Electro Chemical Machining (ECM) Gambar 1. Electro Chemical Machining
• Pengertian Electro Chemical Machining (ECM)
• Electro Chimical Machining (ECM) adalah sebuah metode untuk mengolah bentuk logam melalui proses elektrokimia ( proses elektrolisis dan proses volta).
• Pada ECM proses elektrokimia yang digunakan adalah proses elektrolisis yaitu proses yang dapat mengubah energi lisrik menjadi energi kimia.
• Proses Elektrolisis Menggunakan Hukum Faraday I Dan II Gambar 2. Prinsip Kerja ECM
adalah Prinsip Kerja ECM Benda kerja dihubungkan dengan
sumber arus searah yang bermuatan positif sedangkan pahat dihubungkan dengan sumber arus yang bermuatan negatif dan cairan elektrolit dialirkan diantara pahat dan benda kerja. Sehingga terjadilah proses pengerjaan material benda kerja karena adanya reaksi elektrokimia dan juga reaksi kimia. Electro Chemical Machining (ECM)
• Proses ECM akan Berlangsung apabila memenuhi syarat - syarat sebagai berikut : Pahat bermuatan negatif dan benda kerja bermuatan positif Celah antara pahat dan benda kerja yang berfungsi sebagai aliran cairan elektrolit (sel elektrolit) Sel elektrolit yang terbentuk diantara pahat dengan benda kerja inilah yang membentuk terjadinya reaksi elektrokimia dan reaksi kimia.
Electro Chemical Machining (ECM) Gambar 3. Pahat katoda (di atas) dan anode benda kerja(di bawah).
Electro Chemical Machining (ECM)
• Fungsi dari cairan elektrolit dalam proses ECM, yaitu
• Sebagai media untuk memungkinkan terjadinya proses pengerjaan material.
• Sebagai fluida pendingin selama proses ECM berlangsung
• Untuk menghanyutkan bagian-bagian daripada material benda kerja yang telah dikerjakan.
• Pemilihan cairan elektrolit berdasarkan beberapa faktor sebagai berikut:
• Bersifat sebagai konduktor listrik
• Tidak korosif terhadap peralatan
• Tidak beracun dan tidak membahayakan operator
• Mempunyai sifat kimia yang stabil, sehingga memungkinkan terjadinya reaksi elektro kimia yang stabil selama proses ECM beerlangsung.
Electro Chemical Machining (ECM) Gambar 4 Rotor dibentuk oleh ECM Hasil Produk ECM
Gamba 5. Braket dibentuk oleh ECM Gambar 6.Sensor dibentuk oleh ECM Electro Chemical Machining (ECM)
• Kesimpulan:
• Proses ECM bisa dipergunakan untuk segala macam metal, paduan logam dan material bersifat konduktor listrik. Komposisi dan struktur kimia, titik lelah, kekerasan dan sifat-sifat fisik material lainnya tidak mempengaruhi proses pengerjaan ECM.
• Bentuk permukaanbenda kerja yang kompleks dapat dikerjakan dengan proses ECM sehingga proses ini cocok untuk pembuatan cetakan
• Proses pengerjaan dengan ECM meliputi operasi-operasi, diantaranya: finishing, deburring, honing,countouring,deep hole drilling.
• Proses pengerjaan dengan ECM bebas dari segala bentuk tegangan maupun geramsehingga memungkinkan tidak terjadinya circuit-circuit antara pahat dan benda kerja.
• Surface finish yang bisa dicapai dalam proses ECM berkisar 0,2-0,8 μ m.
Pemesinan elektrokimia (ECM) adalah sebuah metode untuk menciptakan bentuk logam dengan menghilangkan logam menggunakan proses elektrokimia. Sebuah arus langsung dengan kepadatan tinggi dan tegangan rendah melewati antara benda (anoda) dan pra-alat berbentuk
(katoda). Pada permukaan benda kerja anodik, logam dibubarkan dan bentuk alat sehingga akan disalin ke dalam benda kerja.
Pemesinan elektrokimia menciptakan komponen yang tidak dikenakan baik panas atau stres mekanik dan rapuh dengan mesin bahan dapat dengan mudah karena tidak ada kontak antara alat dan benda kerja. Pemesinan elektrokimia normal dan dapat membuat bentuk 3D yang halus. Beberapa contoh bagian yang dibuat menggunakan mesin elektrokimia meliputi dies, molds, turbin dan kompresor pisau, lubang, lubang, slot, dll pemesinan elektrokimia proses dapat melaksanakan sebagian besar jenis bahan dan paduan. Custom tooling yang diperlukan di dalam negatif dari bentuk bagian yang diinginkan.
Pemesinan elektrokimia (ECM) adalah sebuah metode untuk menghilangkan logam dengan proses elektrokimia. Hal ini biasanya digunakan untuk produksi massal dan digunakan untuk bekerja keras materi atau bahan yang sulit untuk mesin dengan menggunakan metode konvensional. Penggunaannya terbatas pada bahan konduktif listrik, namun, ini termasuk semua logam. ECM dapat memotong kecil atau menbentuk sudut aneh, rumit kontur atau rongga dalam sangat keras. baja dan eksotis logam seperti titanium, hastelloy, kovar, inconel dan karbida.
ECM sering dicirikan sebagai "reverse elektroplating," dan yang serupa dalam melaksanakan konsep mesin listrik dalam arus tinggi terjadi antara elektroda dan bagian, melalui proses removal material elektrolit yang mempunyai elektroda bermuatan negatif (katoda), konduktif fluida (electolyte), dan sebuah benda konduktif (anoda), namun pada ECM tidak ada alat yang dipakai. ECM pemotongan alat dipandu sepanjang jalan yang diinginkan sangat dekat dengan pekerjaan, tetapi itu tidak menyentuh bagian. Berbeda dengan EDM , dimana tidak ada percikan api diciptakan. Pemindahan logam sangat tinggi tingkat yang dapat dilakukan dengan ECM, bersama tanpa termal atau tekanan mekanis dipindahkan ke bagian tersebut, dan penutup permukaan cermin mungkin.
Dalam proses deburring, yang ECM menggunakan teknik seperti yang dijelaskan di atas untuk menghapus potongan-potongan besi yang tersisa dari proses permesinan, dan untuk menumpulkan tepi yang tajam. Proses ini sangat cepat dan jauh lebih nyaman daripada metode konvensional deburring dengan tangan atau proses permesinan non-tradisional. Ia akan cenderung
untuk meninggalkan finishing permukaan yang lebih baik, dan tidak ada deformasi logam akan terjadi karena potongan alat tidak benar-benar menyentuh logam.
