Material properties
The effect of the addition of alkanolamide on properties of carbon
black-
fi
lled natural rubber (SMR-L) compounds cured using various
curing systems
Indra Surya
a, H. Ismail
b,*aDepartment of Chemical Engineering, Engineering Faculty, University of Sumatera Utara, Medan, 20155, Sumatera Utara, Indonesia
bSchool of Materials and Mineral Resources Engineering, Universiti Sains Malaysia, Engineering Campus, Nibong Tebal, 14300, Penang, Malaysia
a r t i c l e
i n f o
Article history:
Received 25 November 2015 Accepted 12 January 2016 Available online 21 January 2016
Keywords: Alkanolamide Curing systems Carbon black Filler dispersion Rubber-filler interaction
a b s t r a c t
The properties of carbon black (CB)-filled natural rubber (SMR-L) compounds, with and without the addition of Alkanolamide (ALK), based on various curing systems such as efficient, semi-efficient and conventional vulcanisation systems were investigated. The ALK loading wasfixed at 5.0 phr. It was found that ALK gave improvements to the cure rate, torque difference, crosslink density,filler dispersion, rubber efiller interaction and reinforcing efficiency of CB. ALK also enhanced the tensile modulus, hardness, resilience, tensile strength and elongation at break of CB-filled SMR-L vulcanisates for each curing sys-tem. The degree of improvement of cure characteristics and mechanical properties depended on the level of sulphur and the ratio of accelerator to sulphur in each system. Scanning electron microscopy (SEM) proved that the CB-filled SMR-L vulcanisates with ALK for each curing system displayed a greater matrix tearing line and surface roughness due to greater rubberefiller interaction.
©2016 Elsevier Ltd. All rights reserved.
1. Introduction
Through vulcanisation, weak and plastic raw rubbers are hard-ened or cured by sulphur and converted into strong elastic rubber vulcanisates. When vulcanisation wasfirst discovered, the sulphur reaction with sulphur alone took several hours to be completed. Utilisation of accelerators (organic sulphur donor ingredients), with the combination of other ingredients such as zinc oxide and fatty acids, allowed the sulphur reaction to be accomplished in a shorter time and was recognised as accelerated sulphur vulcanisation
[1e3]. Based on the level of sulphur and the ratio of accelerator to sulphur, accelerated sulphur vulcanisation can be classified into three categories: efficient (EV), semi-efficient (semi-EV) and con-ventional (CV) vulcanisation or curing systems.
The strength and elasticity of a rubber vulcanisate can be further enhanced by the addition of reinforcingfiller. Carbon black (CB) and silica are the most popular reinforcingfillers for rubbers, and have been widely employed in the rubber industry. CB is commonly utilised for producing black rubber products, while silica is used in coloured products. Sometimes, they are also utilised in
combination form (as hybridfiller) for the purpose of achieving their synergistic effect in order to produce better overall mechan-ical properties[4]. However, at a relatively higher loading of CB or silica, thefiller particles tend to form agglomerates and will reduce the properties of the rubber vulcanisates. Practically, to overcome thefiller dispersion problem, special additives such as processing aids, dispersant aids, etc. are utilised.
In our previous work[5], the preparation and application of Alkanolamide (ALK) in silica-filled SMR-L compounds was re-ported. The ALK enhanced the tensile properties and hardness of the silica-filled SMR-L vulcanisates. The enhancement of these properties was attributed to the improvement of silica dispersion and the excelling crosslink density that stemmed from the incor-poration of ALK. The results also indicated that ALK could function as an accelerator and internal plasticiser.
The comparison of ALK and aminopropyltriethoxy Silane (APTES)-silane coupling agent on the properties of silica-filled SMR-L compounds was also reported [6]. Due to its combined and unique function as an accelerator and internal plasticiser, ALK produced a higher reinforcing efficiency than APTES at a similar loading.
A further study regarding the effect of ALK loading on properties of CB-filled SMR-L, epoxidised natural rubber (ENR) and styrene *Corresponding author.
E-mail addresses:ihanafi@usm.my,profhanafi@gmail.com(H. Ismail).
