Proceeding
I nternational Seminar on Sustainable Biomass Production and Utilization Challenges and Oppurtunities (I SOMASS) August, 3-4, 2009
Main Menu
Main Menu ... i
Preface... ii
Seminar Organization ... iii
Editor Organization ... iv
Content Paper’s...v
Collecting Paper’s ... 1-454
Preface
Environmental issues and uncertainly in the future of fossil based energy sources have stimulated global interest in the development of alternative and renewable fuels. In this context, biomass has been identified as a promising resource because of it is abundantly available and convertible into different forms of bio-energy which suits various needs. In addition, bio-energy is acknowledged as environmental friendly because it produces less CO2. There is also a high potential benefits from C-sink for carbon trade in line with Kyoto protocol. However, during the past few years, several controversies have complicated bio-energy development. It has been implicated for exacerbating climate change rather than mitigating and also been cited as a major factor in rising food prices. Such different views reflect that in addition to technical issues, socio-economic issues should be taken into account in pursuing of bio-energy development.
Recognizing the important roles of biomass, The University of Lampung placed biomass development as one of the priorities. In line with this commitment, currently The University of Lampung has established The Biomass Laboratory, under the management of Research Institution. This particular laboratory is devoted to biomass development in a board sense related to biomass utilization, including development of alternative energies. Further development is projected to up-grade this laboratory into A Centre of Excellent on Biomass Research in the next few years. As a part of development plan toward establishment of the centre, Lampung University and Yokohama National University are planning to collaboratively host international seminar on biomass utilization and management, in order to gain more insight on biomass utilization impacts on Green House Gas Emission (GHGE).
The seminar is planned to be carried out to pursue the following three main goals (1) to improve knowledge and understanding of Indonesian scientists (participants) on the ongoing development of science and technology in the field of biomass, (2) to enhance the capacity of the participants in carrying out research on various aspects of biomass, and (3) to enhance understanding on the impacts of biomass utilizations and related issues of C-sink issues for carbon trade in line with Kyoto Protocol and Green House Effects due to CO2 emission.
In this opportunity, on behalf of the Organizing Committee and The University of Lampung, I would like to thank all the participants for their presentation. I would like to extent our sincere thanks and highest appreciation to Yokohama National University for invaluable supports, including financial support for the seminar. Our appreciation also extent to the Government of the Province of Lampung, PT Gunung Madu Plantation, BNI 46, Directorate General of Higher Education Department of National Education, and many others for their supports.
Proceeding
I nternational Seminar on Sustainable Biomass Production and Utilization Challenges and Oppurtunities (I SOMASS) August, 3-4, 2009
Seminar Organization
Organizing Committee:
• Chairman : Dr. Eng. Admi Syarif
• Vice Chairman : Ir. Zainal Abidin, M.Sc. • Secretary : Wasinton Simanjuntak, Ph.D. • Vice Secretary : Dr. Suripto Dwi Yuwono • Treasurer : Dr. Irwan Ginting Suka, M.Sc.
Scientific committee:
• Prof. Dr. Ir. Muhajir Utomo (Indonesia) • Prof. Dr. Koichi Fujie (Japan)
• Prof. Dr. Nobuhiro Kaneko (Japan) • Prof. Dr. Ryohei Kada (Japan) • Prof. Dr. Michel Ritz (USA)*
• Prof. Dr. Ir. Jamalam Lumban Raja (Indonesia) • Prof. Dr. Bustanul Arifin (Indonesia)
• Dr. John Hendri (Indonesia) • Dr. Udin Hasanuddin (Indonesia)
Seminar Committee:
