All sources 39 Internet sources 21
[11] www.sciencedirect.com/science/article/pii/S0142941813001475 1.6% 10 matches
[17] journals.sagepub.com/doi/pdf/10.1177/0892705713486124 1.0% 4 matches
[18] https://www.scribd.com/document/52958715...-EPDM-nanocomposites 1.0% 5 matches
[19] https://www.deepdyve.com/lp/elsevier/eff...teristics-vaB50VP80k 0.9% 3 matches
[20] https://www.sciencedirect.com/science/article/pii/S0142941814001810 0.6% 3 matches
[21] journals.sagepub.com/doi/pdf/10.1177/0095244311405503 0.7% 3 matches
[22] www.tandfonline.com/doi/pdf/10.1080/03602559.2010.496425?needAccess=true 0.5% 2 matches
[23] https://www.researchgate.net/profile/Chr...n=publication_detail 0.5% 3 matches
[24] https://documents.mx/documents/effects-o...triethoxysilane.html 0.4% 2 matches
[25] www.tandfonline.com/toc/yprc20/current 0.3% 2 matches
[26] www.tandfonline.com/doi/abs/10.1080/03602550802355206 0.3% 1 matches
[27] explore.tandfonline.com/page/est/alan-glanville-award 0.2% 1 matches
[28] explore.tandfonline.com/page/est/yprc-composite-award 0.2% 1 matches
[29] https://www.sciencedirect.com/science/article/pii/S0142941802000673 0.2% 1 matches
[30] www.iom3.org/polymer-society/plastics-ru...olecular-engineering 0.2% 1 matches
[33] https://link.springer.com/content/pdf/10.1007/s40090-016-0107-7.pdf 0.1% 1 matches
[34] www.tandfonline.com/doi/pdf/10.1179/146580109X12473409436508 0.2% 1 matches
[35] www.tandfonline.com/loi/yprc20 0.2% 1 matches
[36]
www.tandfonline.com/doi/pdf/10.1179/1743289810Y.0000000010
0.2% 1 matches
1 documents with identical matches
[38] www.tandfonline.com/doi/pdf/10.1179/1743289812Y.0000000013 0.2% 1 matches
[39] www.tandfonline.com/doi/figure/10.1080/1...=top&needAccess=true 0.2% 1 matches
5.1%
Results of plagiarism analysis from 2017-12-05 07:52 UTCAlkanolamide as a novel accelerator and vulcanising agent in carbon black-filled polychloroprene rubber compounds.pdf
Date: 2017-12-05 07:48 UTC
8 pages, 4983 words
PlagLevel: selected / overall
167 matches from 40 sources, of which 28 are online sources.
Settings
Data policy: Compare with web sources, Check against my documents, Check against my documents in the organization repository, Check against organization repository, Check against the Plagiarism Prevention Pool
Sensitivity: Medium
Bibliography: Consider text
--Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=yprc20
Download by: [Indra Surya] Date: 08 June 2016, At: 13:[25]50
Plastics, Rubber and Composites
M
acromolecular
E
ngineering
ISSN: 1465-8011 (Print) 1743-2898 (Online) Journal homepage: http://www.tandfonline.com/loi/yprc20
Alkanolamide as a novel accelerator and
vulcanising agent in carbon black-filled
polychloroprene rubber compounds
I. Surya & H. Ismail
To cite this article: I. Surya & H. Ismail (2016): Alkanolamide as a novel accelerator and vulcanising agent in carbon black-filled polychloroprene rubber compounds, Plastics, Rubber and Composites, DOI: 10.1080/14658011.2016.1187477
To link to this article: http://dx.doi.org/10.1080/14658011.2016.1187477
Published online: 07 Jun 2016.
Submit your article to this journal
View related articles
Alkanolamide as a novel accelerator and
vulcanising agent in carbon black- lled
fi
polychloroprene rubber compounds
I. Surya
1and H.
[11Ismail
] ∗2A feasibility study was carried out on the
utilisation
of Alkanolamide (ALK
) as a novel accelerator
and vulcanising agent in Carbon Black (CB)- lled polychloroprene rubber (CR) compounds.
fi
The
functions of the ALK were compared with those of conventional accelerator and vulcanising
[11]
agents for CR, ethylene thiourea (ETU), and a combination of magnesium and zinc oxides.
