TESIS SK-2401
MODIFIKASI PERMUKAAN PET DENGAN
POLIMER-POLIMER FUNGSIONAL DARI AGEN
RAFT UNTUK MENCAPAI SIFAT ANTIBAKTERI
SALDHYNA DI AMORANRP. 1412 201 901 DOSEN PEMBIMBING
Prof. Dr. Surya Rosa Putra, MS. Dr. Bénédicte Lepoittevin Prof. Philippe Roger
PROGRAM MAGISTER
BIDANG KEAHLIAN BIOKIMIA JURUSAN KIMIA
FAKULTAS MATEMATIKA DAN ILMU PENGETAHUAN ALAM INSTITUT TEKNOLOGI SEPULUH NOPEMBER
THESIS SK-2401
MODIFICATION OF PET SURFACES WITH
END-FUNCTIONALIZED POLYMERS PREPARED FROM
RAFT AGENTS TO ACHIEVE ANTIBACTERIAL
PROPERTIES
SALDHYNA DI AMORA NRP. 1412 201 901
SUPERVISOR
Prof. Dr. Surya Rosa Putra, MS. Dr. Bénédicte Lepoittevin Prof. Philippe Roger
MASTER PROGRAM BIOCHEMISTRY
CHEMISTRY DEPARTMENT
FACULTY OF MATHEMATICS AND NATURAL SCIENCES INSTITUT TEKNOLOGI SEPULUH NOPEMBER
ix
MODIFIKASI PERMUKAAN PET DENGAN POLIMER-POLIMER FUNGSIONAL DARI AGEN RAFT UNTUK MENCAPAI SIFAT
ANTIBAKTERI
Nama Mahasiswa : Saldhyna Di Amora NRP : 1412 201 901
Pembimbing : Prof. Dr. Surya Rosa Putra, MS. Dr. Bénédicte Lepoittevin Prof. Philippe Roger
ABSTRAK
Modifikasi permukaan PET dengan polimer-polimer fungsional dari polimerisasi RAFT telah diteliti sebelumnya. Polimerisasi awal menggunakan stirena telah diteliti untuk mengetahui perbandingan antara polimerisasi radikal bebas konvensional (CFRP) dan polimerassi transfer rantai adisi-fragmenasi secara reversible (RAFT) Tiga tipe dari agen RAFT diantaranya asam pentanoat (4-siano-4-fenilkarbonotioltio), 2-propil dodesil tritiokarbonat, dan 2-siano-2-propil benzoditioat. Ketiga macam agen RAFT tersebut telah diuji coba pada polimerisasi awal dan bisa menghasilkan konversi tertinggi dari monomer-monomer. Agen transfer kontrol (CTA) dari golongan tritiokarbonat terpilih untuk disintesis kemudian difungsionalisasi dengan succinimide..
Monomer-monomer stirena, N,N-dimetilaminoetil metakrilat (DMAEMA) and 2-laktobionamidoetil metacrilat dipolimerisasi dengan teknik polimerisasi RAFT menggunakan succinimid-CTA sebagai agen RAFT. Massa molar terkontrol dan polidispersitas dar polimer-polimer fungsional dikarakterisasi menggunakan kromatografi ekslusi ukuran (SEC).
Permukaan PET diaminolisis terlebih dahulu menggunakan polietilenimin (PEI) dan 1,6-diaminoheksana sebelum proses grafting. Gugus-gugus amin yang terdapat pada permukaan PET dikarakterisasi dengan pengukuran sudut kontak dan spektroskopi fotoelektron X-ray (XPS). Penurunan sudut kontak terjadi antara permukaan PET teraminolisis dan tetesan air (dari Ɵref = 64° ke Ɵ = 48°). Grafting PS dan poli-LAMA sebagai polimer-polimer fungsional pada permukaan PET teraminolisis dilakukan dengan teknik grafting-to. Perubahan sifat permukaan setelah proses grafting dikarakterisasi dengan pengukuran sudut kontak. Grafting PS pada permukaan PET teraminolisis menghasilkan peningkatan sudut kontak (Ɵ = 63°) karena sifat hidrofobik. Di sisi lain, grafting poli-LAMA pada permukaan PET teraminolisis menghasilkan penurunan sudut kontak (Ɵ = 39°) karena sifat hidrofilik.
vii
MODIFICATION OF PET SURFACES WITH END-FUNCTIONALIZED POLYMERS PREPARED FROM RAFT AGENTS TO ACHIEVE
ANTIBACTERIAL PROPERTIES
By : Saldhyna Di Amora
Student Identity Number : 1412 201 901
Supervisor : Prof. Dr. Surya Rosa Putra, MS. Prof. Philippe Roger
Dr. Bénédicte Lepoittevin
ABSTRACT
Modification of PET surfaces with end-functionalized polymers prepared from RAFT polymerization were investigated. Preliminary polymerizations of styrene were prepared to establish the comparison of conventional free radical polymerizations (CFRP) and reversible addition-fragmentation chain transfer (RAFT) polymerizations. Three types of RAFT agents (4-cyano-4-(phenylcarbonothioylthio) pentanoic acid (1), 2-cyano-2-propyl dodecyl trithiocarbonate (2), and 2-cyano-2-propyl benzodithioate (3)) that could obtain the highest conversion of monomers were investigated in the preliminary polymerizations. Controlled transfer agent (CTA) from trithiocarbonate groups were chosen to be synthesized then functionalized with succinimide groups. Monomers of styrene (St), N,N-dimethylaminoethyl methacrylate (DMAEMA), and 2-lactobionamidoethyl methacrylate (LAMA) were polymerized by RAFT polymerization technique using succinimide-CTA (Suc-CTA) as RAFT agent. The controlled molar masses and narrow polydispersities of end-functionalized polymers were characterized by size exclusion chromatography (SEC). PET surfaces were aminolized first by polyethylenimine (PEI) and 1,6-diaminohexane before grafting process. The amine functions on PET surfaces were characterized by contact angle measurements and X-ray photoelectron spectroscopy (XPS). Decreasing of contact angle between aminolized PET surfaces and a droplet of water occured (from Ɵref = 64° to Ɵ = 48°). Then grafting of PS and poly-LAMA as end-functionalized polymers on aminolized PET surfaces were prepared by “grafting-to” technique. The change of surface properties after grafting process was characterized by contact angle measurements. Grafting of PS on aminolized PET surfaces obtained the increasing of contact angle (Ɵ = 63°) because of their hydrophobic properties. In otherwise, grafting of poly-LAMA on aminolized PET surfaces obtained the decreasing of contact angle (Ɵ = 39°) because of their hydrophilic properties.
