealed two recep-tor types in the corpora allata, based on the biphasic inhibitory response of the corpora allata to some of the ASB2 analogs (Pratt et al., 1997). These authors propose that the C-terminal portion of allatostatins contains, in addition to the “message” portion of the neuropeptide (that part responsible for a full effect), a significant amount of “address” information (binding affinity for the receptor). They conclude that a pentapeptide is likely to be the smallest fully active allatostatin structure. Diver-gent evolution of receptor types is proposed to occur together with the evolution of multiple allatostatins from a common ancestor (see below). The recognition of shorter allatostatic peptides by one receptor, and of larger peptides by the other, would allow for cross-talk between the two types of receptors, for the congruence of the “message” of all allatostatins, and for a divergence of function. Cloning and sequencing of allatostatin receptors is clearly the next step in sorting out the basis of the ubiquitous occurrence but varied functions of the multiple allatostatins.
2.8. Effect of allatostatins on the JH pathway
Where in the pathway of JH biosynthesis does the cockroach allatostatin act? Early work by Pratt et al. (1991) demonstrated that the addition of exogenous far-nesol or mevalonate reversed the allatostatic inhibition of JH biosynthesis in vitro, suggesting that the target of the allatostatin existed prior to mevalonate kinase activity. Recent work has shown that the allatostatin is a more effective inhibitor of JH biosynthesis in glands that utilize glucose or amino acids as their carbon source, rather than acetate (Sutherland and Feyereisen, 1996). These results demonstrate further that either the transport of citrate across the mitochondrial membrane (the citrate/malate shuttle) and/or the cleavage of citrate to yield cytoplasmic acetyl-CoA (ATP-citrate lyase) are the probably targets of the allatostatin, i.e., the first commit-ted steps in the synthesis of JH III.
2.9. Evolution of allatostatins
The molecular evolution of the allatostatin precursor in cockroaches has been analyzed recently using the gen-omic DNA sequences of the preproallatostatin precursor for four species of cockroach (Belles et al., 1990), as well as previously published sequences of two others (Donly et al., 1993; Ding et al., 1995). Phylogenetic analysis using parsimony revealed that the allatotropin sequences in these species were generated through a pro-cess of duplication of a single gene, similar to the situ-ation with the FMRFamides. This occurred before the
divergence of these species from each other during evol-ution and led to intragene families of peptides. A deter-mination of the physiological significance of such diver-sity is the next step.
2.10. Neurotransmitter effects on corpus allatum activity
If functional allatotropins/allatostatins cannot be identified in certain cases, such as the lack of Manduca allatotropin to stimulate larval glands, what else might regulate JH biosynthesis? Neurotransmitters are strong candidates, since the axons of non-neurosecretory neu-rons also innervate the corpora allata. There is evidence in both Manduca and Diploptera that neurotransmitters are regulatory factors, and this area of research deserves further attention. Octopamine, a primary insect neuro-transmitter, was shown a decade ago to stimulate JH biosynthesis in Locusta (Lafont-Cazal and Baehr, 1988) and later was found to have the same effect in honey bee adults (Kaatz et al., 1994) and larvae (Rachinsky, 1994). In Diploptera (Thompson et al., 1990) and the cricket Gryllus bimaculatus (Woodring and Hoffmann, 1994), JH production is inhibited by octopamine. Dopa-mine was subsequently found to affect JH synthesis in vitro by the corpora allata of adult female Blatella
germ-anica, and furthermore, its effect could be either
stimu-latory or inhibitory, depending on the stage of the female within the ovarian cycle (Pastor et al., 1991). At the pre-vious JH conference, dopamine was reported to have an effect on the corpora allata of Manduca sexta as well, and in a stage-specific manner during larval-pupal meta-morphosis (Granger et al., 1996). Early in the last larval stadium, dopamine stimulates both JH biosynthesis in vitro and cAMP production. After day 2, when a small ecdysteroid peak was observed prior to the commitment peak (Wolfgang and Riddiford, 1986), its effect on both JH and cAMP production was inhibitory. The source of dopamine affecting gland function has not yet been identified, but like the allatostatin, it has been found by immunocytochemistry in both the brain and corpora allata (Granger, unpublished information). When the corpora allata were screened for neurotransmitter content by electrochemical detection HPLC (Granger et al., 1996; T.C. Sparks, personal communication), the only biogenic amine detected was dopamine.
has not yet been elucidated, persistent levels of JH are known to delay pupation in Lepidoptera. Thus, it is poss-ible that the elevated levels of dopamine may be affect-ing gland function. In the armyworm, Mamestra
bras-sicae, significantly higher levels of dopamine have been
found in the hemolymph and central nervous system of diapause-destined pupae (Noguchi and Hayakawa, 1997), and it would be of interest to know the primary site of dopamine action in this system as well.
2.11. Neurotransmitter receptors in the corpus allatum
Dopamine exerts its effects at the cell membrane via G protein-coupled receptors associated with cAMP (Kababian, 1992). The stage-specific effects of dopam-ine on JH biosynthesis by Manduca corpora allata are mirrored by dopamine effects on cAMP production (Granger et al. 1995b, 1996). This argues for (1) a direct effect of dopamine, rather than indirect (via the release of endogenous allatotropins/allatostatins), and (2) the existence of D1-like and D2-like dopamine receptors, the members of these families of receptors stimulating and inhibiting cAMP production. The results of a pharmaco-logical characterization of the dopamine receptors of the
Manduca corpora allata were presented at this meeting
(Granger et al., this symposium). On the basis of those results, it appears that the D2-like receptor(s) bears con-siderable similarity to those of vertebrates, while the D1-like receptor(s) is unusual in its limited recognition of a wide range of D1 and D2 receptor agonists and antagon-ists active in vertebrates. In this respect, the receptor is strikingly similar to a D1-like receptor isolated and sequenced in Drosophila (Gotzes et al., 1994; Sugamori et al., 1995).