Contents lists available atScienceDirect
Polymer Testing
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / p o l y t e s t
http://dx.doi.org/10.1016/j.polymertesting.2016.01.014
butadiene rubber (SBR) compounds revealed that ALK gave cure enhancement, better filler dispersion and greater rubberefiller interaction to three different types of rubbers[7]. ALK enhanced the mechanical properties, especially up to 5.0 phr of ALK in SMR-L and SBR compounds, and at 1.0 phr of ALK in ENR-25 compound.
It is important to further investigate the applicability of ALK as a new rubber additive in rubber vulcanisation. Hence, through the examination of the properties of CB-filled SMR-L compounds in the presence of ALK, the applicability of ALK in vulcanisation of CB-filled SMR-L compounds with various curing systems was stud-ied. This study focused on the cure characteristics and mechanical properties of CB-filled SMR-L compounds with and without ALK, which were cured by EV, semi-EV and CV systems.
2. Experimental
2.1. Materials
NR grade SMR-L was used and obtained from Guthrie (M) Sdn. Bhd., Seremban, Malaysia, and N330-grade CB was supplied by the Cabot Corporation. Other compounding ingredients such as sulphur (S), zinc oxide (ZnO), stearic acid, N-isopropyl-N'-phenyl-p-phenylenediamine (IPPD), benzothiazyl disulfide (MBTS) were supplied by Bayer Co. (M) Sdn. Bhd., Petaling Jaya, Selangor, Malaysia. The ALK was synthesised in the laboratory using Refined Bleached Deodorized Palm Stearin (RBDPS) and diethanolamine. The reaction procedures and molecular characterisations of the ALK were given in our previous report[5]. The molecular structure of ALK is presented inFig. 1.
2.2. Compounding
The EV, semi-EV and CV vulcanisation recipes were applied in rubber compounding. The compounding procedure was performed on a two-roll mill (Model XK-160).Table 1displays the compound formulation of CB-filled SMR-L compounds with ALK and without ALK at various curing systems.
2.3. Cure characteristics
The cure characteristics of the CB-filled SMR-L compounds with and without ALK at various curing systems were obtained using a Monsanto Moving Die Rheometer (MDR 2000), which was employed to determine the scorch time (ts2), cure time (t90) and
torque difference (MHdML) according to ISO 3417. Samples of the
respective compounds were tested at 150C. The CB-filled SMR-L compounds were subsequently compression-moulded using a stainless steel mould at 150C, with a pressure of 10 MPa, and applying a laboratory hot-press based on respective curing times.
2.4. Measurement of crosslink density
Swelling tests on the CB-filled SMR-L vulcanisates were per-formed in toluene in accordance with ISO 1817. The cured test pieces (30 mm5 mm2 mm) were weighed using an electric balance and swollen in toluene until equilibrium, which took 72 h at room temperature. The samples were taken out from the liquid,
the toluene was removed from the sample surfaces and the weight was determined. The samples were then dried in an oven at 60C until constant weight was obtained. The swelling results were used to calculate the molecular weight between two crosslinks (Mc) by applying the FloryeRehner equation[8].
Mc¼ rpVsV volume fraction of the rubber in the swollen specimen,Qmis the weight increase of the vulcanisate in toluene and
c
is the interac-tion parameter of the rubber networkesolvent (c
of SMR-L¼0.393). The crosslink density is given by;Vc¼2Mc1 (3)
2.5. Tensile, hardness and resilience properties
Dumbbell-shaped samples were cut from the moulded sheets. Tensile tests were performed at a cross-head speed of 500 mm/min using an Instron 3366 universal tensile machine, according to ISO 37. The tensile strength (TS), stress at 100% elongation (M100), stress at 300% elongation (M300) and elongation at break (EB) were determined. The hardness measurements of the samples were performed according to ISO 7691-I, using a Shore A type manual durometer. The resilience was studied by using a Wallace Dunlop Tripsometer, according to BS 903 Part A8. The rebound resilience was calculated according to Equation(4).