The
ALK was incorporated into the
CB- lled CR compounds at 1.0, 2.0, 3.0 and 4.0 phr. By
fi
replacing ETU, it was found that increasing the ALK loading decreased the scorch and cure
times of the CB- lled CR compounds with magnesium and zinc oxides. The incorporation of up
fi
to 3.0 phr of ALK increased the torque differences, and tensile and hardness properties; and
decreased those properties with further increases of ALK loadings. It was also found that ALK
was able to vulcanise the CB- lled CR compound. The 3.0 phr ALK CR compound without the
fi
–
ETU, magnesium and zinc oxides showed a higher tensile strength than that of the control
compound, which was cured by ETU, magnesium and zinc oxides.
Keywords:Alkanolamide, Polychloroprene rubber, Accelerator, Vulcanising agent, Alkanolamide cross-linked polychloroprene
Introduction
Polychloroprene (CR) is one of the most important elas-tomers of all specialty elaselas-tomers. Since it was rst pro-fi
duced in 1932, it has had an outstanding market position due to its favourable combination of technical or engineering properties. CR is classi ed as a high-fi
volume specialty elastomer,1and is used mainly within the rubber industry. Its areas of application within the rubber field are widespread, and include transmission belts, conveyor belts, moulded goods, cable jackets, seals, coated fabrics, etc.
CR has a well-balanced combination of properties that include good mechanical properties, remarkable resist-ance to ozone, oil, and heat; ame retardancy, weather-fl
ability, special cohesiveness, moderate resistance to most chemicals, and ease of processability.2 5– The molecular structure of CR is similar to that of natural rubber; except that chlorine has replaced the methyl groups.1The pres-ence of chlorine causes the cure system of CR to be gen-erally different from that of other diene rubbers.1 6 7, , The chlorine atoms decrease the reactivity of double bonds on the CR backbone; and thus, the reactivity with sulphur becomes less. Metal oxides, thiuram, and thiourea based curing agents; particularly ethylene thiourea (ETU), are widely used as the cure system for CR.7ETU has been
generally used as the vulcanisation accelerator for CR. It is a toxic material and is suspected to be carcinogenic.8 9, The diene rubbers are vulcanised by a single ingredient, such as sulphur or peroxide, while CR is conventionally vulcanised by both magnesium oxide (MgO) and zinc oxide (ZnO), at satisfactory loadings of 4 and 5 phr,
[19]
respectively.The use of many ingredients in rubber com-pounding has the potential to cause several problems, such as an inef cient mixing process,fi additional time and energy consumed during processing, as well as var-ious side effects.10 A reduction of ingredients involved has also been demanded.
Recently, newer curing agents, including thiopho-sphoryl disulphides, dimethyl l-cystine, and cetyltrimethy-lammonium maleate, have been reported.10 12– Although there are many published works on various curing agents for CR, the most practical curing agents are still metal oxides (MgO and ZnO), due to the superior mechanical properties of the cured products. Thus, the appearance of an alternative vulcanisation accelerator and single vul-canising agent for CR that is capable of providing CR products/vulcanisates equivalent or superior to those pro-vided by the ETU and metal oxides has been in demand. In our previous work,13the preparation and application of ALK in silica- lled natural rubber compounds was[20] fi
reported.The incorporation of ALK withinsilicafilled
natural rubber compounds gavebetter mechanical prop-erties namely tensile strength (TS),tensile modulus, and
[17]
hardness. The enhancement of those properties was attrib-uted to the improvement of silica dispersion in the rubber compounds, and the better crosslink density due to the 1Department of Chemical Engineering, Engineering Faculty, University of
Sumatera Utara, Medan 20155, Sumatera Utara, Indonesia
2School of Materials and Mineral Resources Engineering, Universiti Sains
Malaysia, Engineering Campus, Nibong Tebal, Penang14300, Malaysia
∗Corresponding author, email ihana @usm.myfi [27]
incorporation of ALK. The results also indicated that ALK can function as an accelerator.
In this study,the effect of ALK on thecuring
character-[11]
istics and mechanical properties of Carbon Black (CB)- lled CR compounds was compared with thefi conventional accelerator, ETU.The effect of ALK, as a novel vulcanising agent, was also compared with the con-ventional vulcanising agents for CR, of MgO and ZnO.