2.12. Second messenger systems
In Diploptera, the effect of octopamine on corpora allata activity is paralleled by a concomitant effect on cAMP levels (Thompson et al., 1990), while diacylgly-cerol and 1,4,5-inositol triphosphate appear to be involved in transduction of the allatostatin signal (Rachinsky et al., 1994). Mas-AT stimulates inositol phosphate (IP) production in both male and female adult
Manduca corpora allata (Reagan et al., 1992), and it has
been proposed that the allatotropin stimulates JH pro-duction via an increase in 1,4,5-inositol triphosphate (IP3), leading to an increase in intracellular calcium.
High levels of intracellular calcium correlate with biosynthetically active glands in Manduca (Allen et al., 1992). Dopamine, by contrast, functions via cyclic AMP as its second messenger (Granger et al., 1996). The mechanism by which Mas-AS inhibits corpora allata activity in Manduca remains conjectural.
2.13. Interrelationship between neuropeptides and neurotransmitters in the control of the corpus allatum
The possible interrelationship between a neuro-transmitter and a neuropeptide in the control of JH biosynthesis is not a new observation, since it was shown several years ago that both octopamine and Dip-AS down-regulate JH biosynthesis in Diploptera (Thompson et al., 1990; Stay et al., 1994). Mechanisms proposed for the inhibition of JH synthesis by octopamine in
Diplop-tera include roles as (1) a neuromodulator of JH
syn-thesis, regulating ion channels or releasing allatostatin from terminals within the corpora allata; and (2) a neuro-hormone directly affecting JH biosynthesis. Thus, the relationship of dopamine to Mas-AS in inhibiting the corpora allata of Manduca larvae and adults is a critical consideration in the regulation of gland activity in this species.
2.14. Other factors affecting JH biosynthesis
Calcium is another factor impacting on the ability of the corpora allata to synthesize JH, and considerable work has been done in the last decade demonstrating the central and critical role of this ion in both Diploptera and Manduca (Rachinsky et al., 1994). There is a need for further information about the role of calcium in the transduction of neuropeptide and neurotransmitter sig-nals to the corpora allata, as well as of other intracellular events that would modulate the response.
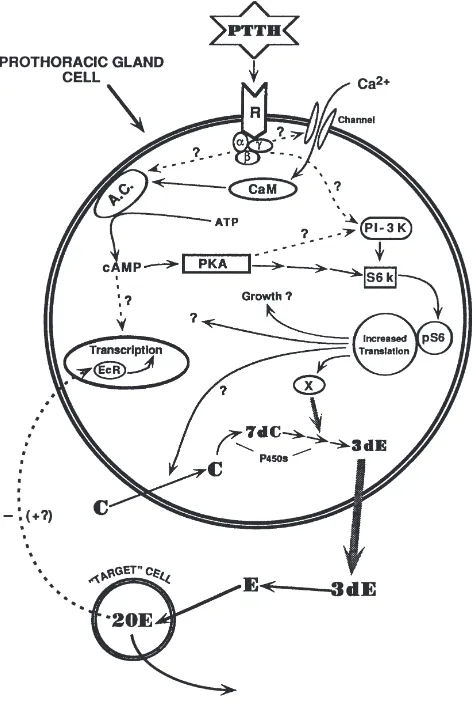
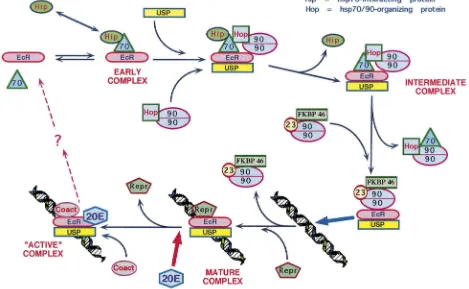
One way to modulate the response of the glands to regulatory ligands is by controlling the kind and/or num-bers of their receptors. Ecdysteroid receptors are known to exist in the nuclei of Manduca corpora allata (Bidmond et al., 1992), and there is preliminary evidence that ecdysteroids control the switch in the responsiveness of the corpora allata to dopamine in Manduca, thus gov-erning the occurrence and/or numbers of D1- and D2-like receptors (Granger et al., 1996). Thus, the ecdys-teroids are strong candidates to figure in the overall scheme of the control of corpora allata function, but there are surprisingly few clues as to the mechanism by which this class of insect hormone is involved. With this decade’s accumulation of information about ecdysteroid receptors as well as transcription factors (Henrich et al., 1999), the stage is set for the investigation of the poss-ible control of corpora allata activity by ecdysteroids at the genomic level.
allatum of Manduca sexta, both in vitro and in vivo (unpublished information). Preliminary data indicate that the USP proteins in the glands are represented by a group of isoforms that probably represent the phos-phorylation states of two primary translation products. In the last larval stadium, the diversity of these isoforms is greatest at wandering, while the highest concentration of USP in the corpora allata occurs several days later, during the premolt peak of circulating ecdysteroids. EcR proteins were also present in multiple isoforms, again probably as the result of multiple phosphorylation states of primary translation products. In contrast to USP, how-ever, the EcRs in the corpora allata show few differences in relative abundance or isoform diversity during the fifth larval stadium. Various other larval tissues were also assayed and were found to be considerably different from the corpora allata in their USP and EcR isoform profiles, and some of these tissues expressed several putative EcR isoforms that varied with developmental stage. These results suggest that USP in the corpora allata and other tissues may form functionally diverse, tissue- and stage-specific homo- and/or heterodimers of the ecdysteroid receptor.
