Cyclitols as cryoprotectants for spinach and chickpea
thylakoids
Birgit Orthen *, Marianne Popp
1Institut fu¨r O8kologie der Pflanzen,Westfa¨lische Wilhelms-Uni6ersita¨t Hindenburgplatz55,48143Mu¨nster,Germany
Received 17 March 2000; received in revised form 25 May 2000; accepted 26 May 2000
Abstract
Thylakoid membranes isolated from either spinach or chickpea leaves were used as a model system for evaluating the capacity of cyclitols to act as cryoprotectants. The effect of freezing for 3 h at−18°C on cyclic photophospho-rylation and electron transport was measured. The cyclitols, ononitol,O-methyl-muco-inositol, pinitol, quebrachitol and quercitol at 50 – 150 mol m−3decreased membrane damage by freezing and thawing to a similar degree as the well known cryoprotectants sucrose and trehalose. On addition of the cryotoxic solute NaCl (100 mol m−3) to the test system these methylated cyclohexanhexols again provided a protection comparable to that of the two disaccha-rides. Quercitol (cyclohexanpentol) was not effective when added in lower concentrations (50 – 100 mol m−3) and in case of this cyclitol a ratio of membrane toxic to membrane compatible solute of 0.66 was apparently needed to prevent a loss of cyclic photophosphorylation. Little difference was observed in the results from spinach or chickpea thylakoids although these plants naturally accumulate different cyto-solutes (spinach: glycinebetaine; chickpea: pinitol). © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Cicer arietinum; Ononitol;O-Methyl-muco-inositol; Pinitol; Quebrachitol; Quercitol;Spinacea oleracea
www.elsevier.com/locate/envexpbot
1. Introduction
It is generally accepted that biomembranes are the main targets of freezing stress, therefore many studies focus either on thylakoid or plasma mem-branes as in vitro model systems. Factors con-tributing to damage of membranes are numerous,
but most of them can be traced back to the reduction of water availability due to ice crystal formation (e.g. Steponkus and Webb, 1992).
During cold acclimation cellular metabolism and membrane structures undergo considerable modifications including alterations in enzyme ac-tivities and metabolic pathways, as well as in-creases in concentrations of sugars, amino acids, alditols, nucleic acids, proteins and phospholipids (Sakai and Larcher, 1987; Guy, 1990). A great number of studies has demonstrated that soluble organic molecules, e.g. sugars (Santarius, 1973), alditols (Santarius and Giersch, 1983), amino
* Corresponding author . Tel.: +49-251-8323832; fax: +
49-251-8321705.
E-mail address:[email protected] (B. Orthen). 1Present address: Institut fu¨r O8kologie und Naturschutz, Universita¨t Wien, Althanstr. 14, 1091 Wien, Austria.
acids (Heber et al., 1971) and glycinebetaine (Coughlan and Heber, 1982) could act as cryopro-tectants for thylakoid membranes.
Another group of low molecular weight organic compounds, whose cryoprotective capacity has yet to be investigated are cyclitols, cyclic polyols with or without methyl groups attached to the oxygen of the hydroxyl groups (Drew, 1984; Loewus, 1990; Loewus and Murthy, 2000).
A number of these compounds (pinitol, quebra-chitol, quercitol, O-methyl-muco-inositol) accu-mulate at low temperatures (Diamantoglou, 1974; Ericsson, 1979; Popp et al., 1997). In mistletoe (Viscum album) cyclitols accounted for more than 25% of the dry matter in winter (Richter, 1989). In a number of tree species onset of the cold season induced enhanced storage of cyclitols in the living bark tissue and buds (Popp and Smirnoff, 1995; Popp et al., 1997). Even in the mediterranean species Mesembryanthemum crys -tallinumexposure to 4°C for 78 h strongly induces the transcription of the myo-inositol-O-methyl transferase (Vernon et al., 1993) a key enzyme on the biochemical pathway to ononitol and pinitol. The accumulation of cyclitols due to cold stress together with the knowledge that a range of low molecular weight compounds act as cryoprotec-tants led us to the hypothesis that cyclitols func-tion as cryoprotective solutes. To check this hypothesis, we tested the cryoprotective capacity of cyclitols on spinach and chickpea thylakoid preparations.