Experimental
Materials
CR [Skyprene B-30] was purchased from TOSOH Co. (Japan), CB N330 was obtained from Malayan Carbon (M) Sdn. Bhd., ethyelene thiourea (ETU), MgO, ZnO, stearic acid, and aromatic oil were all obtained from
[24]
Bayer (M).All materials were used assupplied.The for-mulas for the study of ALK as a novel accelerator are shown inaTble 1, and for the study of ALK as a novel vul-canising agent, in Table 2. The ALK was synthesised in our laboratory using Re ned Bleached Deodorisedfi
Palm Stearin (RBDPS) and diethanolamine. The reaction procedure and molecular characterisation of the ALK was given in our previous report13 and the molecular structure is shown inFig. 1.
Compounding and cure characteristics
The compounding ingredients were mixed using a labora-tory two-roll mill, Model XK-160. The cure character-istics of the CB- lled CR compounds were determinedfi
at 140°C using a Monsanto Moving Die Rheometer (MDR 2000). The respective scorch time (ts2), cure time (t90), and torque difference (Torquemaximum– Torqueminimum) were obtained from the rheograph
[17]
according to ISO 3417.The compound was subsequently compression moulded using a stainless steel mould at 140°C with a pressure of 10 MPa using a laboratory hot-press based on the respective curing times.
Measurement of tensile, hardness properties
[21]
Dumbbell-shaped samples were cut from the moulded
[17]
sheets.Tensile tests were performed at acrossheadspeed of 500 mm min−1using an Instron 3366 universal tensile
[18]
machine according to ISO 37. The TS and st ress at 100% elongation (M100), stress at 300% elongation (M300), andelongation at break(EB) were investigated. The hardness measurements of the samples were obtained
according to ISO 7691-I using a Shore A type manual Durometer.
Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra were obtained using a Perkin-Elmer 2000 ser ies instrument. The spectrum resolution was 4 cm−1 and the scanning range was from 550 to 4000 cm−1. Thin lm (with thicknessfi 0.2 mm) for the FTIR∼
[17]
spectra, was prepared for the CR-VAP3 compound by moulding the rubber compound sample at 140°C at a pressure of10 MPa using a laboratory hot-press, based on the respectivecure time.
Results and discussion
Alkanolamide (ALK) as a novel accelerator in
CB- lled CR compounds
fi
Cure characteristics
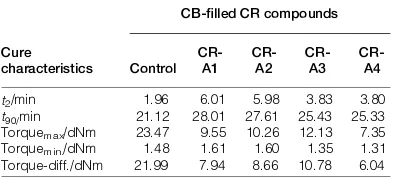
Table 3 shows the comparative effect of ALK as a novel accelerator and ETU as a conventional accelerator on the curing characteristics of the CB- lled CR compounds.fi
In the case of a comparable amount of those ingredients, it can be seen that scorch and cure times of the CR-A1 compound were longer than those of the Control com-pound. It can therefore be considered that ALK gave a longer crosslink process and caused scorch delay to the
[11]
CB- lled CR compound.fi Increasing the ALK loading decreased the scorch and cure timesof the lled CR com-fi pounds.This cure enhancement phenomenon indicates that ALK could act as an accelerator in the vulcanisation of the CB- lled CR compounds. An accelerator is afi
rubber ingredient that enhances the action of a curing or vulcanising agent to speed up the resultant cure, even though it constitutes a very small part of a rubber compound.14
The torque difference value of the CR-A1 compound was lower than that of the Control compound. Increasing the ALK loading, by up to 3.0 phr, increased the torque difference value, while further increases of the ALK load-ing decreased the value.
Theoretically, the torque difference value is an indi-cation of the crosslink density of a rubber com-pound.2 15 17, – The greater the torque difference value, the hig her the crosslink density.18 20– Increased torque differences, of up to 3.0 phr of ALK loading can be attrib-uted to the function of ALK as an accelerator, which enhanced the cure rate and cure state of the CB- lledfi
CR compounds. The deterioration of the value after 3.0 phr ALK loading i.e. the CR-A4 compound was probably due to the softening (or plasticising) effect of the excessive ALK causing a lower torque difference value or crosslink density. The plasticising effect of ALK is derived from the RBDPS, which is a product of the palm oil fractionation process. Palm oil has the effect of plasticising or lubricat-ing rubbers.21
Mechanical properties
Table 4 shows the comparative effect of ALK with that of ETU on the mechanical properties of CB- lled CR vulca-[20] fi
nisates.At a similar loadingi.e. 1.0 phr of accelerator, it can be seen that CR-A1 had lower tensile and hardness properties than the Control, except for M300 and EB. Higher M300 and EB CR-A1 properties may have been Table 1 The compound designation and formulation used to
compare the effect of ALK and ETU as accelerators in CB- lled CR compoundsfi
Ingredients∗
Designation of CB- lled CR compoundsfi
Control CR-A1 CR-A2 CR-A3 CR-A4
CR 100.0 100.0 100.0 100.0 100.0
CB 50.0 50.0 50.0 50.0 50.0
Aromatic oil 10.0 10.0 10.0 10.0 10.0
MgO 4.0 4.0 4.0 4.0 4.0
ZnO 5.0 5.0 5.0 5.0 5.0
Stearic acid 0.5 0.5 0.5 0.5 0.5
ETU 1.0 0 0 0 0
ALK 0 1.0 2.0 3.0 4.0
∗Parts per hundred parts of rubber.