Kegunaan electrochemical machining
Kegunaan electrochemical machining
Deburring, atau merapikan, dari permukaan, adalah sederhana dan penggunaan yang umum ECM. Sebuah pesawat katoda berwajah alat ini ditempatkan berlawanan sebuah benda yang memiliki permukaan yang tidak teratur. Kepadatan arus di puncak ketidakteraturan permukaan lebih tinggi daripada di lembah-lembah. Yang pertama adalah, oleh karena itu, dihapus preferentially dan benda kerja menjadi mulus, diakui dengan mengorbankan saham logam (yang masih dengan mesin dari lembah-lembah dari penyimpangan, meskipun pada tingkat yang lebih rendah). Merapikan elektrokimia adalah satu-satunya jenis ECM di mana bentuk anoda akhir mungkin cocok persis bahwa dari alat katoda.
Elektrokimia deburring adalah proses cepat; khas kali untuk meratakan permukaan komponen yang diproduksi adalah 5 hingga 30 detik. Karena kecepatan dan kesederhanaan operasi,
Pengeboran Lubang
Lubang pengeboran adalah cara pelaku lainnya menggunakan ECM (Gambar 2). Katoda-Alat ini biasanya dibuat dalam bentuk sebuah tabung elektroda. Elektrolit dipompa ke pusat menanggung dari alat, di seberang celah mesin utama, dan keluar di antara sidegap yang terbentuk antara dinding dari alat dan lubang. Pembalikan aliran elektrolit yang cukup sering dapat menghasilkan peningkatan dalam akurasi mesin.
Mesin utama tindakan dilakukan di dalam celah yang terbentuk antara tepi terkemuka alat bor dan pangkal lubang dalam benda kerja. ECM juga hasil lateral antara dinding sisi alat dan komponen, di mana kerapatan arus lebih rendah daripada di tepi terkemuka alat maju. Karena kesenjangan lebar lateral menjadi semakin lebih besar daripada yang di ujung tombak, sisi-ECM tingkat lebih rendah. Dampak keseluruhan dari sisi-ECM adalah untuk meningkatkan diameter lubang yang dihasilkan. Jarak antara dinding sisi benda kerja dan poros tengah dari alat katoda lebih besar
daripada jari-jari eksternal katoda. Perbedaan ini dikenal sebagai "overcut". Jumlah overcut dapat dikurangi dengan beberapa metode. Prosedur umum melibatkan isolasi dinding eksternal dari alat, yang menghambat aliran arus samping. Praktek lain terletak pada pilihan elektrolit seperti natrium nitrat, yang memiliki efisiensi arus terbesar di kerapatan tertinggi saat ini. Dalam pengeboran lubang kepadatan arus tinggi ini terjadi antara tepi terkemuka bor dan dasar benda kerja. Jika elektrolit lain seperti natrium klorida yang menggunakan overcut bisa jauh lebih besar. Efisiensi saat ini untuk natrium klorida tetap stabil pada hampir 100% untuk beraneka ragam kepadatan arus. Jadi, bahkan di sisi celah, pemindahan logam berlangsung pada tingkat yang terutama ditentukan oleh kerapatan arus, sesuai dengan hukum Faraday.
Lubang dengan diameter 0,05-75 milimeter telah dicapai dengan ECM. Untuk lubang 0,5-1,0 milimeter diameter, kedalaman hingga 110 milimeter telah dihasilkan. Pengeboran oleh ECM tidak
terbatas pada putaran lubang bentuk benda kerja ditentukan oleh alat elektroda
Kendali-bentuk membentuk
Kendali-bentuk memanfaatkan membentuk celah yang konstan di seluruh benda dan alat ini bergerak secara mekanis pada tingkat yang tetap ke arah benda kerja agar dapat menghasilkan jenis bentuk yang digunakan untuk produksi bilah kompresor dan turbin. Dalam prosedur ini, arus kepadatan setinggi 100 A / sentimeter persegi akan digunakan, dan di seluruh permukaan benda kerja, kerapatan arus tetap tinggi.
Aliran elektrolit memainkan peran lebih berpengaruh dalam membentuk bentuk penuh daripada pengeboran dan meratakan permukaan. Seluruh besar luas penampang dari benda kerja harus diberikan oleh elektrolit ketika mengalir di antara elektroda. Daerah yang lebih besar elektroda terlibat berarti bahwa tekanan pemompaan yang relatif lebih tinggi dan tingkat aliran volumetrik diperlukan.
Fitur utama dari penggilingan elektrokimia (EKG) adalah penggunaan roda penggiling di mana isolasi kasar, seperti berlian partikel, diatur dalam materi melakukan. Roda ini menjadi alat katoda. Nonconducting partikel yang bertindak sebagai spacer antara roda dan benda kerja, memberikan celah interelectrode konstan, yang melaluinya elektrolit adalah memerah.
Akurasi dicapai oleh EKG biasanya sekitar 0,125 milimeter. Sebuah Kelemahan dari EKG adalah hilangnya akurasi ketika di dalam sudut adalah tanah. Karena efek medan listrik, jari-jari lebih baik dari 0,25-0,375 milimeter jarang bisa dicapai.
Sebuah aplikasi luas elektrokimia grinding produksi alat pemotong tungsten carbide. EKG juga berguna dalam bagian rapuh grinding seperti jarum suntik.
Namun, manfaat Machining elektrokimia lebih baik dipahami dari perspektif manufacturability bukan sekadar dari perspektif fitur. Ketika sebuah komponen mempunyai persyaratan atau fitur materi yang sulit, atau bahkan tidak mungkin, untuk mesin dengan metode tradisional, ECM bisa menjadi alternatif yang memungkinkan untuk alasan berikut:
>>Penghapusan Tool defleksi - Fitur yang memerlukan akut / sudut tumpul pendekatan pemotong dan fitur yang memerlukan panjang tinggi-untuk-rasio pemotong berdiameter kandidat kuat untuk elektrokimia Machining. Hal ini karena perangkat ECM tidak datang ke kontak fisik langsung dengan benda dan sebagai hasilnya, tidak ada kekuatan yg menyebabkan pembelokan yang dapat menyebabkan alat lagu tentunya.
>>Permukaan pelestarian Integritas - Karena proses Machining Elektrokimia benar-benar bebas dari panas, pelestarian integritas permukaan dimaksimalkan. Penggunaan ECM meningkatkan pakai, ketahanan korosi, dan kelelahan logam perlawanan dengan meminimalkan permukaan yang tidak diinginkan berikut cacat
>>Proses penghapusan Menengah - elektrokimia Machining menghasilkan duri-bebas, inspeksi permukaan-siap selesai dalam satu berlalu. Oleh karena itu, proses sekunder seperti deburring, menggiling, dan tangan polishing tidak diperlukan.
>>Pelestarian Dimensional Integritas - Machining elektrokimia Karena benar-benar bebas dari stres mekanik, deformasi plastik dihilangkan. Lembar kerja yang tipis atau mudah dirusak oleh stres mekanis kandidat kuat untuk ECM.