In vitro analysis indicated that USP isoform diversity in the corpora allata is altered in response to changes in physiological ecdysteroid levels, while ecdysteroid-dependent changes in EcRs in the glands are limited to changes in abundance. Very preliminary studies indicate that JH may also influence USP isoform diversity in the corpora allata, although the effect may not be direct. An understanding of the diversity of EcR and USP isoforms in the corpora allata will provide the basis for further study of the complex interactions between the ecydys-teroidogenic and JH biosynthetic pathways, as well as of the possible regulation of putative JH receptors and their downstream targets in the glands. Specific phos-phorylation events and the EcR and USP isoform diver-sity they generate are thus undoubtedly at the core of stage-specific responses of tissues and organs to the ecdysteroid titer. This provides an explanation for how a relatively small number of translation products and a limited number of ligands can be utilized effectively in a large number of permutations.
Thus the answer to the question originally posed by Carroll Williams and discussed at the First International Conference on the Juvenile Hormones in Lake Geneva, Wisconsin — Is JH the handmaiden of ecdysone? — may finally be within reach.
3. JH regulation: binding protein and metabolism
3.1. Nature of JHs
From the structure of JH III, it is obvious that there are a number of challenges that insects face in using this
compound as a hormone, i.e. the necessity to transport a highly lipophyllic molecule in an aqueous environment from its site of biosynthesis, through the insect hemo-lymph, to its site of action in target cells. JH biosynthesis and transport must therefore occur in what can be con-sidered a hostile environment, containing numerous esterases both outside and inside of the cell, capable of removing the conjugated methyl-ester of JH, and mem-brane bound epoxide hydrolases to degrade its stable C10,11 epoxide. Either of these metabolic events can significantly affect the pharmacology of JH and no doubt its biological action, discussed in more detail sub-sequently. Although these considerations of synthesis, transport and degradation are vital to JH function, the presence or absence of JH coordinated with changes in ecdysterioid levels at precise developmental time points is essential for normal growth, metamorphosis and reproduction. Insects have developed a unique system for the regulation of JH concentration which involves dynamic changes in two biochemical processes — JH biosynthesis and JH degradation. When JH biosynthesis levels are high, JH degradation is low, and when JH biosynthesis levels are low, JH catabolism is high. JH binding proteins play a critical role in these processes but at least in respect to the hemolymph, remain in molar excess in comparison to JH (Goodman and Gilbert, 1978). The challenge of JH regulation is further compli-cated by the synthesis of multiple JH homologs and by multiple sites of biosynthesis in some insect species. The biological significance of these observations is conjec-tural.
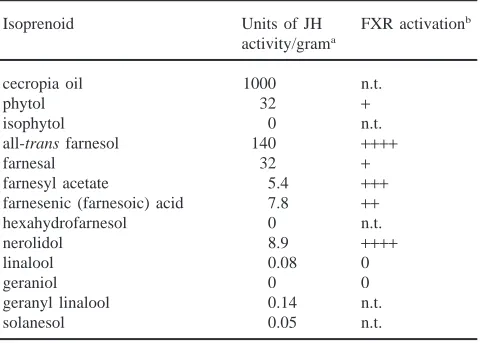
JH has been identified in approximately 100 insect species representing at least ten insect orders, with JH III being the predominant homolog (Baker, 1990). It would be useful in the future, and in terms of under-standing the role of JH in arthropod development in gen-eral, to examine what JH homologs if any are found in the most primitive insect orders, e.g. the Protura and Diplura. The higher homologs isoJH 0, JH I and JH II are found in the more advanced insect orders, i.e. the Lepidoptera, where in some cases multiple homologs are found in the same insect. In the cyclorrhaphous dip-terans, a new JH species, the bis-epoxide of JH III, was identified in Drosphila melanogaster, Calliphora
vom-itoria, Phormia regina and Lucilia cuprina (Richard et
allata of Manduca sexta beginning early in the last stad-ium (Janzen et al., 1991), and the JH acid is the only product at the time of pupal commitment (Bhaskaran et al., 1986; Janzen et al., 1991). JH acid acts as a pro-hormone and is converted to JH by imaginal discs (Sparagana et al., 1985) and may have morphogenetic roles of its own (see subsequent discussion). The de novo synthesis of JH apparently is not limited to the corpora allata since Borosvky et al. (1994) demonstrated the synthesis of JH III from acetate by the ovaries of the mosquito, Aedes aegypti.
We must exercise caution and not limit our view as to what constitutes a “JH”. For example, as discussed elsewhere in this review, the Bhaskaran lab has demon-strated that JH acid is a hormone. In addition, Granger et al. (1995a) discovered a polar JH acid ester synthe-sized and released from the corpora allata of Manduca, but its function is unknown. The Roe laboratory reported at this symposium that both hard and soft ticks have no detectable levels of the common insect JHs as measured by the Galleria wax bioassay, by SIM GC-MS and by radiobiosynthesis with a lower limit of detection in the low fg range. Yet ticks, like insects, have multiple life stages and instars and undergo metamorphosis and reproduction, all of which now appears to be controlled by a hormone other than the common insect JHs. The predominant JH-like molecule in the Crustacea is methyl farnesoate (see Laufer, this symposium).