2. Materials and methods
2.1. Thylakoid preparation and freezing experiments
The method essentially as described by Heber and Santarius (1964) was used. Chloroplasts were isolated from non-hardy spinach (Spinacea oleracea L. cv. Matador) and chickpea (Cicer arietinum L. cv. Phule G5) leaves. Seeds of C. arietinum were soaked 12 h in tap water whereas S. oleracea seeds were directly potted in 1 l of a mixture of garden soil (DIN 11 54 0-F80) and sand 2:1. Cultivation of plants was carried out in
a glasshouse: 13 h of irradiance with 360 mmol m−2s−1at 23°C and and 11 h darkness at 13°C.
Relative humidity was held at 60% during the day and at 95% during the night. Fully expanded leaves from different plants were harvested at the onset of the light period and combined to one sample. After removal of the midrib or the rachis, 20 g of the washed leaf lamina were blended in the cold in 200 ml medium containing 300 mol m−3 sorbitol, 40 mol m−3 MES, 1 mol m−3
cysteine and 1.25 mol m−3
sodium ascorbate (pH 6.1). For homoge-nization of chickpea leaves the buffer volume was increased to 500 ml and 0.5% (w/v) BSA was added. The homogenates were filtered through nylon cloths (20 mm mesh) and centrifuged for 5 min at 4650×g. The chloroplasts were ruptured in 5 mol m−3 MgCl
2 and thylakoids were
sedi-mented at 18 600×g in 5 min. The thylakoids were rewashed and then resuspended in 10 mol m−3 HEPES, 10 mol m−3 MgCl
2. Chlorophyll
content was determined according to Arnon (1949).
For freezing experiments thylakoid suspensions were diluted by adding equal volumes of solutions of cyclitols (OMMI, ononitol, pinitol, quercitol, quebrachitol), sugars (trehalose, sucrose) or glycinebetaine with or without 100 mol m−3
NaCl. The samples were frozen at −18/ −20°C. After 3 h the samples were thawed rapidly in a water bath at 30°C and were stored in the dark at
+4°C until they were measured. Control treat-ments of the preparation with the tested solutes were kept at +4°C in a refrigerator without the addition of NaCl. After the freezing – thawing cy-cle measurements for each solute were performed in the order without NaCl, control, with NaCl. Replicate measurements (]3) of thylakoid activ-ity were done from independent tylakoid prepara-tions. Sample means, with estimate of the S.D., were calculated accoding to Rees (1985).
2.2. Determination of thylakoid acti6ity
Cyclic photophosphorylation (CPP).
in a reaction medium containing 300 mol m−3
sorbitol, 1 mol m−3 HEPES, 2 mol m−3
KH2PO4, 35 mol m
−3 KCl, 1 mol m−3 ADP,
0.065 mol m−3 phenazine methosulfate (pH 7.8)
at 20°C. The light-dependent alkalization was
recorded by a pH electrode according to
Nishimura et al. (1962). Hydroxyl ions were quantified by titration with 10 mol m−3 HCl.
Linear electron transport was measured as O2
uptake mediated by methyl viologen. The reaction medium and conditions were the same as de-scribed for cyclic photophosphorylation except that phenazine methosulfate was replaced by 0.1 mol m−3
methyl viologen and an oxygen elec-trode (Bachhofer, Hansatech, Germany) was used. Evolution of oxygen by catalase reaction was prevented by adding 0.1 mol m−3 KCN.
2.3. Isolation of cyclitols
Dried and ground material from leaves, roots or fruits was extracted with hot water. The hot water extract was treated with yeast for 2 days in order to remove reducing sugars (Richter et al., 1990). The solution was deionized by ion-ex-change (Dowex 1×4, HCOO−-form, 50 – 100
mesh/Dowex 50 W×4, H+-form, 50 – 100 mesh).