>>Tidak terpengaruh oleh Bahan Kekerasan - Karena materi dihapus oleh anodik pembubaran bukan oleh stres mekanik, harga mesin tidak terpengaruh oleh materi kekerasan. Bahan konduktif listrik dapat mesin pada tingkat sampai dengan 0,33 inci (0,84 cm) / menit. Berikut paduan kekuatan-tinggi kandidat kuat untuk ECM.
Keuntungan dari
Electrochemical Machining :
- Komponen terhindar dari panas atau mekanis stres.-Tidak ada alat yang dipakai selama pemesinan elektrokimia.
-Non-kaku dan membuka lembar kerja dapat mesin dengan mudah karena tidak ada kontak antara alat dan benda kerja.
-Bentuk geometris yang kompleks dapat juga harus stabil dan tegas. Elektrolit harus disaring dengan hati-hati untuk mengeluarkan produk dari mesin dan sering kali harus dipanaskan dalam reservoir suhu yang tetap, misalnya 30oC (86oF), sebelum memasuki mesin aparat. Prosedur ini digunakan untuk menyediakan kondisi operasi konstan. Selama mesin memanas elektrolit dari aliran arus. Tindakan pencegahan harus diambil untuk menghindari suhu elektrolit yang tinggi yang dapat menyebabkan perubahan dalam elektrolit konduktivitas spesifik dan selanjutnya efek yang tidak diinginkan pada akurasi permesinan.berulang-ulang dan akurat
-Pemesinan elektrokimia adalah proses hemat waktu bila dibandingkan dengan mesin konvensional
-Bagian yang rapuh tidak bisa mengambil lebih banyak dan juga rapuh bahan yang cenderung untuk mengembangkan retakan pada mesin mesin dapat dengan mudah melalui mesin elektrokimia
KEUNGGULAN :
1. mampu membuat permukaan 3 dimensi yang rumit secara akurat 2. permukaan akhir halus karena ketiadaan bekas pahat/pemotong
3. keausan pahat nol sehingga 1 pahat membuat komponen dalam jumlah besar (produk masal) 4. tidak mempengaruhi benda kerja secara termal
KELEMAHAN :
1. media yang korosif sulit dikendalikan
2. sudut dalam yang tajam (R<0,2 mm) sulit dibuat 3. ongkos perkakas dan perangkat yang mahal 4. konsumsi energinya yang besar
5. laju produksinya dari sedang ke tinggi
6. mesin yang digunakan merupakan mesin – mesin yang berukuran besar
Prinsip kerja electrochemical machining
Pemesinan elektrokimia didasarkan pada prinsip-prinsip yang diuraikan. Seperti ditunjukkan dalam, benda kerja dan alat adalah anoda dan katoda, masing-masing, dari sebuah sel elektrolisis, dan beda potensial yang konstan, biasanya di sekitar 10 V, yang diterapkan di seberang mereka. Elektrolit yang sesuai, misalnya, berair natrium klorida (garam meja) solusi, dipilih sehingga bentuk katoda tetap tidak berubah selama elektrolisis. Elektrolit juga dipompa dengan laju 3-30 meter / detik, melewati celah antara elektroda untuk menghapus produk-produk dari mesin dan untuk mengurangi efek yang tidak diinginkan, seperti yang muncul dengan generasi gas katodik dan pemanas listrik. Tingkat di mana logam kemudian dihapus dari anoda kira-kira dalam proporsi terbalik dengan jarak antara elektroda. Sebagai pemesinan berlangsung, dan dengan gerakan simultan katoda pada tingkat yang khas, misalnya, 0,02 milimeter / detik menuju anoda, kesenjangan lebar sepanjang panjang elektroda secara bertahap akan cenderung ke nilai keadaan
tunak. Dengan kondisi tersebut, sebuah bentuk, kasar saling melengkapi dengan yang ada pada katoda, akan direproduksi di anoda. Celah tipikal lebar maka harus sekitar 0,4 milimeter. Menjadi memahami karakteristik dan prinsip kerja ECM, keuntungan harus dinyatakan dalam waktu singkat sebelum pergi lebih lanjut melalui proses machining:
• tingkat mesin logam tidak bergantung pada kekerasan material,
• bentuk rumit dapat mesin pada logam keras,
February 1, 2009
TUGAS II. TEKNOLOGI & PROSES MANUFAKTUR 1 Created by : dicky’s
8
Proses produksi yang melakukan penggabungan antara Listrik dan kimia disebut
Elektrochemical Machining. Proses produksi ada yang bersifat pengurangan atau penambahan dimensi melalui beragam cara. Sebagai contoh proses finishing banyak dilakukan dengan pelapisan dengan chrome atau nickel yang lebih umum disebut
electroplating. Menurut prinsip kerjanya maka tipe ini pun dapat dibagi menjadi dua yaituElectrochemical Machining dan Electrochemical Deburring and Gerinding
Electrochemical Machining
Electrochemical Machining(ECM) adalah salah satu tipe proses produksi yang
mana pengerjaan/pengolahan benda kerja dilakukan dengan jalan electrolisa dengan energy listrik dan medium elektrolit seperti asam sulphat, coppersulphat dan lainnya. Benda kerja difungsikan sebagai Anode dan bahan yang diuraikan seperti tembaga,chrome sebagai anode seperti gambar dibawah ini.
Besar kecilnya pengurangan atau penambahan dimensi sesuai dengan Hukum Farady yaitu “Massa yang berpindah merupakan fungsi dari arus (amphere),waktu ,jarak, luas permukaan dan sifat dari catode yang terkait dengan “e” atau beda potensial catode-anode maupun resistansi electrolitnya. ECM umumnya digunakan untuk memotong benda logam yang sangat keras dan sulit dimesin atau geometri benda kerja yang rumit.
Electrochemical machining (ECM)
Electrochemical machining (ECM) also uses electrical energy to remove material. An electrolytic cell is created in an electrolyte medium, with the tool as the cathode and the workpiece as the anode. A high-amperage, low-voltage current is used to dissolve the metal and to remove it from the workpiece, which must be electrically conductive. ECM is essentially a deplating process that utilizes the principles of electrolysis. The ECM tool is positioned very close to the workpiece and a low voltage, high amperage DC current is passed between the two via an electrolyte. Material is removed from the workpiece and the flowing electrolyte solution washes the ions away. These ions form metal hydroxides which are removed from the electrolyte solution by centrifugal separation. Both the electrolyte and the metal sludge are then recycled.
Unlike traditional cutting methods, workpiece hardness is not a factor, making ECM suitable for difficult-to-machine materials. Takes such forms as electrochemical grinding, electrochemical honing and electrochemical turning.