% Resilience¼ ½ð1 cosq2Þ=ð1 cosq1Þ 100 (4)
where
q1
is the initial angle of displacement (45) andq2
is the maximum rebound angle.2.6. Scanning electron microscopy (SEM) analysis
The tensile fractured surfaces of the CB-filled SMR-L with and without ALK at various curing systems were examined by using a Zeiss Supra-35VP scanning electron microscope (SEM) to obtain information regarding thefiller dispersion, rubberefiller interac-tion and to detect the possible presence of micro-defects. The
Fig. 1.Molecular structure of Alkanolamide.
Table 1
aparts per hundred parts of rubber.
b5.0 phr was the optimum loading of ALK for CB-filled SMR-L compound with a
fractured pieces were coated with a layer of gold to eliminate electrostatic charge build-up during analysis.
2.7. Measurement of rubberefiller interaction
The rubberefiller interactions were determined by swelling the cured CB-filled SMR-L compounds in toluene, according to ISO 1817. Test pieces with dimensions of (30 mm5 mm2 mm) were prepared from the moulded sheets. The initial weights were recorded prior to testing. The test pieces were then immersed in toluene and conditioned at room temperature in a dark environ-ment for 72 h. After the conditioning period, the weights of the swollen test pieces were recorded. The swollen test pieces were then dried in the oven at 70C for 15 min and were allowed to cool at room temperature for another 15 min before thefinal weights were recorded. The Lorenz and Park's equation[9e11]was applied in this study. The swelling index was calculated according to Equation(5).
Qf=Qg¼ae zþb (5)
where the subscripts f and g referred tofilled and gum vulcanisates, respectively; z was the ratio by weight of filler to hydrocarbon rubber in the vulcanisate; while a and b were constants. The lower the Qf/Qg value, the greater the rubberefiller interaction becomes. In this study, the weight of the toluene uptake per gram of hy-drocarbon rubber (Q) was calculated based on Equation(6).
Q ¼ ½Swollen Dried weight=½Initial weight
100=Formula weight (6)
3. Results and discussion
3.1. The cure characteristics and crosslink density
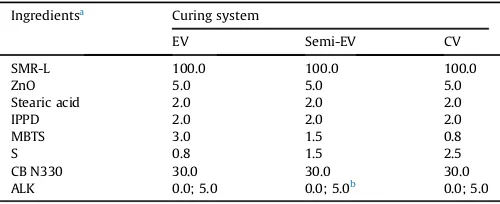
The cure characteristics of CB-filled SMR-L compounds, with and without the presence of ALK at various curing systems, are shown inFigs. 2 and 3andTable 2. The addition of 5.0 phr of ALK into the CB-filled SMR-L compound for each curing system caused a decrease in scorch and cure times and an increase of torque dif-ference. Since amine is an ingredient of accelerators and also an accelerator activator[12], the amine part of ALK, together with ZnO and fatty acid, activated the MBTS-accelerator more pronouncedly and, consequently, improved the rate of sulphur reaction of CB-filled SMR-L compounds. The increase of torque difference value was attributed to the additional function of ALK, as an internal
plasticiser agent, which plasticised and softened thefilled com-pounds. This resulted in reduced viscosity and improved process-ability due to the CB dispersion and SMR-LeCB interaction. The SMR-LeCB interaction may be defined as additional physical crosslinks[13,14]and, together with sulphide crosslinks, contrib-uted to total crosslink density[15,16]of the CB-filled SMR-L com-pound. Degree of crosslink density of a rubber vulcanisate was indicated by its own torque difference value[17e20]. The higher
the torque difference value, the higher the degree of crosslink density.
It was also observed that the scorch times of CB-filled com-pounds, with and without ALK, decreased when the curing system was changed from EV to Semi-EV and CV. All curing systems used MBTS as the accelerator, and it was functionally classified as a primary accelerator which usually provides scorch delay to a rub-ber compound[21]. The lower the amount of MBTS, the lower was the scorch safety. This explained why the scorch times tended to slightly decrease when the curing system was changed through the above sequence.
When the curing system was changed from EV to Semi-EV and CV, the cure time and torque difference tended to increase. A possible explanation may be due to the effect of sulphur content of each curing system. The EV system possesses the least amount of sulphur and the CV system possesses the greatest. The higher amount of sulphur requires longer time to complete the sulphur-isation, or crosslinking reaction, hence it produces higher crosslink density. This explains why the cure time and torque difference of the CB-filled SMR-L compounds, with and without ALK, increased in sequence: EV, Semi-EV and CV.