due to the plasticising effect of the ALK; which provided more free volume, thus allowing more mobility/ exibilityfl
for the CR chains and a break at a higher extension. It can also be seen that the incorporation of up to 3.0 phr of ALK into the CB- lled CR compound increasedfi
the tensile modulus, TS, and hardness. However, further increases of the ALK loading decreased these properties. The modulus (or stiffness/hardness) and tensile properties of rubber vulcanisates are only dependent on the degree of crosslink.22 23, The improvement of tensile modulus, TS, and hardness up to 3.0 phr was attributed to a higher crosslink density; while the deterioration of those proper-ties beyond 3.0 phr was attributed to a lower crosslink density. This explanation is in line with the torque differ-ence value trend shown inbaT le 3.
The EB of the filled CR vulcanisate increased with increasing the ALK loading. EB can be used as a feature
[11]
of the extensibility of rubber vulcanisates.Inc reasingthe ALK loading caused afurthe rincrease in theextensibility (or exibility) of the CR chains.fl This phenomenon can be attributed to ALK functioning as an internal plasticiser to
[11]
the CB- lled CR compounds.fi A plasticiser is a rubber
additive that can beused, not only to improve rubber compound processing, but also to modifyphysical proper-ties, such as the hardness and flexibility of the rubber vulcanisates.2
From the above results, it is clear that ALK can be uti-lised practically as an accelerator in its own right at 3.0 phr of loading for CB- lled CR compounds.fi
ALK as a novel vulcanising agent in CB- lled
fi
CR compounds
Cure characteristics
Table 5 shows the comparative effect of ALK as a novel vulcanising agent and the combination of MgO and ZnO as conventional vulcanising agents, with ETU as the accelerator on the curing characteristics of the
CB-filled CR compounds. CR-VA1, CR-VA2, CR-VA3, and CR-VA4 (CR-VA ser ies) com pounds were used to exam-ine the function of ALK as a vulcanising agent with the addition of an external plasticiser (i.e. the aromatic oil). Meanwhile, compound CR-VAP3 was used to examine the function of ALK as a vulcanising agent without the addition of any external plasticisers.
The incorporation of 1.0 phr ALK into the CB- lledfi CR compound, without MgO, ZnO and ETU, produced CR-VA1 with longer scorch and cure times and a lower torque difference value than that of the control. The increasing ALK loading increased both scorch and cure times. This can be attributed to AL K functioning as a reactant or vulcanising agent, which was involved directly in the crosslink process of the CB- lled CR compounds.fi
The higher the ALK loading, the greater the amount of reactant, and th e longer the time needed to complete the crosslink process.
The incorporation of up to 3.[11]0 phr of ALKinto the CB- lled CR compound increasedfi the torque difference
[11]
value. However, further increases in the ALK loading decreased the torque differencevalue.Since torque differ-ence value relates to the crosslink density of a rubber com-pound, and none of the conventional vulcanising agents existed in the CR-VA series compounds, it can be con-sidered that ALK can function as a crosslinking or vulca-nising agent in the vulcanisation or crosslink process of the CR- lled CR compounds.fi
Based on the torque difference value, 3.0 phr of ALK is the optimum loading for the crosslink process of the
CB-filled CR compound.