Electrochemical deburring is another variation on electrochemical machining designed to remove burrs and impart small radii to corners. The process normally uses a specially shaped electrode to carefully control the process to a specific area. The process will work on material regardless of hardness.
Advantages of Electrochemical Machining (ECM)
1. The components are not subject to either thermal or mechanical stress.
2. There is no tool wear during Electrochemical machining.
3. Non-rigid and open work pieces can be machined easily as there is no contact between the tool and workpiece.
4. Complex geometrical shapes can be machined repeatedly and accurately
5. Electrochemical machining is a time saving process when compared with conventional machining 6. During drilling, deep holes can be made or several holes at once.
7. ECM deburring can debur difficult to access areas of parts.
8. Fragile parts which cannot take more loads and also brittle material which tend to develop cracks during machining can be machined easily through Electrochemical machining
9. Surface finishes of 25 µ in. can be achieved during Electrochemical machining
Joseph McGeough
Institute for Integrated Micro and Nano Systems University of Edinburgh
Edinburgh, EH9 3JL, United Kingdom (July, 2005)
Michael Faraday’s early metallurgic researches, from 1818 to 1824, anticipated the developments which have led to widespread use today of alloy steels. Much effort has been expended to improve their performance for their service as cutting tools in machining. The aim has always been to yield higher rates of machining and to tackle recently developed harder materials on the principle that the tool material must be harder than the workpiece which is to be machined. Much progress has been made; however, in recent years some alloys, which are exceedingly difficult to machine by the conventional methods, have been produced to meet a demand for very high-strength, heat resistant materials. Moreover, these new materials often have to take a complex shape. A search has had to be made for alternative methods of machining since the evolution of suitable tooling has not kept pace with these advances.
Electrochemical machining (ECM) has been developed initially to machine these hard to machine alloys, although any metal can so be machined. ECM is an electrolytic process and its basis is the phenomenon of electrolysis, whose laws were established by Faraday in 1833. The first significant developments occurred in the 1950s, when ECM was investigated as a method for shaping high strength alloys. As of the 1990s, ECM is employed in many ways, for example, by automotive, offshore petroleum, and medical engineering industries, as well as by aerospace firms, which are its principal user.
Metal removal is achieved by electrochemical dissolution of an anodicallypolarized workpiece which is one part of an electrolytic cell in ECM. Hard metals can be shaped electrolytically by using ECM and the rate of machining does not depend on their hardness. The tool electrode used in the process does not wear, and therefore soft metals can be used as tools to form shapes on harder workpieces, unlike conventional machining methods. The process is used to smooth surfaces, drill holes, form complex shapes, and remove fatigue cracks in steel structures. Its combination with other techniques yields fresh applications in diverse industries. Recent advances lie in computer-aided tool design, and the use of pulsed power, which has led to greater accuracy for ECM-produced components.
Theoretical background
Since electrolysis is the basis of ECM, it must be understood before going further through the characteristics and other details of the process.
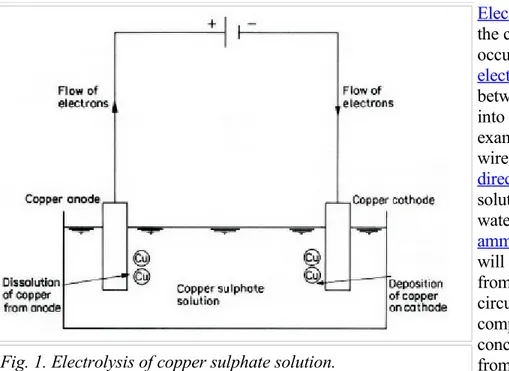
Electrolysis is the name given to the chemical process which occurs, for example, when an
electric current is passed between two conductors dipped into a liquid solution. A typical example is that of two copper wires connected to a source of
direct current and immersed in a solution of copper sulphate in water, as shown in Figure 1. An
ammeter, placed in the circuit, will register a flow of current; from this indication, the electric circuit can be deduced to be complete. A significant conclusion that can be made from an experiment of this sort is that the copper sulphate solution obviously has the property that it could conduct electricity. Such solution is termed an
electrolyte. The wires are called electrodes, the one with positive polarity being the anode, and the one with negative polarity the cathode. The system of electrodes and electrolyte is referred to as the electrolytic cell, whilst the chemical reactions which occur at the electrodes are called the anodic or cathodic reactions or processes.
Electrolytes are different from metallic conductors of electricity in that the current is carried not by electrons but by atoms, or group of atoms, which have either lost or gained electrons, thus acquiring either positive or negative charges. Such atoms are called ions. Ions which carry positive charges move through the electrolyte in the direction of the positive current, that is, toward the cathode, and are called cations. Similarly, the negatively charged ions travel toward the anode and are called
anions. The movement of the ions is accompanied by the flow of electrons, in the opposite sense to the positive current in the electrolyte, outside the cell, as shown also in Figure 2 and both reactions are a consequence of the applied potential difference, that is, voltage, from the electric source.
Fig. 1. Electrolysis of copper sulphate solution.
A cation reaching the cathode is neutralized, or discharged, by the negative electrons on the cathode. Since the cation is usually the positively charged atom of a metal, the result of this reaction is the deposition of metal atoms.
To maintain the cathodic reaction, electrons are required to pass around the external circuit. These are obtained from the atoms of the metal anode, and these atoms thus become the positively charged cations which pass into solution. In this case, the reaction is the reverse of the cathodic reaction.
The electrolyte in its bulk must be electrically neutral; that is, there must be equal numbers of opposite charges within it, and thus there must be equal amounts of reaction at both electrodes. Therefore, in the electrolysis of copper sulphate solution with copper electrodes, the overall cell reaction is simply the transfer of copper metal from the anode to the cathode. When the wires are weighted at the end of the experiment, the anodic wire will be found to have lost weight, whilst the cathodic wire will have increased in weight by an amount equal to that lost by the other wire. Some examples of the reactions occurring in these processes are shown in the Appendix.
These results are embodied in Faraday’s two laws of electrolysis:
1. The amount of any substance dissolved or deposited is directly proportional to the amount of electricity which has flowed.
2. The amounts of different substances deposited or dissolved by the same quantity of electricity are proportional to their chemical equivalent weights.
A popular application of electrolysis is the electroplating process in which metal coatings are deposited upon the surface of a cathodically polarized metal. An example of an anodic dissolution operation is electropolishing. Here, the item which is to be polished is made the anode in an electrolytic cell. Irregularities on its surface are dissolved preferentially so that, on their removal, the surface becomes flat and polished.
ECM is similar to electropolishing in that it also is an anodic dissolution process. But the rates of metal removal offered by the polishing process are considerably less than those needed in metal machining practice.
Some observations relevant to ECM can be made:
• Since the anode metal dissolves electrochemically, its rate of dissolution depends only upon the atomic weight and the ionic charge, the current which is passed, and the time for which the current passes. The dissolution rate is not influenced by the hardness or other characteristics of the metal.