• Dr. F.X. Susilo (Coordinator) • Dr. Ainin Niswati
• Dr. Bartoven Vivit Nurdin
Secretariat Committee:
• Yasir Wijaya, S.Si (Coordinator) • Frensi Riastuti S.Sos
• Adiguna Setiawan
PROCEEDING
International Seminar
ON SUSTAINABLE BIOMASS PRODUCTION AND UTILIZATION:
CHALLENGES AND OPPORTUNITIES
Editor:
Prof. Muhajir Utomo (Indonesia)
Prof. Jamalam Lumban Raja (Indonesia)
Prof. Nobuhiro Kaneko (Japan)
Prof. Ryohei Kada (Japan)
Prof. Koichi Fujie (Japan)
Prof. John Hendri (Indonesia)
Dr. Udin Hasanuddin (Indonesia)
Dr. Irwan Ginting Suka, M.Sc (Indonesia)
Wasinton Simanjuntak, Ph.D (Indonesia)
Dr. Darwin Pangaribuan (Indonesia)
Dr. Suripto Dwi Yuwono (Indonesia)
Ir. Zainal Abidin, M.Sc (Indonesia)
Dr. Eng. Admi Syarif (Indonesia)
Dr. F.X. Susilo (Indonesia)
Organizing Editor: Yasir Wijaya, S.Si
Proceeding of International Seminar : August 2009 / Editor,
John Hendri … [et al.].—Bandar Lampung : Lembaga Penelitian
Universitas Lampung, 2009. viii +437 pg. ; 21 x 29,7 cm
ISBN 978-979-18755-7-8
Published by :
LEMBAGA PENELITIAN UNIVERSITAS LAMPUNG
Jl. Prof. Dr. Sumantri Brojonegoro no. 1 Gedungmeneng Bandarlampung 35145
Proceeding
I nternational Seminar on Sustainable Biomass Production and Utilization Challenges and Oppurtunities (I SOMASS) August, 3-4, 2009
Content:
NO Name
Title
Page
1 Muhajir Utomo
Sustainable Production of Tropical Biomass: Challenges and
Opportunities 1-20
2
Koichi FUJIE1) Naohiro GOTO2) Hirotsugu KAMAHARA2) Udin HASANUDIN3)
Materials and Energy Flow Analyses in Bio-Product Processing of
Plantation 21-27
3 E. Gumbira-Sa’id
Network Development of Research, Development and Application of National Innovation System of Science and Technology With Special Case on The Utilization of Oil Palm Biomass For Food, Feed, Fuel and
Furniture Production 29-39
4 Ryohei KADA Emerging Ecological Risks and Food Security Issues In Asia 40-48
5 Hiroyuki Matsuda Ecological Risk Management In Asian Viewpoints 49-56
6 Jamalam Lumbanraja
Collaborative Program Among International Higher Education: Callenges and Opportunity of Student Mobility and Institutional
Capacity Building 57-70
7 Syamsir Dewang1 , Samsu Arif2
Spectral Reflectance Characteristics of Forest Vegetation Using 7etm
Landsat Satellite Data 71-79
8
Ainin Niswati, Dermiyati, Mas Achmad Syamsul Arif and Sri Yusnaini
Succession of Soil Fauna During The Composting Process of Oil Palm
Empty Fruit Bunch 80-86
9 Arya Wulung, Bambang Puguh , Slameto
The Effects of Ethanol-Gasoline Blends on Performance of Modified
Four Stroke Si Engine 87-94
10 Bambang Sudarmanta
Dual Fuel Engine Performance Using Biodiesel and Syn-Gas From
Rice Husk Downdraft Gasification For Power Generation 95-104
11 Bainah Sari Dewi
The Important Role of Asiatic Black Bear (Ursus Thibetanus) In The
Seed Dispersal Process 105-114
12
Christine Wulandari1, FX Susilo2, Pitojo Budiono3 and Sri Murwani4
Status And Viability of Policy For Supporting Below Ground
Biodiversity and Forest Biomass Sustainability In Indonesia 115-125
13 Donald Irving and Darwin H. Pangaribuan
The Effect of Cultivar and Storage Temperature on Postharvest
Characteristics of Tomato Fruits 126-134
14
Dermiyati, Ainin Niswati, Sri Yusnaini and M.A.Syamsul Arif1
Soil Fauna Succesion, Microbial Respiration and Microbial Biomass During The Composting Process of Bandar Lampung City Municipal
Wastes 135-142
15
Erwanto, Muhtarudin, and Mucharomah Prayuwidayati
Chemical and Biological Treatments of Corn Cob To Improve The Nutritional Content And In Vitro Digestibility As A Feed For
Ruminant 143-149
16 Farida Fathul
Nutrient Content of Rice (Oryza Sativa L.) Herbage As Feed For
Ruminants 150-158
17 Fitriani1 and Suripto Dwi Yuwono 2
The Development Renewable Sugarcane Biomass Energy In Lampung
Province To Support The National Energy Sufficiency 159-169
18 Ita Fitriyana, Indriyati, F.X. Susilo
Bba (Bagasse, Blotong, Ash) Affect The Diversity and Abundance Of
Soil Arthropods In Sugarcane Plantation In Lampung, Indonesia 170-177