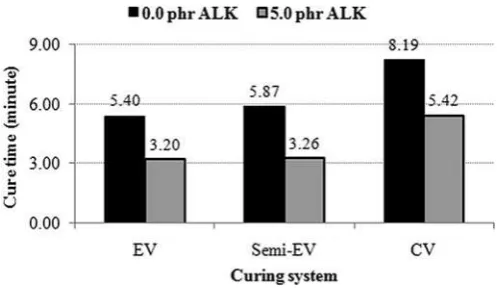
Fig. 4 displays the crosslink density of CB-filled SMR-L com-pounds, with and without the presence of ALK, for various curing systems. The crosslink density was determined by the Flor-yeRehner approach [Eq.(1)]. The addition of 5.0 phr of ALK into the CB-filled SMR-L compounds increased the crosslink density. This observation was in line with the data inTable 2. Torque difference
Fig. 2.Scorch times (ts2) of the CB-filled SMR-L compounds at various curing systems.
Fig. 3.Cure times (t90) of the CB-filled SMR-L compounds at various curing systems.
Table 2
Torque difference properties of the CB-filled and unfilled SMR-L compounds at various curing systems.
Curing systems Loading of ALK (phr) Torque difference properties
values of CB-filled compounds with ALK were higher than those of CB-filled compounds without ALK. This indicated that the crosslink density of CB-filled compounds with ALK was higher than that of CB-filled compounds without ALK.
The crosslink density of thefilled compounds with and without ALK increased when the curing system was changed from EV to Semi-EV and CV. This was simply due to the sulphur content of each curing system. A curing system with higher sulphur content would produce a higher crosslink density[22].
3.2. Thefiller dispersion
The degree of CB dispersion in SMR-L compounds using various curing systems, due to the addition of ALK, can be quantitatively determined by Equation(7) [5e7,23,24].
L¼hr mr (7)
where:
h
r¼[MLf/MLg], and mr¼[MHf/MHg]; where MLfand MHfwere the minimum and maximum torques of thefilled compounds, and MLgand MHgwere the minimum and the maximum torques of
the unfilled/gum rubber compound. A lower value of L, at a particular CB loading, meant a better degree of CB dispersion. The cure characteristics of gum compounds of SMR-L using different curing systems (i.e. EV, Semi-EV and CV; MLGand MHG) were 0.05,
0.07 and 0.05 (MLG); and 4.85, 4.88 and 5.91 (MHG), respectively. Fig. 5presents the values of L for CB dispersion in the SMR-L phase, with and without ALK, using various curing systems. It can be seen that the L values of CB-filled compounds with ALK were lower than those of CB-filled compounds without ALK. The reduced
values of L indicated that ALK had improved the CB dispersion through its plasticisation effect in CB-filled SMR-L compounds.
The L values of CB-filled compounds, with and without ALK, decreased from EV to Semi-EV and CV. This meant that the degrees of CB dispersion were the lowest in EV and the highest in CV. This phenomenon was also attributed to the sulphur content, since CB reacts with sulphur during the curing process and forms CB-sulphur bonds that link the rubber chains and tie thefiller onto the rubber[25]. This is rubber-filler crosslinking which is consid-ered as another type of crosslink to the rubber system, and defined as coupling bonds [25,26]. The higher the sulphur content, the higher the solubility of CB in the sulphur phase, and hence the higher the degree of CB dispersion.
3.3. The rubberefiller interaction
Improved filler dispersion means greater rubberefiller in-teractions. Based on Lorenz and Park's equation (Equation(5)), the rubberefiller interaction of CB-filled SMR-L compounds at various curing systems is presented inFig. 6. It can be seen that the Qf/Qg values decreased with the addition of ALK for all various curing systems. The decreased Qf/Qg indicated that the rubberefiller interaction in CB-filled SMR-L systems became greater, which was attributed to the capability of ALK to plasticise and soften the CB-filled SMR-L compounds and, therefore, improving the CB dispersion.
The rubber-filler interaction of the filled SMR-L compounds, with and without ALK, was enhanced from EV, Semi-EV and CV. Again, this was attributed to the sulphur content of the curing system. The higher the sulphur content, the more pronounced the sulphureCB interaction, and hence the better the CB dispersion and the greater the SMR-L-CB interaction.