• Since only hydrogen gas is evolved at the cathode, the shape that electrode remains unaltered during the electrolysis. This feature is perhaps the most relevant in the use of ECM as a metal shaping process.
In ECM, electrolytes serve as conductors of electricity and Ohm’s law also applies to this type of conductor. The resistance of electrolytes may amount to hundreds of ohms.
Accumulation within the small machining gap of the metallic and gaseous products of the
electrolysis is undesirable. If growth were left uncontrolled, eventually a short circuit would occur between the two electrodes. To avoid this crisis, the electrolyte is pumped through the
interelectrode gap so that the products of the electrolysis are carried away. The forced movement of the electrolyte is also essential in diminishing the effects both of electrical heating of the electrolyte, resulting from the passage of current and hydrogen gas, which respectively increase and decrease the effective conductivity.
Working principles
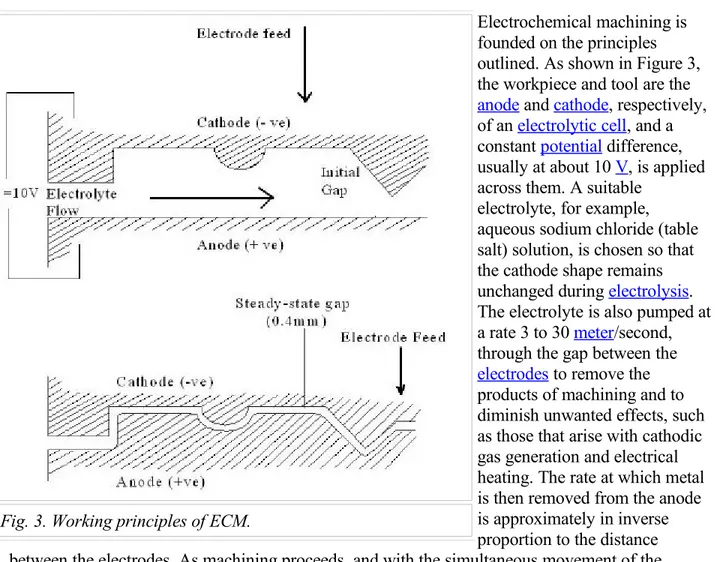
Electrochemical machining is founded on the principles outlined. As shown in Figure 3, the workpiece and tool are the
anode and cathode, respectively, of an electrolytic cell, and a constant potential difference, usually at about 10 V, is applied across them. A suitable
electrolyte, for example, aqueous sodium chloride (table salt) solution, is chosen so that the cathode shape remains unchanged during electrolysis. The electrolyte is also pumped at a rate 3 to 30 meter/second, through the gap between the
electrodes to remove the products of machining and to diminish unwanted effects, such as those that arise with cathodic gas generation and electrical heating. The rate at which metal is then removed from the anode is approximately in inverse proportion to the distance between the electrodes. As machining proceeds, and with the simultaneous movement of the cathode at a typical rate, for example, 0.02 millimeter/second toward the anode, the gap width along the electrode length will gradually tend to a steady-state value. Under these conditions, a shape, roughly complementary to that of the cathode, will be reproduced on the anode. A typical gap width then should be about 0.4 millimeter. Being understood the characteristics and working principles of ECM, its advantages should be stated in short before going further through machining processes:
• the rate of metal machining does not depend on the hardness of the material, • complicated shapes can be machined on hard metals,
• there is no tool wear.
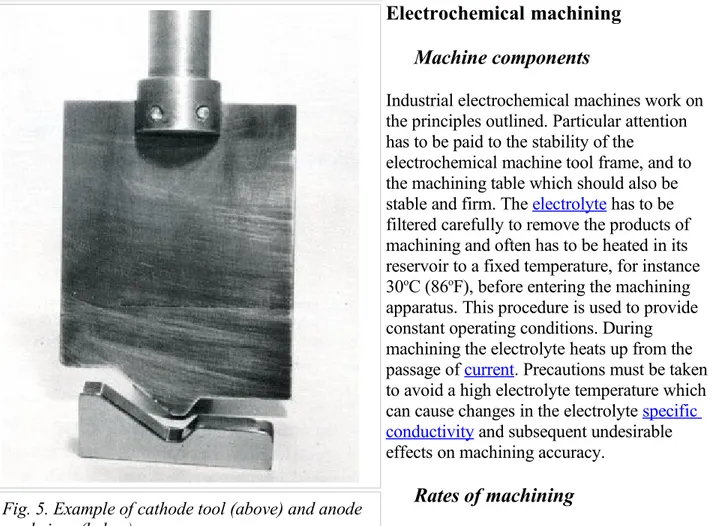
The schematic of an industrial “electrochemical machine” is shown in Figure 4, and an actual example of a cathode tool and anode workpiece are shown in Figure 5.
Electrochemical machining
Machine components
Industrial electrochemical machines work on the principles outlined. Particular attention has to be paid to the stability of the
electrochemical machine tool frame, and to the machining table which should also be stable and firm. The electrolyte has to be filtered carefully to remove the products of machining and often has to be heated in its reservoir to a fixed temperature, for instance 30oC (86oF), before entering the machining apparatus. This procedure is used to provide constant operating conditions. During machining the electrolyte heats up from the passage of current. Precautions must be taken to avoid a high electrolyte temperature which can cause changes in the electrolyte specific conductivity and subsequent undesirable effects on machining accuracy.
Rates of machining
The rates at which metals can be
electrochemically machined is in proportion to the current passed through the electrolyte and the elapsed time for that operation, and is in inverse proportion to the electrochemical equivalent of the anode-metal which corresponds to the
atomic weight of the dissolving ions over the ionic charge times the Faraday’s constant. See the
Appendix for more details.
Many factors other than current influence the rate of machining. These involve electrolyte type, rate of electrolyte flow, and some other process conditions. For example current efficiency
decreases when current density is increased for a certain metal, for example, for nickel.
If the ECM of titanium is attempted in sodium chloride electrolyte, usually very low (10–20%) current efficiencies are obtained. When this solution is replaced by some mixture of fluoride-based electrolytes, to achieve greater efficiencies (>60%), a higher voltage is used.
Fig. 5. Example of cathode tool (above) and anode workpiece (below).
If the rates of the flow are kept too low, the current efficiency of even the most easily
electrochemically machined metal is reduced. Insufficient flow does not allow the products of machining to be so readily flushed from the machining gap. When complex shapes have to be produced the design of tooling incorporating the right kind of flow ports becomes a considerable problem.