It is clear from the few studies that have been conduc-ted, that the different JH homologs have different levels of biological activity. For example, JH I and JH II are more potent morphogenetically than JH III in a number of different biological assays using Manduca (Truman et al., 1973; Nijhout and Riddiford, 1974; Fain and Rid-diford, 1975; Bhaskaran et al., 1986) but this may be due to the greater polarity of JH III, which makes it more prone to degradation (Gilbert and Schneiderman, 1960; Gilbert and Schneiderman, 1961a,b). In addition, the overall peak levels of JH can vary greatly between spec-ies. For example, low concentrations of JH in the range of 10 ng per ml hemolymph elicit the status quo effect in most insects with the exception of the social Hymen-optera (Rachinsky et al., 1990; Huang et al., 1994) and the cockroach, D. punctata (Tobe et al., 1985). The func-tion of the multiplicity of JH products synthesized by the corpora allata (sometimes simultaneously) and other tissues and the significance of peak levels in different species are not fully appreciated. It could be argued that some of these products, i.e. JH III in Lepidoptera, methyl farnesoate in cockroaches or JH III in the higher flies, are evolutionary biochemical remnants. However, the small amount of work in this area suggests otherwise (Yin et al., 1995 and references therein). More research is needed to provide an understanding of both the signifi-cance of JH blends and the differences in JH levels
between species and between developmental stages in some species.
Most JH identification and JH titering in the past has involved either hemolymph in the case of large insects, or the whole organism with small insects, e.g.
Droso-phila. It is apparent now that this approach will not be
adequate in the future. The function of JH cannot be defined by simply studying JH levels in hemolymph or in a whole insect homogenate. It is critical to our under-standing of the mode of action of JH to study JH at the level of individual tissues. In the past, this perspective has essentially been ignored, and at this juncture, surpris-ingly, we know almost nothing about the quantitative nature of JHs in individual tissues, with the exception of hemolymph. Careful quantitative analysis of JH in specific tissues during development using at least a few insect models is requisite for a real understanding of JH function. We should also confirm data obtained from in vitro assay systems by developing in vivo assays to study JH biosynthesis, transport, degradation and func-tion. It is necessary to prove that data from in vitro stud-ies are not artifacts of the incubation conditions.
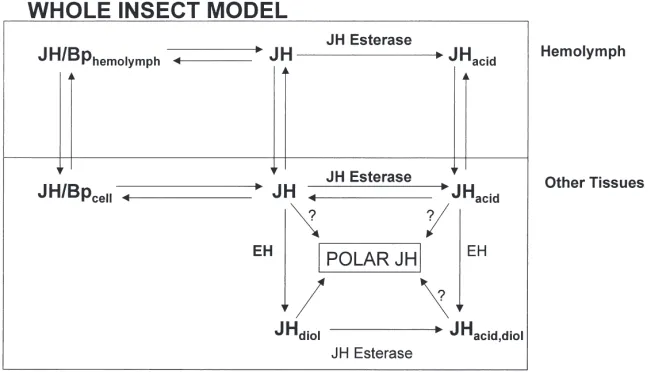
3.2. JH esterase
Fig. 1. A model for JH metabolism. Bp, binding protein. (From M. Roe, unpublished information).
known, other primary pathways for the degradation of JH cannot be ruled out.
Most of the research on JH metabolism has focused on hemolymph esterases and the interaction of JH ester-ase with hemolymph JH binding proteins. The hypoth-esis by Sanburg et al. (1975) noted that there were two classes of esterases involved in the metabolism of JH, JH specific esterase and “general” (a-naphthyl acetate) esterase. As will be discussed below, there is no question that insects have a JH-specific esterase, and that this esterase is involved in reducing the JH titer. However, with all of the research conducted on the metabolism of JH by JH esterases, the significance of non-specific esteratic action is still not clear. It is assumed that most of the JH in insects is bound to JH binding proteins and is in that way protected from the non-specific esterases with low binding affinities (Touhara and Prestwich, 1993; Touhara et al. 1993, 1995). This conclusion is based in large measure on research with hemolymph, rather than with other tissues where the interaction of JH with binding proteins, JH receptors, metabolic enzymes and JH synthetic processes must be considered. One definitive study with Manduca has even shown that JH levels fell prior to the increase in JH esterase activity (Baker et al., 1987). The general conclusion reached based on the research utilizing hemolymph is that since insects have a JH-specific esterase and high affinity, hemolymph binding proteins, JH esterase is the only esterase important in JH degradation. Whether this con-clusion is warranted and applicable to all tissues and insects in general is not known.