19 Ganjar Oki Widhanarto
Biomass and Carbon Sequestration Potential of Plantation Forest In West Kalimantan ( Case Study In Plantation Forest Pt. Finnantara
Intiga) 178-186
20
Hendro Risdianto, Kevin Jonathan C., Yoke Christine V.M., Tjandra Setiadi
Evaluation of Crude Laccase Enzyme Performance At Pulp Bleaching
Pre-Treatment Process 187-196
21
Komariah1, Masateru SENGE2, Kengo ITO2,
John Tawiah ADOMAKO2
Soil Physical Properties Affected By Combinations of Soil Solarization
and Organic Amendment 197-209
22 Kumala Dewi
Prospect of Canna (Canna Edulis Ker.) Rhizome as A Feedstock For
Bioethanol Production 210-221
23
Maria Transiska Mahvie Syahroh1, Sri Yusnaini2, Ainin Niswati2, Dermiyati2
The Effect of Vermicompost Extract on The Growth and Nutrition
Uptake of Caisim (Brassica Camprestris L-Spp.) 222-233
Proceeding
I nternational Seminar on Sustainable Biomass Production and Utilization Challenges and Oppurtunities (I SOMASS) August, 3-4, 2009
24 Muhtarudin, Liman, Ali Husni
Effect of Amino Acid Restricted Supplementation In Rations Based on Sugarcane Forage Ammoniated on Production of Rumen Vollatil
Fatty Acid, Rumen Ammonia, and Nutrient Digestibility on Cattle 234-237
25
Munandar1), R.A.Suwignyo1), Akihiro Nose3), Sarno2), and Sabaruddin1
Model Cdm of Mangrove Forest: an Estimation of Net Co2 Fixation Capacity In Mangrove Forest By Using
Methods of Co2 Gas Exchange and Allometric Method 238-249
26 Rachmad Edison*, Sri Hidayati**
Production Surfactant Methyl Ester Sulfonate (Mes) of Jatropha Oil (Jatropha Curcas L.) With Temperature and Time Sulfonation,
Temperature Purification, and Concentration Methanol Settings 250-263
27 Rahman1, Azrul Sulaiman Karim Pohan2 Rice Husk Stove As An Effective Solution of Oil Crisis and Environmental Pollution Which Is Efficient and Economical 264-270
28
Rizki Maharani1,2*, Yutaka Tamai1,3, Yajima Takashi1,3, Minoru Terazawa3
An Alternative Utilization of Tropical Wood Sawdust As an Artificial Soil Matrix: Effects of Different Mills on The Physical Properties of
Sawdust 271-282
29
Rudy Situmeang, R. Supryanto, Wasinton
Simanjuntak, and Joko Susilo Nio/Lacro3 Catalyst For Converting Methane To Methanol 283-292
30
Rusdi Evizal1, Tohari2, Irfan D. Prijambada2,
Jaka Widada2, Donny Widianto2 Biomass Production of Shade-Grown Coffee Agroecosystems 293-303
31 Sri Yusnaini, Ainin Niswati, Dermiyati
Changes in Organic – C, N, P And K in Vermicompost of
Biodegradable Organic Wastes 304-310
32 S. Mujim and J. Prasetyo
The Effects of Some Corn Purelines And Varieties on Rust (Puccinia
Polysora) Severity and Yield of Corn (Zea Mays L.) 311-316
33 Sutikno1 and Udin Hasanuddin 2
Production of Activated Carbon From Solid-Coffee Waste By Na2so4
Activation 318-329
34 Sutrasno Kartohardjono and Andri Krestianto
Utilization of Natural Solvent From Noni’s Fruit (Morinda Citrifolia)
For Co2 Absorption Using Hollow Fiber Membrane Contactor 330-338
35
Sutrasno Kartohardjono and Muhammad Haikal Nur
Utilization of Natural Solvent From Leaves of Noni (Morinda Citrifolia) in Co2 Absorption Through Hollow Fiber Membrane
Contactor 339-347
36
Widodo Wahyu Purwanto; *Dijan Supramono;
**Yulianto S. Nugroho; *Dwi Endah Lestari Characteristics of Biomass Pellet As Fuel 348-362
37 Yosef Manik Mass and Energy Balance of A Municipal Solid Waste Incinerator 363-369
38 Yusmiati1,Udin Hasanudin1 Biogas Production From Tapioca Wastewater in Pilot Scale Bioreactor 370-379
39 Yusuf Widodo and Arif Qisthon
Optimalization of Utilizing Cassava Waste (Onggok) Through
Biological Processing on Rumen Parameters by In Vitro Method 380-386
40 Zainal Abidin
Proftitability of Jatropha Farming to Support Desa Mandiri Energi
(Village Self Reliance On Energy) In Lampung Province 387-395
41
Irwan Ginting Suka, Wasinton Simanjuntak, and Kamisah D. Pandiangan
Potential Utilization of Rice Husk Silica as an Alternative for
Mineral-Derived Silica 396-403
42
Wasinton Simanjuntak, Irwan Ginting Suka, and Kamisah. D. Pandiangan.