3.4. The reinforcing efficiency (RE)
The degree of reinforcement provided by the filler can be calculated through its reinforcing efficiency (RE), which in its simplest form, was given by Equation(8) [6].
RE¼ ðMH MLÞf ðMH MLÞg=ðMH MLÞg (8)
in which:
(MH ML)f¼difference in torque value offilled compound
(MH ML)g ¼ difference in torque value of unfilled/gum
compound
A higher RE value meant greater rubber-filler interaction, which
Fig. 4.Crosslink density of the CB-filled SMR-L compounds at various curing systems.
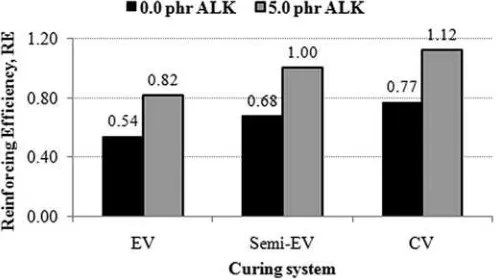
was influenced by the degree offiller dispersion. The improved filler dispersion provided a greater surface area for rubberefiller interactions. RE of CB on SMR-L compounds, with and without ALK, at various curing systems is shown inFig. 7.
As presented inFig. 7, ALK increased the RE of CB on SMR-L compounds. This was attributed to the combined effects of better filler dispersion and greater rubberefiller interaction.
The RE values of CB, with and without ALK, were the lowest in EV and were the highest in CV. This was due to the lowest degree of CB dispersion and the weakest rubber-filler interaction in EV, and the highest degree of CB dispersion and the greatest rubber-filler interaction in CV.
3.5. The mechanical properties
Table 3showed the mechanical properties of CB-filled SMR-L, with and without the addition of ALK, for various curing systems. Obviously, the tensile modulus (M100 and M300), hardness, resil-ience, tensile strength and elongation at break were significantly increased using various curing systems with the addition of ALK.
Tensile modulus and hardness of a rubber vulcanisate are mainly dependent on the degree of crosslinking[27,28]. Resilience is enhanced, to some extent, as the crosslink density rises[21,29]. Hence, the enhancements of M100, M300, hardness and resilience were attributed to the enhancement of crosslink density, as dis-played inFig. 4.
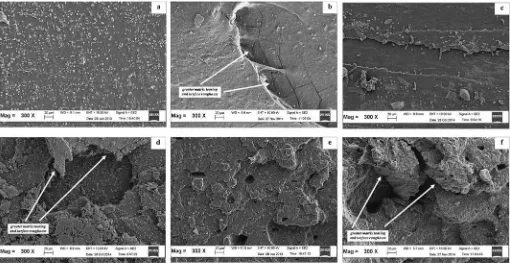
The enhancement in tensile strength was attributed to a higher RE, or the combined effects of betterfiller dispersion and greater rubberefiller interaction. This explanation was in line with the results inFigs. 5e7and the SEM micrographs later inFig. 8. The micrographs of CB-filled SMR-L vulcanisates with ALK exhibited greater matrix tearing lines and surface roughness. This indicated greater rubberefiller interaction which altered the crack paths, leading to increased resistance to crack propagation, thus causing an increase in tensile strength.
The elongations at break of CB-filled SMR-L compounds with ALK were higher than those of CB-filled compounds without ALK. Again, this was attributed to the function of ALK as an internal plasticiser agent which modified theflexibility of CB-filled SMR-L vulcanisates. The ALK provided a free volume which allowed more flexibility for the SMR-L chains to move.
The mechanical properties of CB-filled SMR-L vulcanisates, with and without ALK, of the CV system were the greatest due to the highest degree offiller dispersion, greatest rubber-filler interaction, and hence highest RE. The mechanical properties of CB-filled SMR-L vulcanisates, with and without ALK, of the EV system were the lowest due to the lowest degree of filler dispersion, weakest rubber-filler interaction, and hence lowest RE.