Surface finish
Type of electrolytes used in the process affects the quality of surface finish obtained in ECM. Depending on the material, some electrolytes leave an etched finish. This finish results from the nonspecular reflection of light from crystal faces electrochemically dissolved at different rates. Sodium chloride electrolyte tends to produce an etched, matte finish with steels and nickel alloys. The production of an electrochemically-polished surface is usually associated with the random removal of atoms from the anode workpiece, whose surface has become covered with an oxide film. This is governed by the metal-electrolyte combination used. Nonetheless, the mechanisms controlling high-current density electropolishing in ECM are still not completely understood. For example, with nickel-based alloys, the formation of a nickel oxide film seems to be a prerequisite for obtaining a polished surface; a finish of this quality, of 0.2 µm, has been claimed for Nimonic (a nickel alloy) machined in saturated sodium chloride solution. Surface finishes as fine as 0.1 µm have been reported when nickel-chromium steels are machined in sodium chlorate solution. The formation of an oxide film on the metal surface is considered the key to these conditions of polishing.
Sometimes the formation of oxide film on the metal surface hinders efficient ECM and leads to poor surface finish. For example, the ECM of titanium is rendered difficult in chloride and nitrate electrolytes because the oxide film formed is so passive. Even when higher voltages about 50 V
are applied to break the oxide film, its disruption is so non-uniform that deep grain boundary attack of the metal surface can occur.
Occasionally, metals that have undergone ECM have a pitted surface while the remaining area is polished or matte. Pitting normally stems from gas evolution at the anode; the gas bubbles rupture the oxide film.
Process variables also affect surface finish. For example, as the current density is raised the finish generally becomes smoother on the workpiece surface. A similar effect is achieved when the electrolyte velocity is increased. In tests with nickel machined in hydrochloric acid solution the surface finish has been noted to improve from an etched to a polished appearance when the current density is increased from about 8 to 19 A/square centimeter with constant flow velocity.
Accuracy and dimensional control
Electrolyte selection plays an important role in ECM. Sodium chloride, for example, yields much less accurate components than sodium nitrate. The latter electrolyte has far better dimensional control owing to its current efficiency - current density characteristics. Using sodium nitrate electrolyte, the current efficiency is greatest at the highest current densities. In hole drilling these
high current densities occur between the leading edge of the drilling tool and the workpiece. In the side gap there is no direct movement between the tool and workpiece surface, so the gap widens and the current densities are lower. The current efficiencies are consequently lower in the side gap and much less metal than predicted from Faraday’s law is removed. Thus the overcut in the side gap is reduced with this type of electrolyte. If another electrolyte such as sodium chloride solution was used instead, then the overcut could be much greater. Using sodium chloride solutions, its current efficiency remains steady at almost 100% for a wide range of current densities. Thus, even in the side gap, metal removal proceeds at a rate which is mainly determined by current density, in accordance with Faraday’s law. A wider overcut then ensues.
Shaping
Most metal-shaping operations in ECM utilize the same inherent feature of the process whereby one electrode, generally the cathode tool, is driven toward the other at a constant rate when a fixed
voltage is applied between them. Under these conditions, the gap width between the tool and the workpiece becomes constant. The rate of forward movement between the tool and the workpiece becomes constant. The rate of forward movement of the tool is matched by the rate of recession of the workpiece surface resulting from electrochemical dissolution.
Three practical cases are of interest in considering some equations derived for the variation of the interelectrode gap width:
1. When there is no tool movement, the gap width increases indefinitely with the square root of machining time. This condition is often used in deburring by ECM when surface irregularities are removed from components in a few seconds, without the need for mechanical movement of the electrode.
2. When the tool is moved mechanically at a fixed rate toward the workpiece, the gap width tends to a steady value. This inherent feature of ECM, whereby an equilibrium gap width is obtained, is used widely in ECM for reproducing the shape of the cathode tool on the workpiece.
3. Under short-circuit conditions the gap width goes to zero. If some process conditions, such as too small equilibrium gap width caused by a too high movement of the tool toward the workpiece, occur, contact between the two electrodes ensues. This causes a short circuit between the electrodes and hence premature termination of machining.
The equilibrium gap is applied widely in the shaping process. Studies of ECM shaping are usually concerned with three distinct problems:
1. The design of the cathode tool shape needed to produce a required profile geometry of the
anode workpiece.
2. For a given cathode tool shape, prediction of the resultant anode workpiece geometry, for example, hole drilling by ECM.
3. Specification of the shape of the anode workpiece, as machining proceeds. This is most readily predicted for the smoothing of surfaces, although for actual shaping of components by ECM, estimates of the machining times as the shape develops provide useful
Applications
Smoothing of rough surfaces
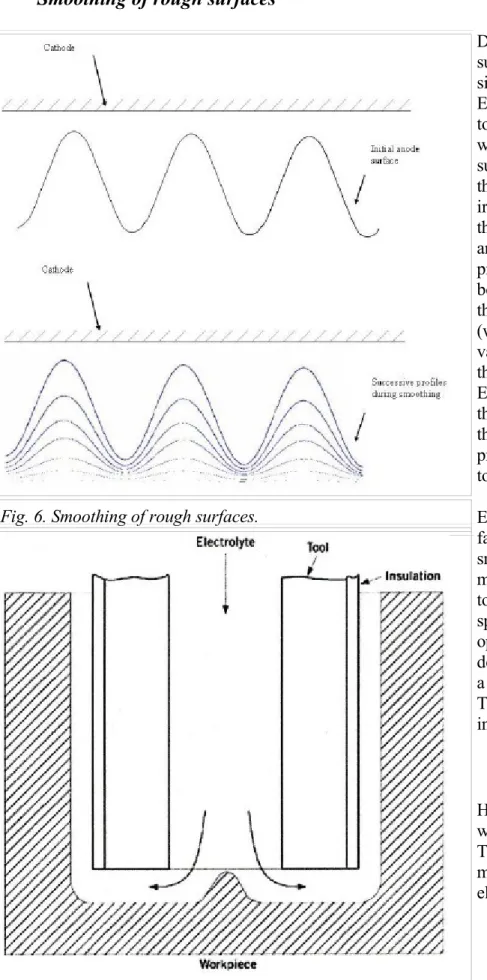
Deburring, or smoothing, of surfaces (Figure 6), is the simplest and a common use of ECM. A plane-faced cathode tool is placed opposite a workpiece that has an irregular surface. The current densities at the peaks of the surface
irregularities are higher than those in the valleys. The former are, therefore, removed
preferentially and the workpiece becomes smooth, admittedly at the expense of stock metal (which is still machined from the valleys of the irregularities, even though at a lower rate).
Electrochemical smoothing is the only type of ECM in which the final anode shape may match precisely that of the cathode tool.
Electrochemical deburring is a fast process; typical times for smoothing the surfaces of manufactured components are 5 to 30 seconds. Owing to its speed and simplicity of operation, electrochemical deburring can be performed with a fixed, stationary cathode tool. The process is used in many industries.