Evidence for the substrate specificity of JH esterase and its functional role in the reduction of the JH titer is clear. Potent and highly selective transition state analog inhibitors of hemolymph JH esterase have been synthe-sized (Hammock et al. 1982, 1984; Linderman et al., 1987; Roe et al., 1997). Detailed structure/activity
stud-ies and molecular modeling of the interaction of these trifluoromethylketones with JH esterase from the hemo-lymph of Trichoplusia ni have shown that the active site of JH esterase is hydrophobic, with a molecular volume almost identical to that of JH (Hammock et al., 1984). There is also a hydrophobic site near the active site of the enzyme which apparently accommodates the C-3 methyl branch of JH III (Linderman et al., 1987). Mim-icry of the JH backbone is a critical factor in inhibition of the esterase (Hammock et al., 1984; Roe et al., 1990). Interestingly, the trifluoromethylketones mimicking the alpha,beta unsaturation of JH were poorer inhibitors than the sulfur containing OTFP (1-octylthio-1,1,1-trifluoro-propan-2-one) or acetylenic trifluromethylketones (Linderman et al., 1989). This was explained when 19
sulfone) demonstrated persistent, essentially complete selectivity for JH esterase versus a-naphthyl acetate esterases in last instars of the cabbage looper (Roe et al., 1997). The application of OTFP sulfone to last instars and virgin female adults of T. ni delayed meta-morphosis and initiated egg production, respectively (Roe et al., 1997), demonstrating the importance of JH esterase in the regulation of JH titer. Hammock et al. (1990) also showed that recombinant baculovirus expressing insect JH esterase could reduce JH levels in the Lepidoptera. These anti-JH effects of JH esterase provided a model system to demonstrate the usefulness of the baculovirus expression system as a novel insecti-cide. Although these studies show clearly that JH ester-ase has a significant role in the regulation of JH titer, it does not mean that the metabolism of JH by esterase results in a loss of biological activity, e.g. JH acid can act as a hormone or pro-hormone (see subsequent discussion). There may also be other functions for JH esterase in insects. For example, the highly selective JH esterase inhibitor, OTFP, was shown by Anspaugh et al. (1995) and Devorshak and Roe (unpublished observation) to be an excellent inhibitor of insecticide resistance-associated esterases from the Colorado potato beetle, Leptinotarsa decemlineata, and the tufted apple budmoth, Platnota idaeusalis, respectively. In the tufted apple budmoth, structure/activity studies with trifluor-omethylketones showed that the optimum inhibitor of resistance-associated esterases was OTFP, as was also the case in similar studies conducted with T. ni (Hammock et al., 1984). We must take care not to assume that the only function of JH esterase is JH metab-olism.
Numerous studies have measured JH esterase activity both in hemolymph as well as other tissues during insect development (Vince and Gilbert, 1977; reviewed by Hammock, 1985; Roe and Venkatesh, 1990). The corre-lation of JH esterase activity with developmental changes has been used extensively in the literature to argue for a role of JH esterase in the regulation of embryogenesis (Roe et al., 1987; Share et al., 1988), lar-val development and metamorphosis (reviewed by Ham-mock, 1985; Hanzlik and HamHam-mock, 1988; Roe and Venkatesh, 1990; Jesudason et al., 1990), reproduction (Lessman and Herman, 1984; Venkatesh et al., 1987), diapause (Kramer and de Kort, 1976; Mane and Chip-pendale, 1981; Bean et al., 1983), and other processes. Jesudason et al. (1990) showed that both the peak levels of JH esterase activity as well as the timing of peak activity were regulated precisely in each stadium of
Manduca (see also Vince and Gilbert, 1977). The peak
levels of JH esterase activity were positively correlated with insect size from the first through the last instar. Delineating the mechanism by which JH esterase activity is regulated relative to the animal’s size and for an indi-vidual tissue, should be of general interest to
biochem-ists. Lassiter et al. (1996) found that a mechanism was in place for the tissue specific, differential expression of JH esterase in Culex quenquefaciatus adults during reproduction. This has been observed for other insect species during larval development (see Roe and Venka-tesh, 1990).
Our understanding of the mechanism(s) regulating JH esterase activity is meager considering that this is one of the critical unanswered questions in the field of JH metabolism. At least two parameters are presumed to be important in the regulation of JH esterase activity–JH esterase biosynthesis and degradation. It appears that one of the main sources of hemolymph JH esterase activity is the fat body (Wing et al., 1981). Several studies have shown that JH esterase activity can be increased by the addition of JH and JH mimics at the time of pupal com-mitment through pupation in last stadium Lepidoptera (Jones et al., 1981; Jones and Hammock, 1983; Venka-tesh and Roe 1988, 1989). However, prior to commit-ment, JH esterase activity is somewhat, but not absol-utely, refractory to JH. Venkatesh and Roe (1988, 1989) found that an inhibitory factor found only in the insect head reduced JH esterase activity but not a-naphthyl acetate esterase activity in the hemolymph and other tissues of T. ni and Manduca, respectively (see also Vince and Gilbert, 1977). Inhibition was dose responsive and occurred even when insects were treated with a juvenoid. Jones et al. (1981) characterized a JH esterase induction factor in T. ni in experiments using isolated abdomens. Venkatesh and Roe (1989) found that it occurred only in the hemolymph of ligated abdomens and not in unligated insects. Both hemolymph JH ester-ase anda-naphthyl acetate esterase were increased, and the increase in JH esterase activity occurred only in the hemolymph and not in a whole abdomen homogenate. While there is currently insufficient information on the regulation of JH esterase activity to develop a reasonable hypothesis, a recent step toward such an hypothesis was taken by Jones et al. (1998, personal communication) who showed that the JH esterase gene from the cabbage looper has a composite promoter consisting of an AT-rich motif (TATA box), an initiator motif for tion and at least one additional binding site for transcrip-tional factors.
appears to accumulate in the pericardial cells (Booth et al., 1992; Ichinose et al., 1992), suggesting that this may be the primary site of inactivation, but whether this is a mechanism common to other insect species is conjec-tural. Bonning et al. (1997) discovered that the injected radiolabelled JH esterase enters the pericardial cells by receptor-mediated endocytosis, in association with a putative heat shock cognate protein (HSC) and then undergoes lysosomal degradation. We have much more to learn about this process. How specific is the mech-anism for JH esterase inactivation? Is this process found in other insects or used for the regulation of other pro-teins (enzymes)? Is JH esterase degradation itself regu-lated and by what means? With the availability of excel-lent biochemical and molecular probes for studying JH esterase, e.g. specific antibodies, recombinant enzyme, affinity ligands, etc., JH esterase would be a good model system to study the functional inactivation of a protein in insects. Considering that we know so little about protein degradation in insects, this would be a worthwhile goal for the future.