Cyclitols were separated by using an anion-ex-change column (Dowex 1×4, OH−-form, 50 –
100 mesh) with distilled water as eluent. Final purification of single cyclitols was achieved on a cellulose column (elution with 85% v/v acetone). OMMI (D-1-O-methyl-muco-inositol) was iso-lated from roots ofRhizophora mangleL., ononi-tol (4-O-methyl-myo-inositol) from leaves of Hymenea 6errucosa L., pinitol (L-3-O -methyl-chiro-inositol) from fruits ofCeratonia siliqua L., quebrachitol (L-2-O-methyl-chiro-inositol) from
leaves and fruits of Acer sp. and quercitol (L
-1,2,3,4/2,5 cyclohexane-pentol) from leaves and fruits of Quercus roburL.
3. Results
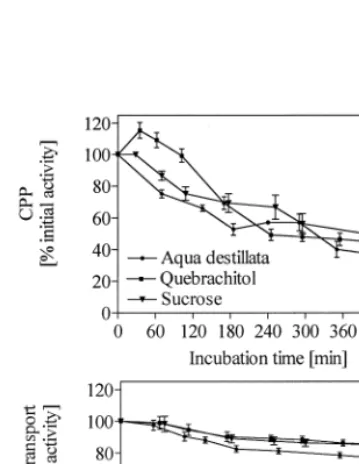
As reported by Heber et al. (1971) the capacity for cyclic photophosphorylation and uncoupled electron transport of thylakoids prepared either from spinach or chickpea leaves was reduced by storing them in the dark at 4°C for the period necessary to conduct the freezing experiments (Fig. 1). The composition of the medium also influenced the rate of aging so the values obtained after the freeze – thaw cycle were routinely com-pared with controls kept in the corresponding solute at 4°C in the dark without addition of NaCl. While freezing retarded the aging of the membrane activities, aging proceeded in thawed samples as in unfrozen controls. Photophosphory-lation (Fig. 1A) was much more strongly affected during the 8 h experimental period than electron transport (Fig. 1B).
No cyclic photophosphorylation was observed in frozen thawed thylakoid membranes of spinach regardless of whether NaCl was added or not.
Addition of 50 mol m−3 cyclitols to the
Fig. 2. Capacity for cyclic photophosphorylation of spinach thylakoid preparations after a freeze thaw cycle (3 h,−18°C) in the presence of various organic solutes at concentration of 50, 100 and 150 mol m−3; A without NaCl, B with addition of 100 mol m−3NaCl. The values were calculated as percentage of the activity of control samples that were incubated at 4°C in the same solution without addition of NaCl and were mea-sured at the same time. Columns represent the mean of at least three measurements, bars indicate S.D.
effect at the lower concentration range was pro-portionally greater than at the higher range (150 mol m−3).
The well known cryoprotectants sucrose and trehalose showed a similar pattern of cryopreser-vation (Fig. 2A). Addition of sucrose and tre-halose prior to freezing in the concentration range from 50 to 150 mol m−3 protected the
mem-branes to a similar extent as most of the cyclitols. Addition of 50 mol m−3sucrose led to a retention
of 93% of the activity of the aged control, while increasing the concentration up to 150 mol m−3
resulted in 158% of control activity. Trehalose was more efficient in preventing membrane dam-age especially at a concentration of 150 mol m−3
(235%).
Addition of 100 mol m−3NaCl to the samples
prior to the freeze – thaw cycle had a profound effect. In a concentration from 50 to 100 mol m−3
quercitol failed to prevent total loss of cyclic photophosphorylation activity during freeze – thawing when NaCl was present. However, all other solutes tested in this study diminished the cryotoxic effect of NaCl. The addition of 50 mol m−3
organic solute leading to a molar ratio of cryotoxic to cryoprotective agent of 2 but a osmo-lar ratio nearer 4, slightly reduced the cyclic pho-tophosphorylation compared with the values obtained from thylakoids suspended just in cry-oprotective solutes. While at higher organic solute concentrations the effect of NaCl was abolished and some of the aging effects also multiplied.
In contrast to cyclic photophosphorylation un-coupled electron transport activity was only re-duced to 30% of the controls by the freeze – thaw cycle even in the absence of any cryoprotectant, however, addition of 50 mol m−3 of the various
cyclitols prior to freezing was insufficient to retain the control levels (Fig. 3A). Concentrations of 100 mol m−3
abolished the freezing – thawing effects and higher concentrations protected the mem-branes even better.