3.6. Scanning electron microscopy (SEM) study
Fig. 8displays the SEM micrographs of fractured surfaces of the vulcanisates of CB-filled SMR-L, with and without ALK, for various curing systems, taken at 300 magnification. It can be clearly observed that the CB-filled SMR-L vulcanisates with 5.0 phr of ALK for each curing system (micrographs of Fig. 8(b), (d) and (f)) exhibited greater matrix tearing lines and surface roughness compared to those of CB-filled SMR-L vulcanisates without ALK (Fig. 8(a), (c) and (e)). This indicated betterfiller dispersion and greater rubberefiller interaction, and the micrographs of the tensile fractured surfaces were in good agreement with the graphs in
Figs. 5 and 6, which showed the lower L and Qf/Qg values of CB-filled SMR-L compounds with ALK. An enhancement in rupture energy, due to a greater rubberefiller interaction, was responsible for the roughness and the matrix tearing line of the fractured sur-face. The micrographs of the tensile fractured surfaces were in good agreement with the results obtained by other researchers[30,31]
who reported that an increase in rupture energy was responsible for the roughness and the matrix tearing line of the fractured surfaces.
4. Conclusions
From this study, the following conclusions were drawn:
1. Alkanolamide increased the cure rate, torque difference value, crosslink density, degree of filler dispersion, rubberefiller interaction and reinforcing efficiency of efficient, semi-efficient and conventional curing systems of carbon black-filled natural rubber (SMR-L) compounds.
2. Alkanolamide also improved the tensile modulus, hardness, resilience, tensile strength and elongation at break of the effi -cient, semi-efficient and conventional curing systems of carbon black-filled natural rubber (SMR-L) compounds.
3. Degree of improvement of the cure characteristics and me-chanical properties of carbon black-filled natural rubber (SMR-L) compounds with Alkanolamide depended on the curing
Fig. 7.Reinforcing efficiency of the CB-filled SMR-L compounds at various curing systems.
Table 3
The mechanical properties of CB-filled SMR-L compounds at various curing systems.
Curing systems Loading of ALK (phr) Mechanical properties
M100 (MPa) M300 (MPa) TS (MPa) EB (%) Hardness (Shore A) Resilience (%)
system, especially the level of sulphur and ratio of accelerator to sulphur of each curing system.
4. Morphological studies of the tensile fractured surfaces of carbon black-filled natural rubber (SMR-L) vulcanisates of each curing system with Alkanolamide exhibited a greater matrix tearing line and surface roughness due to greater rubberefiller interaction.
Acknowledgements
The authors would like to thank Universiti Sains Malaysia for providing the research facilities for carrying out the experiment and for making this research work possible. One of the authors (Indra Surya) is grateful to the Directorate General of Higher Edu-cation (DIKTI) Tahun Anggaran 2011, Ministry of EduEdu-cation and Culture (Kemdikbud) of the Republic of Indonesia, for the award of a scholarship under thefifth batch of the Overseas Postgraduate Scholarship Program.
References
[1] M. Morton, Introduction to Rubber Technology, Reinhold Pub. Corp., New York, 1959.
[2] M. Morton, Rubber Technology, Van Nostrand Reinhold Company, New York, 1987.
[3] M. Akiba, A. Hashim, Vulcanization and crosslinking in elastomers, Prog. Polym. Sci. 22 (1997) 475e521.
[4] N. Rattanasom, T. Saowapark, C. Deeprasertkul, Reinforcement of natural rubber with silica/carbon black hybridfiller, Polym. Test. 26 (2007) 369e377.
[5] I. Surya, H. Ismail, A. Azura, Alkanolamide as an accelerator,filler-dispersant and a plasticizer in silica-filled natural rubber compounds, Polym. Test. 32 (2013) 1313e1321.
[6] I. Surya, H. Ismail, A. Azura, The comparison of alkanolamide and silane coupling agent on the properties of silica-filled natural rubber (SMR-L) com-pounds, Polym. Test. 40 (2014) 24e32.
[7] I. Surya, H. Ismail, A. Azura, The effect of alkanolamide loading on properties of carbon black-filled natural rubber (SMR-L), epoxidised natural rubber (ENR), and styrene-butadiene rubber (SBR) compounds, Polym. Test. 42 (2015) 208e214.