The incorporation of 3.0 phr ALK into the CB- lledfi
CR compound, without the addition of MgO, ZnO, Table 2 The compound designation and formulation used to compare the effect of ALK and a combination of MgO and ZnO as
vulcanising agents in CB- lled CR compoundsfi
Ingredients∗
Designation of CB- lled CR Compoundsfi
Control CR-VA1 CR-VA2 CR-VA3 CR-VA4 CR-VAP3
CR 100.0 100.0 100.0 100.0 100.0 100.0
CB 50.0 50.0 50.0 50.0 50.0 50.0
Aromatic oil 10.0 10.0 10.0 10.0 10.0 0.0
MgO 4.0 0.0 0.0 0.0 0.0 0.0
ZnO 5.0 0.0 0.0 0.0 0.0 0.0
Stearic acid 0.5 0.5 0.5 0.5 0.5 0.5
ETU 1.0 0 0 0 0 0
ALK 0 1.0 2.0 3.0 4.0 3.0
∗Parts per hundred parts of rubber
Table 3 Cure characteristics of the CB- lled CR compoundsfi with ETU and ALK as accelerators
Cure characteristics
CB- lled CR compoundsfi
Control
t90/min 21.12 28.01 27.61 25.43 25.33 Torquemax/dNm 23.47 9.55 10.26 12.13 7.35
Torquemin./dNm 1.48 1.61 1.60 1.35 1.31
Torque-diff./dNm 21.99 7.94 8.66 10.78 6.04 1 Molecular structure of ALK
ETU, and aromatic oil, produced a CR-VAP3 compound with a more superior torque difference value than the CR-VA series compounds. This can be attributed to a higher degree and state of curing caused by ALK; since the absence of aromatic oil made the interaction between the ALK and the CB- lled CR compound stronger andfi
more intense.
Mechanical properties
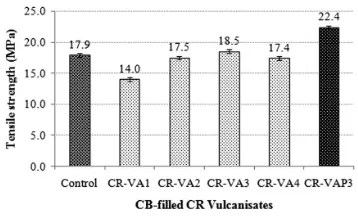
Table 6andFig. 2show the effect of ALK as a vulcanising agent on the tensile modulus, hardness and TS of the
CB-filled CR vulcanisates. It can be seen that the tensile mod-ulus increased up to the maximum level, at 3.[11]0 phr of ALK loading, which then decreased with further increases of loading. The results of hardness and TS also exhibited a similar trend.
The enhancement of tensile modulus, TS and hardness up to 3.0 phr ALK loading were attributed to a higher reinforcement level of CB to the CR rubber, due to the crosslink density improvements of the CR-VA series com-pounds. The deterioration of those properties beyond 3.0 phr can be attributed to a lower degree of crosslink den-sity and a more pronounced softening effect of the exces-sive ALK.
The addition of 3.0 phr AL K into the CB- lled CRfi
compound, without any external plasticiser, produced the CR-VAP3 vulcanisate with superior tensile modulus (especially the M300) and TS, and a comparable hard-ness to Control vulcanisate. Both modulus and tensile reinforcements were observed. The modulus/stiffness reinforcement was attributed to ALK's ability to interact with CB ller, which became stronger and more intense,fi
since no plasticising agent was present in thefilled CR compound. M300 displays the degree of rubber- llerfi
interaction.24The greater the M300, the stronger the l-fi ler-rubber interaction. The strong interaction between the CB ller and the CR can be attributed to the naturefi
of the ALK molecule. As shown inFig. 1, the ALK is Table 4 Mechanical properties of the CB- lled CR vulcanisates with ETU and ALK as acceleratorsfi
Mechanical properties
CB- lled CR vulcanisatesfi
Control CR-A1 CR-A2 CR-A3 CR-A4
M100/MPa 4.084 2.16 2.30 2.45 2.06
M300/MPa N/A∗ 7.95 9.05 9.22 7.61
TS/MPa 17.94 11.74 12.13 12.99 10.95
EB/% 261 362 405 410 417
Hardness/Shore A 75 59 60 62 57
∗N/A = Not available.
Table 5 Cure characteristics of the CB- lled CR compounds with ALK and a combination of MgO and ZnO as vulcanisingfi agents
Cure characteristics
CB- lled CR compoundsfi
Control CR-VA1 CR-VA2 CR-VA3 CR-VA4 CR-VAP3
t2/min 1.96 5.51 6.26 6.32 6.34 6.87
t90/min 21.12 22.23 22.39 25.16 25.43 24.03
Torquemax/dNm 23.47 6.84 9.52 11.49 10.72 20.74
Torquemin./dNm 1.48 1.34 1.42 1.50 1.32 4.67
Torque-diff./dNm 21.99 5.50 8.10 9.99 9.40 16.07
Table 6 Tensile modulus, and hardness of the CB- lled CR vulcanisates with ALK and a combination of MgO and ZnO asfi vulcanising agents
Properties
CB- lled CR vulcanisatesfi
Control CR-VA1 CR-VA2 CR-VA3 CR-VA4 CR-VAP3
M100/MPa 4.08 1.19 1.87 1.90 1.75 4.16
M300/MPa N/A∗ 4.84 6.87 9.12 9.05 17.96
Hardness/Shore A 75 55 57 59 56 75
∗N/A = Not available.