Addition of 100 mol m−3NaCl (Fig. 3B) to the
test media had less effect on electron transport than on cyclic photophosphorylation. Even at a molar ratio of 2 (cryotoxic to cryoprotective so-lutes) slight reduction in membrane protection was noted only in two cases (pinitol and sucrose) suspension prior to freezing protected the
thy-lakoids against the loss of cyclic photophosphory-lation (Fig. 2A). The extent to which the cyclitols protected the membranes against the impact of freezing and thawing differed. Addition of 50 mol m−3 quebrachitol and ononitol led to a retention
of 45% respectively 57% of the unfrozen controls (4°C), while OMMI, pinitol and quercitol addi-tions resulted in a protection of up to 80% of the cyclic photophosphorylation. Increasing cyclitol concentration up to 150 mol m−3led to a greater
compared to the treatment without NaCl. All other solutes protected the thylakoids to an equiv-alent extent as the treatments without NaCl. Changing the ratio in favour of the cryoprotec-tant up to 0.66 resulted in greater electron trans-port in case of most solutes.
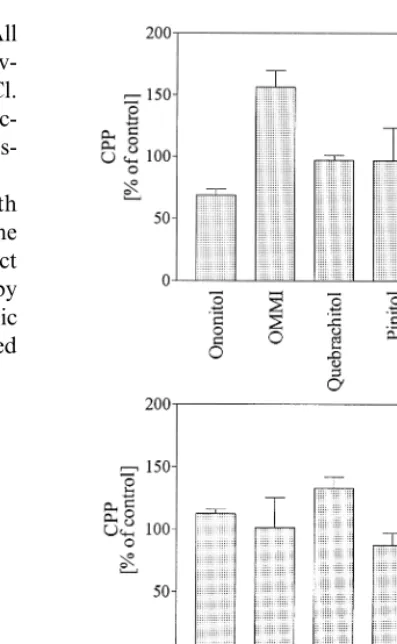
A set of parallel freeze – thaw experiments with chickpea thylakoids (Figs. 4 and 5) supported the results from spinach showing that cyclitols protect isolated membranes against damage caused by freezing and thawing and NaCl. Again, cyclic photophosphorylation was completely inhibited
Fig. 4. Capacity for cyclic photophosphorylation of chickpea thylakoid preparations after a freeze thaw cycle (3 h,−18°C) in the presence of various organic solutes at concentration of 100 mol m−3; A without NaCl, B with addition of 100 mol m−3 NaCl. The values were calculated as percentage of the activity of control samples that were incubated at 4°C in the same solution without addition of NaCl and were measured at the same time. Columns represent the mean of at least three measurements, bars indicate S.D.
Fig. 3. Capacity for uncoupled electron transport of spinach thylakoid preparations after a freeze thaw cycle (3 h,−18°C) in the presence of various organic solutes at concentration of 50, 100 and 150 mol m−3; A without NaCl, B with addition of 100 mol m−3NaCl. The values were calculated as percentage of the activity of control samples that were incubated at 4°C in the same solution without addition of NaCl and were mea-sured at the same time. Columns represent the mean of at least three measurements, bars indicate S.D.
when thylakoids were subjected to −18°C sus-pended in HEPES – MgCl2 with or without
addi-tion of 100 mol m−3NaCl. In the presence of 100
mol m−3cyclitol cyclic photophosphorylation
ac-tivity (Fig. 4A) varied from 70 to 160% of the controls (4°C). The addition of 100 mol m−3
the presence of an organic solute was much less marked although there were some differences in detail with the various cyclitols (Fig. 4B).