3.3. JH epoxide hydrolase
The general assumption has been that JH epoxide hydrolase has a secondary role in the metabolism of JH acid (Roe and Venkatesh, 1990). However, measure-ments of JH III epoxide hydrolase activity in vitro from last stadium T. ni (Kallapur et al., 1996) revealed levels equivalent to that of JH III esterase activity. When 10R,11S-JH II was injected at physiological levels, as high as 40% of the known primary metabolites of JH metabolism was JH diol. Kallapur et al. (1996) also found that larval T. ni epoxide hydrolase preferred JH II to JH II acid at physiological cencentrations. Hal-arnkar et al. (1993) found that the major in vivo metab-olite of JH I in last stadium Manduca was the phosphate conjugate of JH diol, suggesting that JH esterase may be unimportant in the metabolism of this homolog. The Roe laboratory also demonstrated at this symposium that most of the metabolism of JH in vivo in last stadium
T. ni results in a water soluble metabolite, although the
structure is not yet known. Chromatography, enzyme derivatization and FAB-MS analysis showed that this product of JH metabolism was not the phosphate-diol found in Manduca. Lassiter et al. (1995) showed that JH III epoxide hydrolase was the predominant route of in vitro metabolism throughout the last stadium of the southern house mosquito, Culex quinquefasciatus, with two peaks in whole animal JH epoxide hydrolase activity occurring near the time of commitment and pupation.
Three lepidopteran epoxide hydrolases have now been cloned and sequenced: GenBank Accession Number U46682 from Manduca eggs (Wojtasek and Prestwich, 1996); U73680 from fat body of T. ni, and AF03542 from the gut of T. ni (Harris et al., 1999). These epoxide
hyrolases demonstrated very high homology and con-tained the exact catalytic triad (Asp, Glu, His) as that of mammalian microsomal epoxide hydrolases. Although there is evidence from differential centrifugation studies that insects have a soluble epoxide hydrolase (Roe and Venkatesh, 1990), this has not been confirmed by sequence analyses. However, based on Southern blot analysis, it is likely that additional epoxide hydrolases are present in T. ni that have not yet been sequenced.
The mechanism of JH epoxide hydration involves a nucleophilic attack by the active site Asp-residue of the least substituted oxirane carbon of JH which produces an enzyme-substrate adduct/ester intermediate (Linderman et al., 1995; Roe et al., 1996).18O labeling studies using
microsomal epoxide hydrolase from T. ni in a multiturn-over reaction, showed clearly the exclusive addition of the label to the C10 position of JH. The enzyme-sub-strate adduct is broken by the hydrolysis of water which restores the Asp-carboxylate and produces JH diol. In this scheme, the oxygen that is added to the C10 position comes from aspartate and not from water, unlike the situ-ation for JH esterase. Until recently, there have been no effective inhibitors of insect epoxide hydrolase. Com-pounds designed to mimic a polarized and ionic tran-sition state were poor epoxide hydrolase inhibitors, while aliphatic epoxides like 10,11-epoxy-11-methyldode-canoate (MEMD, Roe et al., 1996) demonstrated an I50
of 80µM. In these studies, mimicry of the JH backbone appeared to be an important factor in JH epoxide hydro-lase inhibition, as was the case for JH esterase. The Lind-erman and Roe laboratories described (this symposium) S-MEMD with an I50of 12 nM. This is the most potent
insect JH epoxide hydrolase inhibitor to date. Interest-ingly, this compound contains the unnatural enantiom-eric configuration of JH. One explanation may be that the S-enantiomer results in a more stable ester intermedi-ate as compared to the R configuration, resulting from a conformational change in the epoxide hydrolase. It may be necessary in the future to modify the methyl-ester of S-MEMD to reduce its metabolism by esterases in vivo. The in vivo inhibition of JH epoxide hydrolase is critical to understanding the significance of these enzymes in the regulation of JH titer and insect development.
al. (1990) showed that changes in JH epoxide hydrolase activity were positively correlated with changes in JH esterase activity during the last stadium of Manduca. By contrast, Lassiter et al. (1995) demonstrated two peaks of JH epoxide hydrolase activity during the last stadium of C. quinquefasciatus, but no changes in JH esterase activity during the same period. These data argue that at least one endogenous function of this enzyme is JH degradation. Anspaugh et al. (this symposium) reported that baculovirus expressing recombinant fat body epox-ide hydrolase from T. ni was more virulent in last stad-ium cabbage loopers than the wild-type virus. The use of epoxide hydrolase recombinant baculoviruses as well as stable genomic transformations in Drosphila and novel EH inhibitors, will be useful in the future to increase our understanding of the role(s) of this enzyme in JH regulation.