Electrochemical Pretreatment For Enhanced Production of Reducing Sugar From Fresh Cassava Starch to Improve Bioethanol Production
Efficiency. 404-414
43
Dijan Supramono; *Widodo Wahyu Purwanto; **Yulianto S. Nugroho; *R. Febry
Rizqiardihatno
Design of High Efficiency and Environmental Friendly Biomass Pellet
Stove Using Heat Recovery Principle 415-428
44
R.A. Bustomi Rosadi1), Diding Suhandy1), Ahmad Tusi1) and Teguh Wiyono2)
The Effect of Salinity to The Growth and Yield of Tomatoes
(Lycopersicon Esculentum Mill) on Hydrophonic System 429-437
45 Wan Abbas Zakaria
Economic Efficiency of Cassava (Manihot Esculenta) Farming In
Terbanggi Besar Subdistrict of Central Lampung District 438-446
46
Yasir Wijaya, Asihing Kustanti, and Imam Akbar
Potential of Coastal Region as a source Biomass in Lampung
Mangrove Center 447-454
!" #$$%
ISBN : 978-979-18755-7-8 I-283
Horisawa, S., Sunagawa, M., Tamai, Y., Matsuoka, Y., Miura, T., Terazawa, M., 1999. Biodegradation of nonlignocellulosic substances II: physical and chemical properties of sawdust before and after use as artificial soil. Journal of Wood Science 45 (6), 492-497. Houghton, J.I., Burgess, J.E., Stephenson, T., 2002. Off-line particle size analysis of
digested sludge. Water Resources 36, 4643-4647.
Hwang, S.I., Lee, K.P., Lee, D.S., Powers, S.E., 2002. Models for estimating soil particle-size distributions. Soil Science Society of America Journal 66 (4), 1143-1150.
Kitsui, T., Terazawa, M., 1999. Bio-Toilet environmentally-friendly toilets for the 21 century (dry closet using sawdust as an artificial soil matrix). In: Proceedings of international symposium of bio-recycling/composting. 6-8 september, Sapporo, Japan. Paulrud, S., Mattsson, J.E., Nillson, C., 2002. Particle and handling characteristics of wood
fuel powder: effects of different mills. Fuel Processing Technology 76, 23-39.
Rühlmann, J., Körschens, M., Graefe, J., 2006. A new approach to calculate the particle density of soils considering properties of the soil organic matter and the mineral matrix. Geoderma 130, 272-283.
Terazawa, M., Horisawa, S., Tamai, Y., Yamashita, K., 1999. Biodegradation of Lignocellulosic substance I: System for complete degradation of garbage using sawdust and aerobic soil bacteria. Journal of Wood Science 45 (4), 354-358.
Terazawa, M., 2003. Sawdust Saves Globe―Bioconversion of biomass wastes into multifunctional recyclates using sawdust as an artificial soil matrix―. In: Proceedings of the 1st International Symposium on Sustainable Sanitation, Nanjing, China (1), 9-12. The Ministry of Forestry Republic of Indonesia., 2007. Forestry Statistics of Indonesia year
2006. Jakarta, Indonesia.
http://www.dephut.go.id/INFORMASI/STATISTIK/2006/IV24_06.pdf>.