[8] P.J. Flory, J. Rehner Jr., Statistical mechanics of crosslinked polymer networks II. Swelling, J. Chem. Phys. 11 (1943) 521.
[9] O. Lorenz, C. Parks, The crosslinking efficiency of some vulcanizing agents in natural rubber, J. Polym. Sci. 50 (1961) 299e312.
[10] H. Ismail, M. Nasaruddin, U. Ishiaku, White rice husk ashfilled natural rubber compounds: the effect of multifunctional additive and silane coupling agents, Polym. Test. 18 (1999) 287e298.
[11] H. Ismail, S. Shaari, N. Othman, The effect of chitosan loading on the curing characteristics, mechanical and morphological properties of chitosan-filled natural rubber (NR), epoxidised natural rubber (ENR) and styrene-butadiene rubber (SBR) compounds, Polym. Test. 30 (2011) 784e790.
[12] H. Long, Basic Compounding and Processing of Rubber, Rubber Division, American Chemical Society Inc. The University of Akron, Ohio, USA, 1985. [13] B. Boonstra, G. Taylor, Swelling offilled rubber vulcanizates, Rubber Chem.
Technol. 38 (1965) 943e960.
[14] R. Nunes, J. Fonseca, M. Pereira, Polymerefiller interactions and mechanical
properties of a polyurethane elastomer, Polym. Test. 19 (2000) 93e103.
[15] G. Kraus, Reinforcement of Elastomers, John Wiley&Sons Inc., New York, 1965.
[16] K. Polmanteer, C. Lentz, Reinforcement studies-effect of silica structure on properties and crosslink density, Rubber Chem. Technol. 48 (1975) 795e809.
[17] B. Boonstra, H. Cochrane, E. Dannenberg, Reinforcement of silicone rubber by particulate silica, Rubber Chem. Technol. 48 (1975) 558e576.
[18] H. Cochrane, C. Lin, The influence of fumed silica properties on the processing, curing, and reinforcement properties of silicone rubber, Rubber Chem. Tech-nol. 66 (1993) 48e60.
[19] H. Ismail, C. Ng, Palm oil fatty acid additives (POFA's): preparation and application, J. Elastom. Plast. 30 (1998) 308e327.
[20] P. Teh, Z. Mohd Ishak, A. Hashim, J. Karger-Kocsis, U. Ishiaku, Effects of epoxidized natural rubber as a compatibilizer in melt compounded natural rubbereorganoclay nanocomposites, Eur. Polym. J. 40 (2004) 2513e2521.
[21] B. Rodgers, Rubber Compounding: Chemistry and Applications, CRC, New York, 2004.
[22] H. Ismail, I. Salmiah, Y. Tsukahara, Palm oil fatty acid as an activator in carbon blackfilled natural rubber compounds: effect of vulcanization system, Polym. Int. 44 (1997) 523e529.
[23] B. Lee, Reinforcement of uncured and cured rubber composites and its rela-tionship to dispersive mixing-an interpretation of cure meter rheographs of carbon black loaded SBR and cis-polybutadiene compounds, Rubber Chem. Technol. 52 (1979) 1019e1029.
[24] P. Pal, S. De, Effect of reinforcing silica on vulcanization, network structure, and technical properties of natural rubber, Rubber Chem. Technol. 55 (1982) 1370e1388.
[25] M.Q. Fetterman, The unique properties of precipitated silica in the design of high performance rubber, Elastomerics 116 (1984) 18e31.
[26] M. Fetterman, Precipitated silicaecoming of age, Rubber World 194 (1986)
38e40.
[27] D.L. Hertz Jr., S.E. INC, Theory &practice of vulcanization, Elastomerics (November 1984) 1e7.
[28] H. Ismail, H. Chia, The effects of multifunctional additive and epoxidation in silicafilled natural rubber compounds, Polym. Test. 17 (1998) 199e210.
[29] W. Hofmann, F. Bayer, Vulcanization and vulcanizing agents, Maclaren (1967) 143e147.
[30] H. Ismail, M. Mathialagan, Comparative study on the effect of partial replacement of silica or calcium carbonate by bentonite on the properties of EPDM composites, Polym. Test. 31 (2012) 199e208.