3.3.1. JH binding protein (JHBP)
JH binding proteins were first described by Whitmore and Gilbert (1972) and most recently reviewed by de Kort and Granger (1996). Three types of hemolymph binding proteins have been described in insects. The first fully characterized JHBP was from Manduca and has a low molecular weight of approximately 30, 000 (Kramer et al., 1976; see also Goodman and Gilbert, 1978). This binding protein has subsequently been found in several other lepidopterans and consists of a single polypeptide and a single JH binding site per protein molecule. The lepidopteran JHBP is a minor hemolymph component comprising less that one percent of total hemolymph pro-tein. A second, high molecular weight binding protein, lipophorin, was found in the hemolymph of L.
decemli-neata, Periplaneta americana and Locusta migratoria
(de Kort and Koopmanschap 1986, 1987, 1989). Lipo-phorin is the predominant hemolymph protein and the main hemolymph carrier of lipids in the insect. It con-tains about 40–50% lipid and is composed of two apop-roteins–apolipophorin I and II at 250 and 80 kDa, respectively. Because the hemolymph lipophorin con-centration is high, a high concon-centration of JH binding sites are available. The third class of JH binding protein is a 566 kDa hexameric protein first described from L.
migratoria by Koopmanschap and de Kort (1988). The
native protein contains 15% lipid, has 6 JH binding sites, and is composed of 77 kDa subunits. Although there are three categories of JH binding proteins, they can be gen-erally characterized as having high affinity for JH, but little affinity for JH degradation products like JH acid or JH diol, and high enantioselectivity for the natural occurring JHs.
The presence of a hemolymph JHBP offers several advantages to insects in respect to the use of JH as a hormone. Because of the lipophilicity of JH, JHBP pro-vides an effective mechanism for downloading JH into the aqueous hemolymph and most likely reduces the
problem of JH sequestration in lipophillic membranes. Similarly, JHBP reduces non-specific sequestration into liphophillic compartments or lipid-aqueous interfaces and facilitates the uniform circulation of JH to all of the insect tissues. These proteins may also be storage sites for JH. Binding protein may be an important factor in downloading JH from the corpora allata to the hemo-lymph, and from the hemolymph to target tissues. The reverse may be important for the tissue clearance of JH and JH degradation in the hemolymph. At physiological concentrations of JH, only a fraction of the high affinity JH binding sites in the hemolymph are occupied by JH (Goodman and Gilbert, 1978).
Most of the research to date on JH transport and degradation has focused on JH binding protein and JH esterase in a single tissue, hemolymph (Fig. 1). For example, we know little about intracellular JHBPs (see subsequent discussion of JH receptors) and the role of binding proteins in general in the transport of JH from hemolymph to the cellular JH receptor. The same is true for JH metabolism. Although extensive studies have been conducted on hemolymph JH esterase, the signifi-cance of cellular JH esterases, JH epoxide hydrolases and polar JH metabolites remains unresolved. This is a considerable concern since JH analogs that were once thought to be JH metabolites are now found to act as JH prohormones or hormones. Certainly the view that JH esterase is the most important factor in JH inactivation needs to be re-examined. We can no longer assume that JH acid, which has a low affinity for JH binding protein, is devoid of biological activity or cannot be recycled back to JH. We also must be careful in extrapolating data from studies on hemolymph to other insect tissues. A case in point is the critical work of the Prestwich lab-oratory (Touhara and Prestwich, 1993; Touhara et al., 1995) showing on strictly kinetic grounds that hemo-lymph binding protein in Manduca effectively protected JH from JH epoxide hydrolase (and probably non-spe-cific esterases) but not JH esterase. This suggests that JH acid is the primary metabolic route for JH in
Mand-uca, and that epoxide hydrolase is important in the
whole organism (Figs. 1 and 2). It may also be useful to reexamine the organ culture experiments of Hammock et al. (1975) and the in vivo approaches, e.g. Slade and Zibitt (1972) and others (see reviews by Hammock, 1985; Roe and Venkatesh, 1990). Since these older approaches more closely approximate the organism as a whole, they have less possibility of leading us in direc-tions and to conclusions that may have little to do with the insect’s physiology.
3.4. Comparative regulation by JH and JH-like compounds (other arthropods)
The consensus view has been that the insect model for the regulation of metamorphosis and reproduction applies to the Arthropoda in general. This view has been heightened by work during the last ten years on the Crus-tacea. Apparently, crustaceans have the same biosyn-thetic pathway as insects for the synthesis of farnesyl pyrophosphate and methyl farnesoate (MF) [the pre-sumed JH of the Crustacea (Laufer, this symposium)], but unlike insects, do not make the C10,11 epoxide of juvenile hormone (Smith and Sedlmeier, 1990; Charm-antier et al., 1991; Cusson et al., 1991a; Chang et al., 1993; Laufer et al., 1993; Chang, 1993; Homola and Chang, 1997). Crustacean methyl farnesoate is synthe-sized by the mandibular organ (Fig. 2) with the rate of synthesis controlled by the peptidic mandibular organ inhibitory hormone from the X-organ/sinus gland (XO/SG) complex. Ecdysteroids are also present in the Crustacea and are synthesized by the Y-organ and con-trolled by the peptidergic molt inhibitory hormone. Although the details are still under investigation, and there is some variability between species, MF promotes the production of ecdysone and reduces the duration of the intermolt period. Evidence was presented in these
Fig. 2. Hormones involved in the regulation of arthropod development. (From M. Roe, unpublished information.)
proceedings by the Laufer laboratory that MF also can act as a “status quo” hormone, as JH does in insects. MF in adult Crustacea is believed to initiate the synthesis of the yolk protein precursor in the hepatopancreas or other tissues, just as JH controls vitellogenesis in many adult female insects.