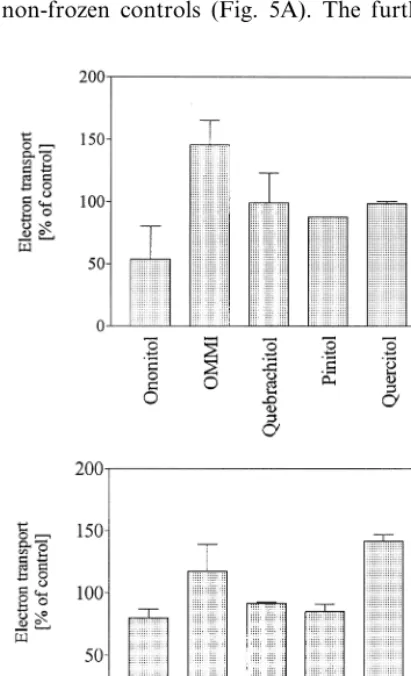
Electron transport in chickpea thylakoids is again much less affected by freeze – thawing than cyclic photophosphorylation with a reduction of only 34% being observed. Suspending thylakoids in media containing 100 mol m−3 of various
cyclitols resulted in retention up to 150% of the non-frozen controls (Fig. 5A). The further
addi-tion of 100 mol m−3 NaCl previous to freezing
(Fig. 5B) did not have such a marked effect as found for cyclic photophosphorylation. For most of the solutes no significant difference in electron transport between the treatment with and without NaCl could be observed.
4. Discussion
As pointed out in Section 3, quantitative analy-sis of the data is limited due to aging of thylakoid preparation. After 3 h the CPP activity of con-trols with organic solutes declined about 50%. This decline in activity was effected by the type of organic solute and by time and was prevented by freezing in an appropriate cryoprotectant. Thus single comparisons were difficult, however, our aim in this study was to compare the cryoprotec-tive effect of purified cyclitols with the well estab-lished cryoprotectants, sucrose and trehalose in a simple in vitro system.
The observed inactivation of cyclic photophos-phorylation and reduction of electron transport in response to a freeze – thaw cycle without any cry-oprotectant is caused by mechanical stress. This type of cryo-injury could be due either to direct damage to the thylakoids of ice crystals (Santarius and Giersch, 1983) or to marked volume changes during freezing and thawing (Hincha et al., 1984). Both effects could result in mechanical rupture of the membrane vesicles. Another reported conse-quence is the loss of the electron transport protein, plastocyanin, from the membrane (Hin-cha and Schmitt, 1988, 1992). The presence of cryoprotectants may diminish the mechanical stress by decreasing the osmotic potential thus reducing the freeze induced shrinkage since less water is removed from the system for ice forma-tion. All sugars tested so far for their ability to prevent freeze thaw damage act by reducing the freezing-associated solute influx into the mem-brane vesicles leading to reduced osmotic swelling during thawing and thereby minimizing danger of membrane rupture (Hincha, 1986; Bakaltcheva et al., 1992, 1994).
Although the five cyclitols tested in this study varied somewhat in their cryoprotective efficiency
it was evident that they had a similar capacity to sucrose and trehalose for preventing the mechani-cal damage (Fig. 2A, Fig. 3A, Fig. 4A). Another important perturbation during freeze – thaw cycles was the chemical damage due to an increase of cryotoxic solute concentration caused by crystal-lization of water. Chemical stress leads to irre-versible changes in conformation as well as in composition of the membranes (Schmitt et al., 1985). Cryoprotective additives may prevent these alterations through two different modes of action. The colligative theory first described for erythro-cytes (Lovelock, 1953) assumed a quite simple mode of action: irrespective of the chemical na-ture of the cryoprotectants, but depending on the molar fractions of cryoprotective and cryotoxic substances the concentration of the latter in the remaining solution during ice formation is bal-anced non-specifically by the presence of the cryoprotectants.
Several observations can not be explained by this hypothesis, for instance the effect of low concentrations of high molecular weight compo-nents like proteins (Volger and Heber, 1975) and dextrans (Santarius, 1982). Even low molecular weight osmolytes (sugars, alditols) applied in equimolar concentration may differ so much in their degree of cryoprotection that an additional, non-colligative mode of action has to be assumed (Santarius and Giersch, 1983). Only such a sub-stance-specific mechanism of membrane protec-tion can explain the differences in the extent of cryoprotective effect between the methylated cy-clohexanhexols (OMMI, ononitol, pinitol and quebrachitol) and the non-methylated cyclohexan-pentol (quercitol) in the NaCl treatment of our experiments (Fig. 3B, Fig. 4B). It is feasible from the different chemical structure of these two cycli-tol groups that they have a different capacity for interaction with the membrane components.