structured by a hydrophobic long chain hydrocarbon and polar terminal groups. The hydrophobic long chain had an af nity towards the CB, and wetted andfi
dispersed the filler agglomerates; thus, reducing the interaction with the rubber. This interaction scheme allows the breakdown of the ller into smaller sized par-fi
ticles through mechanical shearing during the early stage of mixing. The smaller the particles sizes, the greater the available surface area for the interaction, and the stron-ger is the interfacial interaction/bonding between the ALK and the CB. Interfacial bonding is a feature of modulus reinforcement25that increases the total cross-link density of rubber vulcanisates.26 27,
Tensile reinforcement is attributed to ALK functioning as a vulcanising agent. As discussed earlier, the ALK mol-ecule also has polar terminal groups. Based on the FT-IR study (which will be discussed later), the hydroxyl groups of the ALK interacted with the chlorine atom of the CR. Speci cally, the hydrogen atoms of the hydroxyl groupsfi
interacted chemically wit h the allylic chlorine of the CR, formed hydrogen chloride (HCl); which was released as a vapour during the crosslinking process. Simul-taneously, the oxygen atoms of the hydroxyl groups reacted with the carbon atoms of the CR, and cross-linked the CR molecule chains. Through this mechanism, an ALK cross-linked the CR. The probable crosslinking reaction between the ALK and the CR is presented in Fig. 3.
Menon and Visconte found that the crosslinking of CR could take place in the presence of functionally active chemicals, such as Phosphorylated Cashew Nut Shell Liquid (PCNSL).28Their work shows that at a high temp-erature, the hydroxyl group in the PCNSL phosphate group could react with the chlorine atom of the CR and form a primary chemical bond.
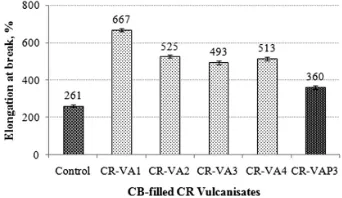
Figure 4 shows the effect of ALK on the EB of the
CB-filled CR vulcanisates. As can be seen, the EB of CR-VA series vulcanisates tend to decrease with an increasing ALK loading of up to 3.0 phr. EB corresponds to the crosslink density/torque difference value. This is simply due to the increasing crosslink density, which immobilises the CR segments from the CB surface. However, EB is found to increase beyond the 3.0 phr ALK loading. The explanation is again given by the plasticising effect of the excessive ALK, which lowers the crosslink density and causes the CR segments to move more freely.
Owing to a higher crosslink density, the EB of CR-VAP3 was lower than that of the CR-VA series. A higher EB of CR-VAP3 than the control can be attributed to the more pronounced plasticising effect of the ALK than that of the aromatic oil.
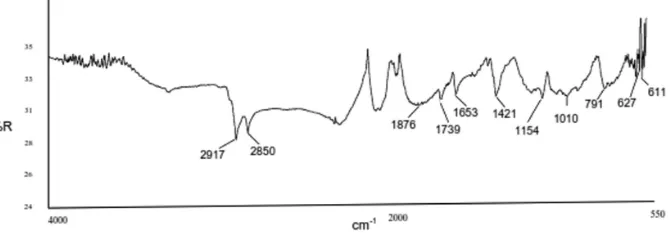
Infrared spectroscopic study
Table 7andFig. 5show the wave numbers of the func-tional groups and the result of the FTIR spectroscopic 3 The probable crosslinking reaction between ALK and CB- lled CR rubberfi
4 EB of the CB- lled CR vulcanisatesfi
Table 7 The wavenumbers of functional groups of CR-VAP3 vulcanisate
Vibration Wavenumber (cm−1)
C Cl– 627
C Cl stretch– 791
C O Alcohol stretch– 1010
study on the CR-VAP3 vulcanisate. The stretching fre-quency of the numerous C H modes of the CR molecule– is normally placed between 3100 and 2800 cm−1. The strong infrared bands that appear at 2850 and 2917 cm−1, respectively, were attributed to the =C H stretching– mode. The C Cl stretching region between 800 and 600– cm−1is well known for the speci c resonance in the con-fi formational structure of CR.29The strong band at 627 cm−1in the infrared was assigned to the C Cl stretching– mode, and the chlorine groups.