Zavala, M.A.L., Funamizu, N., Takakuwa, T., 2004. Modeling of aerobic biodegradation of feces using sawdust as a matrix. Water Resources 38 (5), 1327-1339
NiO/LaCrO
3Catalyst for converting methane to Methanol
Rudy Situmeang, R. Supryanto, Wasinton Simanjuntak, and Joko Susilo Department of Chemistry, FMIPA University of Lampung, BandarLampung 35415
ISBN : 978-979-18755-7-8 I-284
Abstract
NiO/LaCrO3 catalyst was prepared using citric acid method from its’ precursor of nitrates. The preparation
steps consist of wet impregnation of varied percentages (b/b) NiO into LaCrO3 , evaporation and calcination.
To examine its activity, the catalyst was used in CH4 conversion experiment at different temperatures. The
experimental results indicated the dependency of CH4 conversion on temperature, with the highest methanol
formation (176.05 µL) was achieved at 100 oC under Ar/CH
4 (40/10, 50 mL min-1) as a feed. Adding O2 gas
into feed increasing the methanol formation and the increment achieved 68%. X-ray diffraction examination proved that its crystalline phases consist of NiO cubic structure in a bulk crystalline of LaCrO3 perovskite
structure. Furthermore, increasing Ni content enlarging unit cell volume of catalyst. FTIR analysis appeared to indicate that both the Lewis and Brønsted-Lowry acid sites were involved in the catalytic conversion of CH4.
Keywords: CH4 Conversion, NiO/LaCrO3 activity, CH3OH formation, Lewis and Brønsted-Lowry acid properties
1. Introduction
Now days, air emission regulation has been applied a broadly across the nations, especially on activities resulting pollutant gases such as NOx, SOx, and COx. In addition, source of fuel energy is limited for 20 – 30 years. Therefore, finding out the energy resource dealt with environment is really interesting and challenging (Bell et al., 2004, [email protected], www.acrion.com, and www.methanol.org ).
One of alternative energy resources is biomass with its marvelous abundance. Biomass can be change simply to biogas by catalytic pyrolyse or microbial process. The main product of these processes is methane gas. Furthermore, methane gas is processed to methanol by these methods, first forming synthesis gas and then methanol or direct methane oxidation to oxygenates excluding the synthesis gas generating step. In the first method, including synthesis gas forming, unfortunately is a highly endothermic process, thus also expensive.
!" #$$%
ISBN : 978-979-18755-7-8 I-285
of research used water as a feed due to increase the methanol formation (Alptekin, et al., 2000).
In this moment, we study the conversion of methane into methanol using LaCrO3 doped NiO. Here, we emphasize our study on how increasing Ni content into LaCrO3 can give a synergic effect in directly partial oxidation of methane to methanol at low reaction temperature.
2. Experimental
2.1. Catalysts Characterization
X-rays power diffraction pattern of NiO/LaCrO3 were recorded from 2θ = 10 to 90o on a Philips diffractometer Model PW 1710 using Cu Kα radiation at a step 0.02o . Electron micrographs were obtained with Scanning Electron Microscope . To determine Lewis and BrØnsted – Lowry sites, catalysts were previously contacted to ammonia saturated vapor at ambient temperature, using FT-IR spectrophotometer.
2.2. Catalysts Preparation
Solid LaCrO3 was prepared by dissolving Cr(NO3)3 . 9 H2O, and La(NO3)3 . 6 H2O in citric solution. Then, these solutions (1:1) was mixed and evaporated until sol – gel solution formed. Furthermore, this precursor was calcinated at 600 oC for 6 hours (increased by 2 oC min-1). Solid formed was crushed into a fine grain. Catalyst LaCrO
3 supported NiO were prepared by dissolving nickel nitrate solid in citric solution and then adding LaCrO3 solid with ratio 5 to 7% (NiO : LaCrO3). After solvent evaporated, sol – gel form was dried and calcinated as before. Solid NiO was also prepared as follow as mentioned above.
2.3. Catalytic Measurements
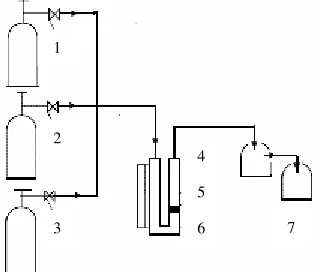
ISBN : 978-979-18755-7-8 I-286 mixing reagents were passed by valve (6) to the quartz reactor (7) of an inside diameter 12 mm. The catalyst bed was placed on quartz grains. The reactor was heated by furnace. The measurement and control of temperature was ensured by thermocouple and temperature regulator with a digital meter. The gas products are passed to dichloromethane solution as a trapper. The reaction was held at 100 – 250oC. The contact time was carried out for 30 minutes. The composition of mixture products was analyzed chromatographically using a Varian apparatus equipped with mass spectrometer detector with argon as a carrier gas.