In the case of compatible solutes it has been postulated that only those solutes originally stored in a plant exhibit a beneficial effect (Mane-tas et al., 1986; Gibson et al., 1997). We therefore compared glycinebetaine-storing Spinacea oler -acea (Storey and Wyn Jones, 1977) and pinitol-storing Cicer arietinum (Ford, 1984). How-ever there was no marked difference in the
cryoprotective efficiency of the different type of solutes in the two thylakoid preparations.
It seems likely that cyclitol concentrations simi-lar to those used in our experiments are present in vivo in the vicinity of thylakoids, as for example demonstrated for M. crystallinum chloroplasts containing pinitol up to 230 mol m−3 (Paul and
Cockburn, 1989).
Acknowledgements
The financial support for this study by the DFG (Po323/1-2) is gratefully acknowledged. Thanks are due to Christiane Terjung (Mu¨nster) for methodical help. We wish to thank Judith Krepke (Mu¨nster) for the excellent preparation of ononitol.
References
Arnon, D.I., 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase inBeta6ulgaris. Plant Physiol. 24, 1 – 5. Bakaltcheva, I., Schmitt, J.M., Hincha, D.K., 1992. Time and temperature-dependent solute loading of isolated thy-lakoids during freezing. Cryobiology 29, 607 – 615. Bakaltcheva, I., Williams, W.P., Schmitt, J.M., Hincha, D.K.,
1994. The solute permeability of thylakoid membranes is reduced by low concentrations of trehalose as a co-solute. Biochim. Biophys. Acta 1189, 38 – 44.
Coughlan, S.J., Heber, U., 1982. The role of glycinebetaine in protection of spinach thylakoids against freezing stress. Planta 156, 62 – 69.
Diamantoglou, S., 1974. U8ber das Verhalten von Cycliten in vegetativen Teilen ho¨herer Pflanzen. Biochem. Physiol. Pflanz. 166, 511 – 523.
Drew, E.A., 1984. Physiology and metabolism of cyclitols. In: Lewis, D.H. (Ed.), Storage of Carbohydrates in Vascular Plants. Cambridge University Press, Cambridge, pp. 132 – 155.
Ericsson, A., 1979. Effects of fertilization and irrigation on the seasonal changes of carbohydrate reserves in different age-classes of needle on 20 year-old scots pine trees (Pinus sil6estris). Plant Physiol. 45, 270 – 280.
Ford, C.W., 1984. Accumulation of low molecular weight solutes in water stressed tropical legumes. Phytochemistry 23, 1007 – 1015.
Gibson, Y., Bressieres, A., Larher, F., 1997. Is glycine betaine a non-compatible solute in higher plants that do not accumulate it? Plant Cell Environ. 20, 329 – 340.
Heber, U., Santarius, K.A., 1964. Loss of adenosine triphos-phate synthesis caused by freezing and its relationship to frost hardiness problems. Plant Physiol. 39, 712 – 719. Heber, U., Tyankova, L., Santarius, K.A., 1971. Stabilisation
of biological membranes during freezing in the presence of amino acids. Biochim. Biophys. Acta 241, 578 – 592. Hincha, D.K., Schmitt, J.M., 1988. Mechanical freeze – thaw
damage and frost hardening in leaves and isolated thy-lakoids from spinach. I. Mechanical freeze – thaw damage in an artificial stroma medium. Plant Cell Environ. 11, 41 – 46.
Hincha, D.K., Schmitt, J.M., 1992. Freeze – thaw injury and cryoprotection of thylakoid membranes. In: Somero, G.N., Osmond, C.B., Bolis, C.L. (Eds.), Water and Life: Com-parative Analysis of Water Relationship at the Organismic, Cellular and Molecular Levels. Springer, Berlin, pp. 316 – 337.
Hincha, D.K., Schmidt, J.E., Heber, U., Schmitt, J.M., 1984. Colligative and non-colligative freezing damage to thy-lakoid membranes. Biochim. Biophys. Acta 769, 8 – 14. Hincha, D.K., 1986. Sucrose influx and mechanical damage by
osmotic stress to thylakoid membranes during an in vitro freeze – thaw cycle. Biochim. Biophys. Acta 861, 152 – 158. Loewus, F.A., Murthy, P.P.N., 2000.myo-Inositol metabolism
in plants. Plant Sci. 150, 1 – 19.