The strong bands that appear at 1421 and 1653 cm−1, respectively, were attributed to the C N and C=O– stretches belonging to the ALK.
The stretching frequency of the numerous C=O modes of CB ller is normally placed between 1600 and 1800fi
cm−1. The strong bands that appear at 1739 and 1876 cm−1, respectively, were attributed to the C=O stretching mode and C=O carbonyl groups of the CB.
These assignments are in good agreement with the lit-erature30and the spectra clearly show the characteristic wave numbers of a mixture of CR, ALK and CB in the CR-VAP3 vulcanisate.
In addition, a new strong band was observed at 1154 cm−1and was assigned to the C O C stretching mode.– – This spectral feature indicates that a new chemical bond-ing occurred durbond-ing the vulcanisation of the CR-VAP3 compound. It was proposed that the ALK interacted strongly with the CR. The interaction was an ionic reac-tion i.e. the hydrogen atoms of the OH groups of the ALK, which had locally positive charges, interacted chemically with the chlorine atoms of the CR, which had locally negative charges, and formed hydrogen chlor-ide (HCl), which was released as a vapour during the vul-canisation. Simultaneously, several C O C chemical– – bonds were formed that cross-linked the CR molecules, instead of the non-polar hydrocarbon of the ALK inter-acted physically through moment dipole interaction with the CB ller. The CB/ALK/CR interaction/bondingfi
was formed, the probable crosslinking reaction of which was presented previously inFig. 3.
Conclusions
ALK can be utilised, not only as a novel accelerator, but also as novel vulcanising agent in the vulcanisation of
CB-filled CR rubber compound. In the replacement of the conventional accelerator (ETU), ALK, together with the conventional vulcanising agents (MgO and ZnO),
vulcanised the CB- lled polychloropene rubber com-fi
pounds. Increasing the ALK loading in the CB- lledfi
compound decreased both scorch and cure times. In the replacement of the conventional vulcanising agents, and without the presence of ETU, ALK can func-tion practically as a novel vulcanising agent in its own right at 3.0 phr, and vulcanise the CB- lled CR rubberfi
compounds. The tensile modulus, TS and hardness of the CB- lled CR rubber vulcanisates were improved;fi
especially up to a 3.0 phr loading.
Acknowledgements
The authors would like to thank Universiti Sains Malay-sia for providing the research facilities for carrying out the experiment and for making this research work possible. One of the authors (Indra Surya) is grateful to the Direc-torate General of Higher Education (DIKTI), Ministry of Education and Culture (Kemdikbud) of the Republic of Indonesia, for the award of a scholarship under the fthfi
batch of the Overseas Postgraduate Scholarship Program.
References
1. J. S. Dick and R. A. Annicelli: Rubber technology: compounding‘ and testing for performance ;' 2001, Munich, Hanser Gardner Publications.
2. B. Rodgers: Rubber compounding: chemistry and Applications ;‘ '
2004, New York CRC Press.
3. R. Schatzel and G. Cassell: Synthetic elastic polymers in the cable‘ industry ,'Ind. Eng. Chem.,1939,31, (8), 945 949.–
4. S. K. De and J. R. White: Rubber technologists handbook ;‘ '2001, Shawbury, Rapra Technology.
5. A. Ciesielsky: ‘An introduction to rubber technology ;' 1999, Shawbury, iSmithers Rapra Publishing.
6. W. Hofmann: ‘Vulcanization and vulcanizing agents ;' 1967, London, MacLaren and Sons.
7. M. Akiba and A. Hashim: Vulcanization and crosslinking in elas-‘ tomers ,'Prog. Polym. Sci.,1997,22, (3), 475 521.–
8. D. M. Smith: Ethylene thiourea: thyroid function in two groups of‘ exposed workers ,'Brit. J. Ind. Med.,1984,41, (3), 362 366.– 9. R. Chhabra, S. Eustis, J. Haseman, P. Kurtz and B. Carlton:
‘Comparative carcinogenicity of ethylene thiourea with or without perinatal exposure in rats and mice ,' Fund. Appl. Toxicol.,1992,
18, (3), 405 417.–
[19]
10. H. Ismail, Z. Ahmad and Z. Mohd Ishak:‘Effects of cetyltrimethy-lammoniummaleate on curing characteristics and mechanical prop-ertiesof polychloroprene rubber',Polym.Test.,2003,22, (2), 179– 183.