Figure 1. A schematic diagram of the experimental set up : Methane (1), oxygen or hydrogen (2), and Argon (3) were supplied from the gas cylinders equipped by micrometric valves. The mixing reagents were passed by valve to the quartz reactor (4) and catalyst is located on (5), furnace with temperature program (6). The gas products was collected on trapper containing dichloromethane (7) and gas plastic bag before analyzing.
3. Results and Discussion
3.1. Catalyst Characterizations
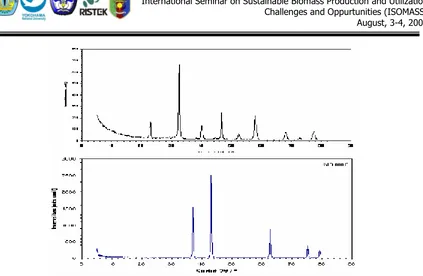
In Figs. 2 and 3 XRD powder patterns of the prepared LaCrO3, NiO and 6% NiO/LaCrO3 catalysts are presented. All the patterns of prepared solids exhibit the typical lines for LaCrO3, NiO and 6% NiO/LaCrO3 solids respectively.
1
3 2
6 5 4
!" #$$%
ISBN : 978-979-18755-7-8 I-287
Figure 2. Diffractogram LaCrO3 and NiO calinated at 600oC and reduced by H2 gas
In case of NiO/LaCrO3 solid, there are two lines at 2θ = 44.5 and 62o which are the strong lines of Cubic NiO, JCPDF 47 – 1049. No evidence of any other phase besides LaCrO3 and NiO was found in all samples. For catalyst impregnated various NiO, the obtained line intensities and patterns are not depending on NiO content.
0 100 200 300 400 500 600 700 800
0 10 20 30 40 50 60 70 80 90
Sudut 2θθθθ / o
In
te
ns
it
as
(
ar
b.
u
ni
t)
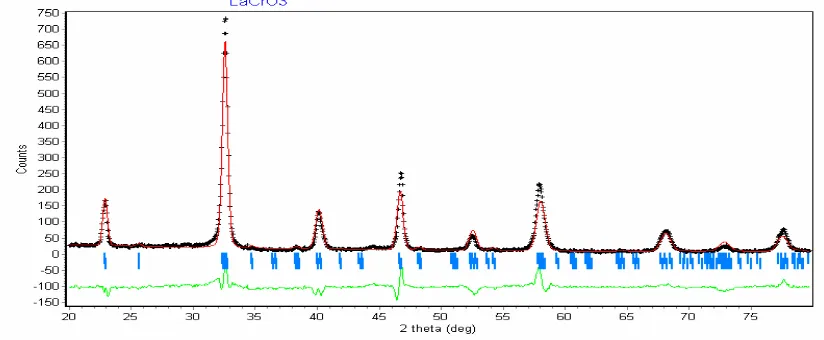
ISBN : 978-979-18755-7-8 I-288 Further investigation using Rietveld program to 6%NiO/LaCrO3 solid, proved that its unit cell change such as parameter a = 5,536Å , b=5,4794Å, dan c = 7,7941Å (goodness of fitting, χ2 = 1,306 and unit cell - volume = 236,426Å3) as shown on Figure 5 and Table 1 below. Increasing NiO content in LaCrO3 enlarging unit cell volume of NiO/LaCrO3 catalyst.
Figure 5. XRD diffractogram 6% NiO/LaCrO3 calculated by Rietveld method
Table 1. Unit cell Parameter of 5 – 7% NiO/LaCrO3 obtained by Rietveld method
NiO/LaCrO3 a (Ǻ) b (Ǻ) c (Ǻ) Volum (Ǻ3)
5% 5,5300 5,4867 7,7850 236,208
6% 5,5360 5,4794 7,7941 236,426
7% 5,5390 5,4800 7,7950 236,607
Note: calculated cell volume, V = a x b x c because simple orthorhombic structure
!" #$$%
ISBN : 978-979-18755-7-8 I-289
Figure 6. Electron micrograph 6% NiO/LaCrO3
Gravimetric acidity of NiO/LaCrO3 increases if it is compared to those of both NiO and LaCrO3 as shown on Figure 7 below. Quantitatively, acidity increment caused of NiO addition to LaCrO3 is about 24%. Acidity distribution of brønsted-Lowry and Lewis sites respectively is further discussed.