Loewus, F.A., 1990. Inositol biosynthesis. In: Morre´, D.J., Boss, W.F., Loewus, F.A. (Eds.), Inositol Metabolism in Plants. Wiley-Liss, New York, pp. 193 – 216.
Lovelock, J.E., 1953. The protective action of glycerol against haemolysis of erythrocytes by freezing and thawing. Biochim. Biophys. Acta 11, 28 – 36.
Manetas, Y., Peropoulou, Y., Karabourniotis, G., 1986. Com-patible solutes and their effect on phosphoenolpyruvate carboxylase of C4-halophytes. Plant Cell Environ. 9, 145 – 151.
Nishimura, M., Itoh, T., Chance, B., 1962. Studies on bacte-rial photophosphorylation. III. A sensitive and rapid method for determination of phosphorylation. Biochim. Biophys. Acta 59, 177 – 182.
Paul, M.J., Cockburn, W., 1989. Pinitol, a compatible solute in Mesembryanthemum crystallinum L.? J. Exp. Bot. 40, 1093 – 1098.
Popp, M., Smirnoff, N., 1995. Polyol accumulation and metabolism during water deficit. In: Smirnoff, N. (Ed.), Environment and Plant Metabolism: Flexibility and
Acclimation. Environmental Plant Biology Series. BIOS, Oxford, pp. 199 – 215.
Popp, M., Lied, W., Bierbaum, U., Gross, M., Große-Schulte, T., Hams, S., Oldenettel, J., Schu¨ler, S., Wiese, J., 1997. Cyclitols-stable osmotica in trees. In: Rennenberg, H., Escherich, W., Ziegler, H. (Eds.), Trees-Contribution to Modern Tree Physiology. Backhuys, Leiden, pp. 257 – 270. Rees, D.G., 1985. Essential Statistics. Chapman and Hall,
London.
Richter, A., Thonke, B., Popp, M., 1990. 1D-1-O -methyl-muco-inositol inViscum albumand members of the Rhi-zophoraceae. Phytochemistry 29, 1785 – 1786.
Richter, A., 1989. Osmotisch wirksame Inhaltsstoffe in ein-heimischen Mistelarten und ihren Wirten. University of Vienna Ph.D. Thesis.
Sakai, A., Larcher, W., 1987. Frost Survival of Plants. Ecolog-ical Studies, vol. 62. Springer, Berlin.
Santarius, K.A., Giersch, C., 1983. Cryopreservation of spinach chloroplast membranes by low-molecular-weight carbohydrates. II. Discrimination between colligative and noncolligative protection. Cryobiology 20, 90 – 99. Santarius, K.A., 1973. The protective effect of sugars on
chloroplast membranes during temperature and water stress and its relationship to frost, desiccation, and heat resistance. Planta 113, 105 – 114.
Santarius, K.A., 1982. Cryoprotection of spinach chloroplast membranes by dextrans. Cryobiology 19, 200 – 210. Schmitt, J.M., Schramm, M.J., Pfanz, H., Coughlan, S.,
Heber, U., 1985. Damage to chloroplast membranes dur-ing dehydration and freezdur-ing. Cryobiology 22, 93 – 104. Steponkus, P.L., Webb, M.S., 1992. Freeze-induced
dehydra-tion and membrane destabilisadehydra-tion in plants. In: Somero, G.N., Osmond, C.B., Bolis, C.L. (Eds.), Water and Life: Comparative Analysis of Water Relationship at the Organ-ismic, Cellular and Molecular Levels. Springer, Berlin, pp. 338 – 362.
Storey, R., Wyn Jones, R.G., 1977. Quaternary ammonium compounds in plants in relation to salt resistance. Phyto-chemistry 16, 447 – 453.
Vernon, D.M., Ostrem, J.A., Bohnert, H.J., 1993. Stress per-ception and response in a facultative halophyte: the regula-tion of salinity-induced genes in Mesembryanthemum crystallinum. Plant Cell Environ. 16, 437 – 444.
Volger, H.G., Heber, U., 1975. Cryoprotective leaf proteins. Biochim. Biophys. Acta 412, 335 – 349.