11. A. Das, N. Naskar and D. K. Basu: Thiophosphoryl disul des as‘ fi crosslinking agents for chloroprene rubber ,' J. Appl. Polym. Sci., 2004,91, (3), 1913 1919.–
5 The infrared spectrum of CR-VAP3 vulcanisate
12. A. Das, N. Naskar, R. Datta, P. Bose and S. Debnath: Naturally‘ occurring amino acid: Novel curatives for chloroprene rubber ,' J. Appl. Polym. Sci.,2006,100, (5), 3981 3986.–
[11]
13. I. Surya, H. Ismail and A. Azura:‘Alkanolamide as an accelerator,
filler-dispersantand a plasticizer insilica- lled natural rubber com-fi
pounds ,'Polym.Test.,2013,32, (8), 1313 1321.–
14. H. Long:Basic Compounding and Processing of Rubber, Rubber
Division. 1985, American Chemical Society Inc. The University of Akron, Ohio, USA.
15. B. Boonstra, H. Cochrane and E. Dannenberg: Reinforcement of‘ silicone rubber by particulate silica ,'Rubber Chem. Technol.,1975,
48, (4), 558 576.–
16. H. Cochrane and C. Lin: The in uence of fumed silica properties on‘ fl the processing, curing, and reinforcement properties of silicone rubber ,'Rubber Chem. Technol.,1993,66, (1), 48 60.–
17. H. Ismail and C. Ng: ‘Palm loi tyfat ac id vesadditi (POF A's): Pre pa rati on and ap pl iatc o ni ',J. Eom.tasl tPasl .,1998,30, (4), 308–327.
18. J. S. Dick: Basic rubber testing: selecting methods for a rubber test‘ program ;'2003, Pennsylvania, ASTM International.
19. P. Sae-Oui, C. Sirisinha, K. Hatthapanit and N. Phewthongin: ‘In uence of magnesium carbonate loading on the compound prop-fl erties of polychloroprene, natural rubber, and their blend ,'J. Appl. Polym. Sci.,2008,110,(5), 2763 2769.–
20. P. Kundu, D. Tripathy and B. Gupta: Blends of poly [ethylene (vinyl‘ acetate)] and polychloroprene: Studies on capillary and dynamic flows',J. Appl. Polym. Sci.,1997,63, (2), 187 193.–
21. F. W. Barlow: Rubber compounding: principles, materials, and‘ techniques ;'1993, New York, CRC Press.
22. D. L. Hertz Jr and S. E. INC:Elastomerics,1984,116, (November), 17 21.–
[24]
23. H. Ismail and H. Chia:‘The effects of multifunctional additive and vulcanization systems on silicafilledepoxidized natural rubber com-pounds ,'Eur.Polym. J.,1998,34, (12), 1857 1863.–
24. S. Wolff: Optimization of silane-silica OTR compounds. Part 1:‘ Variations of mixing temperature and time during the modi cationfi of silica with Bis-(3-Triethoxisilylpropyl)-Tetrasul de ,fi ' Rubber Chem. Technol.,1982,55, (4), 967 989.–
25. E. Dannenberg: Filler choices in the rubber industry ,‘ ' Rubber Chem. Technol.,1982,55, (3), 860 880.–
26. G. Kraus: Interactions of elastomers and reinforcing llers ,‘ fi 'Rubber Chem. Technol.,1965 38, (5), 1070 1114., –
27. K. Polmanteer and C. Lentz: Reinforcement studies-effect of silica‘ structure on properties and crosslink density ,' Rubber Chem. Technol.,1975,48, (5), 795 809.–
28. A. Menon and L. Visconte: Self crosslinkable blends of polychlor-‘ oprene and phosphorylated cashew nut shell liquid prepolymer ,' J. Appl. Polym. Sci.,2004,91, (3), 1619 1625.–
29. V. Arjunan, S. Subramanian and S. Mohan: Vibrational spectro-‘ scopic studies on trans-1, 4-polychloroprene ,' Turk. J. Chem., 2003,27, (4), 423 432.–
![Table 4 shows the comparative effect of ALK with that ofETU on the mechanical properties of CB- lled CR vulca-finisates.[20] At a similar loading i.e](https://thumb-ap.123doks.com/thumbv2/123dok/3907883.1860193/5.595.76.273.582.672/table-comparative-effect-mechanical-properties-nisates-similar-loading.webp)