0 1 2 3 4 5 6 7
m
m
ol
N
H
3 / (
g
ka
t *
1
0
-1 )
NiO LaCrO3 NiO/LaCrO3
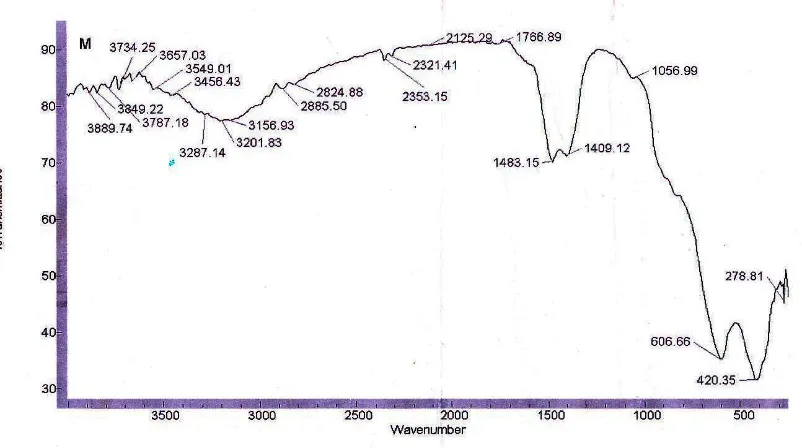
ISBN : 978-979-18755-7-8 I-290 Infra red spectra analysis of 6% NiO/LaCrO3 absorbed ammonia have done in order to knowing acid distribution on both brønsted-Lowry and Lewis sites . Vibration of –NH3 and –NH4+ forms is referred BrØnsted-Lowry and Lewis acid sites respectively. This spectra is showed on Figure 8, scanned on the range 450 – 4000 cm-1. Wave number range of 3100 – 3700 cm-1 refers to stretching vibration and below 2500 cm-1 is indicated to bending and rocking vibrations.
Figure 8. FTIR spectra of 6% NiO/LaCrO3 treated ammonia for 24 hours
Formation ammonium ion, –NH4+ (BrØnsted-Lowry acid) is showed on wave number of 1483,15 and 1409,12 cm-1, whereas coordinated species is referred to Lewis acid site and is showed on wave number of 1600 cm-1. Peak appeared at 606,66 dan 420,35 cm-1 is referred respectively to O-Cr-O deformation and Cr-O stretching vibration (Zheng et al., 1999). However, peaks appeared on wave number 498; 488; dan 510 cm-1 as a characteristic adsorption of NiO is not exist .
3.2. Methane oxidation
!" #$$%
ISBN : 978-979-18755-7-8 I-291
0 20 40 60 80 100 120 140 160 180
C
H3
O
H
,
m
ic
ro
L
1 2 3 4
Temperature
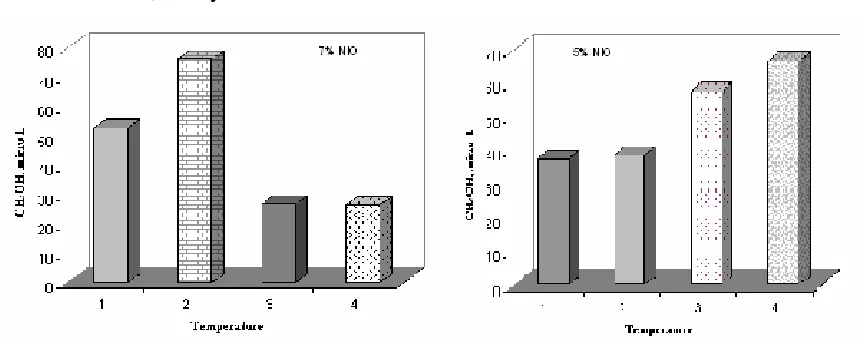
Figure 9. Methanol formation in various temperature reaction on 6% NiO/LaCrO3 ( Label 1 = 100oC, 2= 150oC, 3= 200oC, 4= 250oC)
The same pattern of tendency is also happened on LaCrO3 doped by 7% NiO as shown on Figure 10. However, the tendency on LaCrO3 doped by 5% NiO is the methanol formation increases as temperature reaction increases even though its quantity is lesser than that of 6% NiO/LaCrO3 catalyst.