Hole drilling
Hole drilling is another principal way of using ECM (Figure 7). The cathode-tool is usually made in the form of a tubular electrode. Electrolyte is pumped Fig. 6. Smoothing of rough surfaces.
down the central bore of the tool, across the main machining gap, and out between the sidegap that forms between the wall of the tool and the hole. Reversal of the electrolyte flow can often produce considerable improvement in machining accuracy.
The main machining action is carried out in the gap formed between the leading edge of the drill tool and the base of the hole in the workpiece. ECM also proceeds laterally between the side walls of the tool and component, where the current density is lower than at the leading edge of the advancing tool. Since the lateral gap width becomes progressively larger than that at the leading edge, the side-ECM rate is lower. The overall effect of the side-ECM is to increase the diameter of the hole produced. The distance between the side wall of the workpiece and the central axis of the cathode tool is larger than the external radius of the cathode. This difference is known as the "overcut". The amount of overcut can be reduced by several methods. A common procedure involves the insulation of the external walls of the tool (Figure 7), which inhibits side-current flow. Another practice lies in the choice of an electrolyte such as sodium nitrate, which has the greatest
current efficiency at the highest current densities. In hole drilling these high current densities occur between the leading edge of the drill and the base of the workpiece. If another electrolyte such as sodium chloride were used the overcut could be much greater. The current efficiency for sodium chloride remains steady at almost 100% for a wide range of current densities. Thus, even in the side gap, metal removal proceeds at a rate that is mainly determined by the current density, in accordance with Faraday's law.
Holes with diameters of 0.05 to 75 millimeter have been achieved with ECM. For holes of 0.5 to 1.0 millimeter diameter, depths of up to 110 millimeter have been produced. Drilling by ECM is not restricted to round holes; the shape of the workpiece is determined by that of the tool electrode.
Full-form shaping
Full-form shaping utilizes a constant gap across the entire workpiece and the tool is moved mechanically at a fixed rate toward the workpiece in order to produce the type of shape used for the production of compressor and turbine blades. In this procedure, current densities as high as 100
A/square centimeter are used, and across the entire face of the workpiece, the current density remains high.
Electrolyte flow plays an even more influential role in full-form shaping than in drilling and smoothing of surfaces. The entire large cross-sectional area of the workpiece has to be supplied by the electrolyte as it flows between electrodes. The larger areas of electrodes involved mean that comparatively higher pumping pressures and volumetric flow rates are needed.
Electrochemical grinding
The main feature of electrochemical grinding (ECG) is the use of a grinding wheel in which an
insulating abrasive, such as diamond particles, is set in a conducting material. This wheel becomes the cathode tool. The nonconducting particles act as a spacer between the wheel and workpiece, providing a constant interelectrode gap, through which electrolyte is flushed.
Accuracies achieved by ECG are usually about 0.125 millimeter. A drawback of ECG is the loss of accuracy when inside corners are ground. Because of the electric field effects, radii better than 0.25 – 0.375 millimeter can seldom be achieved.
A wide application of electrochemical grinding is the production of tungsten carbide cutting tools. ECG is also useful in the grinding of fragile parts such as hypodermic needles.
Electrochemical arc machining
A process that relies on electrical discharges in electrolytes, thereby permitting metal erosion as well as ECM in that medium, has been developed. Because this process relies on the onset of arcs rather than sparks, it has been named electrochemical arc machining (ECAM). A spark has been defined as a sudden transient and noisy discharge between two electrodes; an arc is a stable thermionic phenomenon. Duration discharges of approximately 1 second to 1 millisecond are described as sparks, whereas for durations of about 0.1 second said discharges can be considered arcs. Because in the ECAM process duration, energy, and time of ignition of sparks are under control, it is valid to regard them as arcs.
An attraction of the ECAM technique is the very fast rates of metal removal attainable by the combined effects of sparking and ECM. The ECAM technique can be applied in all the ways discussed for ECM, thus surfaces can be smoothed and drilled. Turning is also possible, as is wire machining.
One form of this process relies on a pulsed direct current, that is, full-wave rectifiedacpower supply that is locked in phase with a vibrating tool head. The oscillation of the tool gives rise to a set of conditions whereby ECM occurs over each wave cycle. The interelectrode gap narrows as the tool vibrates over one cycle. During the same period the current rises until sparking takes place by breakdown of the electrolyte and/or generation of electrolytic gas or steam bubbles in the gap, the production of which aids the discharge process.
For drilling, the discharge action occurs at the leading edge of the tool, whereas ECM takes place on the side walls between the tool and the workpiece. The combined spark erosion and ECM action yields fast rates of metal removal. Because ECM is still possible, any metallurgical damage to the components caused by the sparking action can be removed by a short period of ECM after the main ECAM action. Currents of 250 A at 30 V are typically used in the process.
Economic aspects
The industrial sectors utilizing ECM technology fall into five main categories: tool and die,
automotive, aerospace, power generation, and oil and gas industries. Leading the world’s principle machine tool manufacturing nations in production and export of tools in the 1980s were Japan followed by the former West Germany. The United States led in imports and consumption; consumption was high for both Japan and W. Germany as well. Unconventional machine tools including ECM are generally considered to account for only 1% of total production.
Electrodischarge machining (EDM) holds the largest share, possibly as much as 50% and ECM about 15% lagging behind laser processes which are 20%.
Manufacturing engineers wishing to use ECM processes in industry need to address the challenge of proper tool design. The cost of design can be as much as 20% of the cost of an electrochemical machine for complex components. Predictability of overcuts obtained for specific applications and the particular electrolytes to be used for the alloy metals that have to be machined must also be considered along with specific controls and limits on the ECM equipment needed.
Computer-controlled equipment and sensors are available for electrochemical machining systems. However in the 1990s practical ECM systems are often favored because the amount of control and/or monitoring of the process is far less than that which was required in the 1970s. Thus machines are used successfully in which electrical spark detection is eliminated and machining products control, for example, pH monitoring, is nonexistent.
The present and future status of ECM
High-rate anodic electrochemical dissolution is a practical method of smoothing and shaping hard metals by employment of simple aqueous electrolyte solutions without wear of the cathodic tool. ECM can offer substantial advantages in a wide range of cavity-sinking and shaped-hole
production operations.
Control of the ECM process is improving all the time, with more sophisticated servo-systems, and better insulating coatings. However there is still a need for basic information on electrode
phenomena at both high current densities and electrolyte flow-rates.
Tool design continues to be of paramount importance in any ECM operation. The use of computer-aided design to predict cathode tool profiles will continue to advance.