The general assumption has been that the chelicerates, and more specifically ticks, regulate their metamorphosis and reproduction in a manner similar to insects. How-ever, in comparison to our knowledge of insect endocrin-ology, and even that of Crustacea, there is a dearth of data on tick endocrinology (Fig. 2). The retrocerebral organ complex (RCO) and associated brain neurosecre-tory cells of the tick synganglion have been hypothesized to be the counterpart of the corpus cardiacum-corpus allatum complex of insects and the XO/SG complex of the Crustacea (reviewed by Sonenshine, 1991). How-ever, the function of the RCO is unknown and no neuro-endocrine products have been traced to this complex.
of JHs, JH mimics and anti-JHs, and the results from these experiments have been equivocal. It was reported at this symposium that no JH could be detected in eggs, larvae, nymphs and adults of the hard tick, Dermacentor
variabilis, by the Galleria wax test bioassay; no JH
could be detected in the hemolymph of adult D.
varia-bilis and the adult soft tick, Ornithodoros parkeri, by
GC/MS; and no JH, MF or farnesol was synthesized by the synganglion or any other tissues of nymphs and adults of these two species (Roe et al., 2000; Neese et al., 2000). These results are not the final word on the subject of JH in ticks but do question whether the com-mon insect JHs have a role in tick development and per-haps in chelicerates in general. Certainly, the processes of metamorphosis and/or reproduction are regulated by a hormone, but the structure of this hormone and its origin remain conjectural.
4. Future research and speculation
4.1. Introduction
Concurrent with our expanding knowledge of the gen-eral field of endocrinology has come the revelation that what was accepted as a central dogma is no longer true. In the case of steroid hormones, we now know that some can act at the level of the cell membrane in addition to acting as classic nuclear hormones at the level of the genome (Christ et al., 1999). We predict that the same will be true of JH because of the patency studies of Davey (this symposium) and the accumulated evidence indicating that JH can act at the level of the genome. Indeed, it is known that membrane-oriented target sys-tems can influence gene regulation since kinases traverse the cytoplasm and enter the nucleus.
It may be of interest to this readership and perhaps recall memories to Professor Applebaum, who organized JH VII, that Applebaum and Gilbert (1972) authored a paper showing that 20-hydroxyecdysone stimulates adenylate cyclase in lepidopteran pupal wing epidermis. This study together with those of Leenders et al. (1970), who showed that Drosophila salivary glands previously exposed to 20-hydroxyecdysone (20E) contained increased quantities of endogenous cAMP, were cer-tainly among the first studies to indicate the multivalent action of steroid hormones. We note these early studies for two reasons. First, it is a way to acknowledge Shalom Applebaum both as a scientist and friend and for hosting this wonderful meeting in Jerusalem, and second, to emphasize an important fact that is now known to occur commonly in biological systems. The papers mentioned above on the stimulation of adenylate cyclase by 20E have not been cited more than a dozen times in the last twenty-five years, probably because mammalian endoc-rinologists have only recently read papers on insect
endocrinology and, of course, because it was only disco-vered relatively recently that progesterone can act at the level of the cell membrane of the amphibian oocyte.
What can we learn about this experience as far as JH research is concerned? First, discoveries that seem so important and earth shattering to insect researchers are usually not applauded by individuals working on higher organisms. They forget the ground-breaking work of Ulrich Clever, Keilin, etc. and, in most cases, do not read the insect literature. It is therefore important that this community of scholars working on JH acknowledge the excellent research conducted by their peers and work toward having the best of the projects funded by extra-mural agencies. Secondly, we must continue to publish some of our most excellent papers in journals dedicated to insect research, rather than publishing our best work in JBC, PNAS, etc. It is important to demonstrate that insects and their molecules are wonderful tools for eluci-dating basic developmental biological questions and that insect hormones still have promise as insect growth regulators.
There are many problems to solve in the future, some of which have been raised in the preceding portion of this paper, but two emerge as quite basic: the JH receptor and the question of elucidating the nature and, perhaps, the function of JH “metabolites”.
4.2. JH metabolites
It has been known for several decades that JH is degraded to JH acid and then perhaps to JH acid diol (see previous discussion). Recent studies from the Bhaskaran laboratory (Ismail et al., 1998) indicate that the major yolk protein in Manduca, as well as its mRNA, are detectable in the prepupal stage, and its production is enhanced by methoprene. The ability of the cells to respond to methoprene is acquired after the ecdysteroid-initiated commitment to metamorphosis. They have shown further that the acquisition of competence requires in addition to ecdysteroid, prior exposure to JH II acid, as shown by the injection of JH II acid and 20E into isolated abdomens. Methoprene acid also induces competence to respond to methoprene. Their analyses confirmed that vitellogenic transcripts are present in fat body only if the isolated larval abdomens were pre-treated with both ecdysteroid, JH acid or methoprene acid. They conclude that JH acid is not simply a metab-olite of JH, but is a hormone in its own right. This is of real interest since L.G. has proposed for several years that ecdysone metabolites may have morphogenetic roles of their own and may not be simple degradation pro-ducts, as is now proposed for the JH acid.