Figure 10. Methanol formation in various temperature reaction on 5 and 7% NiO/LaCrO3 ( Label 1 = 100oC, 2= 150oC, 3= 200oC, 4= 250oC)
ISBN : 978-979-18755-7-8 I-292
0.00 50.00 100.00 150.00 200.00 250.00
C
H
3
O
H
,
1
0
-6 L
5%NiO 6%NiO 7%NiO
LaC rO3 dope d
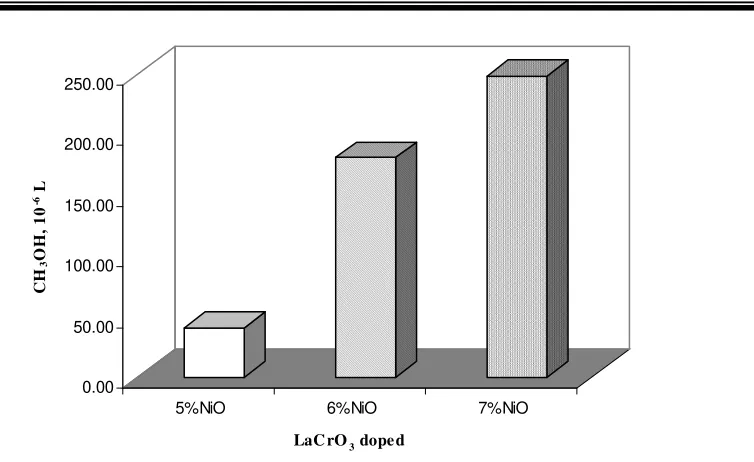
Figure 11. Methanol formation under the feed of Ar/CH4/O2 (35/10/5) at 100oC
In general, by adding O2 gas on the feed, the methanol formation is increased. In addition, as NiO quantity as dopant increases the methanol formation also increases. The biggest increment is happened on 7% NiO/LaCrO3 catalyst. The increment is almost achieved 68%. It means that oxygenous supply on transform CH4 gas into CH3OH is working.
Conclusions
Based on this experiment, we can conclude that the temperature reaction affect the formation of methanol from methane gas. In general, all catalyst of LaCrO3 doped NiO is active on this reaction. In the reaction under Ar/CH4 as a feed, the bulk oxygen of catalyst played a role on transforming methane gas into methanol. Increasing the Ni content in LaCrO3 catalyst giving a negative effect to methane conversion. Furthermore, adding O2 gas on the feed increasing methanol formation.
Acknowledgements
!" #$$%
ISBN : 978-979-18755-7-8 I-293
would like to thank Andi Setiawan Ph.D for chromatography – mass spectroscopy data discussion.
References
1.Alexis T. Bell, Benjamin R. Wood, Jeffrey A. Reimer, Michael T. Janicke and
Kevin C. Ott. 2004. Methanol formation on Fe/Al-MFI via the oxidation of methane by nitrous oxide. J. Catal Vol. 225, issue 2, 2004, p. 300 –
3006.
2.Alptekin, Gokhan O., Andrew M. Herring, D.L. Williamson, Tim R. Ohno and
Robert L. McCormick. 2000. Methane partial oxidation by unsupported and silica supported iron phosphate catalysts : Influence of reaction conditions and co-feeding of water on activity and selectivity. J. Catal. Vol. 181, issue 1, p. 104 – 112.
4.Han S. , Martenak D.J., Palermo R.E., Pearson J.A. and Walsh D.E. 1994.
The Direct partial oxidation of methane over ZSM-5 catalyst : Metals effects on Higher Hydrocarbon Formation . J. Catal Vol. 148, issue
1, p. 134-137.
5.Han S. , Martenak D.J., Palermo R.E., Pearson J.A. and Walsh D.E. 1992.
The Direct partial oxidation of methane to liquid hydrocarbons over ZSM-5 catalyst. J. Catal Vol. 136, issue 2, p. 578-583.
6.Michalkiewicz Beata. 2004. Partial oxidation of methane to formaldehyde and methanol using molecular oxygen over FeZSM-5. Appl. Catal. A : General, Vol.
277, issues 1 – 2, p. 147 – 153.
7. www.acrion.com
8. www.methanol.org
9. Zheng Wenjun., Wenqin Pang., GuangYao Meng and Dingkun Peng 1999.