Recently developments in ECM practice have dwelt on the replacement of constant dc by pulsed currents (PECM). Significant improvements in surface quality have been claimed. Much smaller electrode gaps may be obtained, for example, below 0.1 millimeter leading to improved control of accuracy, for example to 0.02 to 0.10 millimeter, with dies, turbine blades, and precision electronic components. The key to further advancement in PECM lies in development of a low cost power supply. Successful development of technique will enable on-line monitoring of the gap size, enabling closer process control.
Despite these attractions, PECM should be regarded as complementary to, and not a substitute for, established ECM technology; the former is expensive and metal removal rates can be lower than these of the latter.
The advent of new technology for controlling the ECM process and the development of new and improved metal alloys, which are difficult to machine by conventional means, will assure the future of electrochemical machining.
Appendix
Reactions that occur during the electrolysis of copper sulphate (Figure 1) are as follows. The
anodic reaction is ionizing of copper: Cu ==> Cu2+(aq) + 2e
-While at the cathode the copper ions are discharged to form copper metal: Cu2+(aq) + 2e- ==> Cu
Reactions that occur during the electrolysis of iron (Figure 2) are as follows. The anodic reaction is ionizing of iron:
Fe ==> Fe2+(aq) + 2e
-At the cathode, the reaction is likely to be the generation of hydrogen gas and the production of hydroxyl ions:
H2O + 2e- ==> H2 + 2OH -The net reaction is thus:
Fe + 2H2O ==> Fe(OH)2(s) + H2
The ferrous hydroxide may react to form ferric hydroxide: 4Fe(OH)2 + 2H2O + O2 ==> 4Fe(OH)3
Characteristics of ECM
By use of Faraday’s laws, if “md” (kg) is the mass of metal dissolved, and because “md = vd” where “v” (m3) is the corresponding volume and “d” (kg/m3) the density of the anode metal, the volumetric removal rate of anode metal (m3/second) is given by:
Where “a” (kg/mol) is the atomic weight of the anode metal, “I” (ampere) is the current flowing, “z” is the ionic charge of the anode metal, and the Faraday constant “F” equals 96,487
coulombs/mol. If a machining operation has to be carried out on an iron workpiece at a typical rate of 2.6 × 10-8 kg/C, for this removal rate to be achieved by ECM, the current in the cell must be about 700 A, because “a/zF” = 29 × 10-8 and “d”= 7,860 kg/m3 for iron.
Rates of machining
By use of Faraday’s laws the rates at which metals can be electrochemically machined can be calculated.
Where “md” (kg) is the mass of metal electrochemically machined by current “I” (ampere) passed for a time “t” (second). The quantity “a/zF” is called the electrochemical equivalent of the anode metal as mentioned before.
Table I shows the metal machining rates that can be obtained when a current of 1000 A is used in ECM. Metal removal rates in terms of volumetric machining are often more useful than mass removal rates, and both quantities are included. (It is assumed that the anodic current efficiency is 100%, that is all the current is used to remove metal, which is not always the case.)
Electrochemical Machining
This is really cool. You know how a battery works, roughly? 3 components -- two dissimilar metals and a conductive liquid. The positive metal dissolves and plates itself on the negative side(or reacts with the liquid), and an electric current flows between the two metals if joined with a wire.
One can also FORCE the reaction to happen by driving power through it. The positive side will dissolve and plate itself on the negative(or react with the liquid) as per above. If you've heard of electroplating, that's how it works.
Electrochemical machining is another use of the process. By carefully choosing the shape of the negative metal, one can electrochemically 'drill' into the positive metal. Choose the right fluid and it won't plate itself on the negative side, but just become a suspended powder; pump the fluid around to get the powder out of the way.
My first attempt at this was bill-nye-style kitchen science. Two AA batteries, a penny, and a piece of iron in saltwater with plasticine to hold stuff in place.. I screwed up, making the penny the
positive side. The water rapidly filled with foamy yellow crap as the penny dissolved and ate into the zinc inside.
My second attempt reversed the two and used a copper wire instead of a penny. It slowly dug a divot into the metal. Partial success!
My third attempt was the second on a larger scale, using an insulated copper wire so as to drill a small hole instead of a wide divot. Generated hydrogen gas(this is normal) blew the varnish off the wire(good way to do it, maybe) ruining the narrow hole and digging a wide divot again.
My fourth attempt was a half-assed attempt at pumping water though the center of a gear and out the underside, and using that as the neative electrode to dig an inside gear in metal. Since it was half-assed, stuff kept moving around, I didn't have enough water for stuff to settle out, my electrolyte wasn't very good, and the pump was a retarded modified mister sprayer.
Which brings me to my fifth attempt.
Labels for left image:
A. Ancient 200W pre-ATX power supply
B. Even ancienter adjustable constant-current supply, 600ma max C. Milling Machine mechanism
E. 9.5L of distilled water dissolved with as much pickling salt as physically possible. That's three kilograms of salt for ten liters of water, so don't skimp when you buy.
F. Rubber stopper -- may be illegal in some regions that outlaw glassware. Because only meth heads use glassware, you know. It has no legitimate uses 9.9
G. Fish bubbler tubing
H. The hard-to-make bit -- gear with a pipe through the hub to force water out under it. i.e. the 'cutting tool'
I. A freaking plastic tub
J. Cathode(-), goes to the cutting tool K. Anode(+), goes to the workpiece
L. Hard-drive magnet to hold the windshield wiper motor in place M. Magnet to hold the inlet hose in place inside the plastic tank
N. Duct-tape banding to hold the plastic tank in shape when it bulges disturbingly, threatening to explode and spray electrically-conductive saltwater on the perilously nearby electric outlets and power supplies(don't do what I do!)
For a bonus 500 points, find Waldo in this pic.
In the settling tank diagram, note how the input tube is way up in the top. This is so that if any bubbles get pumped in somehow, they get pushed right back out by pressure from the pump. I keep the top tube held there with a paperclip on the end and a magnet on the outside of the bottle. I like magnets. Instant fasteners without the holes or glue.
Windshield washer pumps have most impressive pressure. Even running it undervolted(5V instead of the normal 12), it bulged the settling tank in a weird and worrying way. I had to drop it to 3V before the volume of water leaving the tank balanced the volume entering. This was convenient though, since windshield washer pumps are not designed for continuous operation; even at 5V it would slowly overheat, but several hours on 3V only got it slightly warm.
Anyway, here's a bunch of images of the thing:
• Gear-shaped divot after an hour of operation
• Settling tank with mostly clear water
• Mechanism in action; the white stuff in the water is iron hydroxide, the white stuff coming up around the gear is hydrogen gas. Ventilate well!
• After 5 hours, the central hole comes free!
• The settling tank is really grungy now though it still delivered fairly clear water to the tool. I've shaken it up to dislodge the stuff sticking to the top, we'll see how well it settles over a week or three.
Conclusions: Electrochemical machining is not good for jagged shapes, it tends to round them out. It might do better for much larger gears, perhaps.