xi
ACKNOWLEDGEMENTS
Many thanks to education ministry of Indonesia for one year scholarship that backed up my Master 2 programme in France. Unforgettable person that always supported me to try hard study in French, Syaiin, and also the personnes helped me to get the scholarship, Ria and Aini. My proffesor in chemistry departement of Institut Teknologi Sepuluh Nopember (ITS) Surabaya, Rosa Surya Putra, also as co-proffesor in my internship. Then, special thanks for Sandrine Lacombe, director of Master SERP-Chem Université Paris-sud, has accepted me with her fully wisdom. Eva Renouf and Béatrix Piredda, secretary of Master SERP-Chem, also have helped me to organize all of the registration requirements in Université Paris-sud.
My sincere appreciation goes to Philippe Roger and Bénédicte Lepoittevin who supervised me as it should be and gave me the chance of polymers world. Philippe has accepted me to his research group in synthesis laboratory of bioactive molecules and macromolecules for the internship although I still didn’t know much about polymers. Unforgettable supervisor ever I met, Bénédicte, has taught me patiently about how prepared the well-structured polymers. She didn’t only give me the laboratory skills but also many knowledges of science specially polymers chemistry. I also could study from my faults because she always advised me directly when I was doing the faults in laboratory. Having many special moments with all of the staff and students in the laboratory make me want to say thanks to Sophie, Nathan and Ludovic. When I had some difficulties, Sophie always helped me. She has taught me how to use nuclear magnetic resonance, size exclusion chromatography, infra-red spectroscopy, and drop shape analysis. Also for Wenqing, Lu, and Valentine that invited me to play badminton every Friday night as facility to get refreshing after working hard one week in the laboratory.
xiii
Table of contents xiii
List of figures xvii
Lists of table xix
Lists of abbreviation xxi
1. Introduction 1
2.3 Reversible Addition-fragmentation chain transfer (RAFT) Polymerization
8
2.3.1 Addition-fragmentation chain transfer 9 2.3.2 Reversible addition-fragmentation chain transfer
(RAFT)
2.4.2 Surface Characterization 13
2.4.2.1 Water Contact Angle 13
2.4.2.2 X-ray photoelectron spectroscopy (XPS) 14
3. Methodology 17
xiv
3.2 Methods 18
3.2.1 Preliminary Polymerizations 18
3.2.1.1 Conventional Free Radical Polymerization (CFRP) of Styrene
18
3.2.1.2 RAFT Polymerization of Styrene 18 3.2.2 Synthesis of Functional Chain Transfer Agent (CTA) 18
3.2.2.1 Synthesis of 2-(1-isobutyl) sulfanylthiocarbonyl-sulfanyl-2-methyl propionic acid (CTA), (4)
18
3.2.2.2 Synthesis of Succinimide based CTA (Suc-CTA), (5)
19
3.2.3 Synthesis of 2-Lactobionamidoethyl methacrylate (LAMA), (6)
19
3.2.4 Preparation of End-Functionalized Polymers via RAFT Polymerization
3.2.5.2 “grafting-to” of End-functionalized Polymers in Aminolized PET Surfaces
21
3.2.6 Characterization 22
4. Results and Discussion 23
4.1 Preliminary Polymerizations 23
4.1.1 Conventional Free Radical Polymerization (CFRP) of Styrene
23
4.1.2 RAFT Polymerization of Styrene 24
4.2 Synthesis of Functional Chain Transfer Agent (CTA) 30 4.2.1 Synthesis of 2-(1-isobutyl)
sulfanylthiocarbonyl-sulfanyl-2-methyl propionic acid (CTA), (4)
30
xv
4.3 Synthesis of 2-Lactobionamidoethyl methacrylate (LAMA), (6)
31
4.4 End-Functionalized Polymers via RAFT Polymerization
32
4.4.1 RAFT Polymerization of Styrene using Suc-CTA (5) 32 4.4.2 RAFT Polymerization of DMAEMA using
xix different temperatures ([St]:[ CTA (2)]:[AIBN] = 100:1:0.1)
26
4.4 Results for RAFT polymerization of St with CTA (3) at different temperature ([St]:[ CTA (3)]:[AIBN] = 100:1:0.1)
26
4.5 Results for RAFT polymerizations of LAMA using CTA (4) and Suc-CTA (5) at 80 °C ([LAMA]:[CTA (4)]:[ACVA] = 100:5:1 and [LAMA]:[Suc-CTA (5)]:[ACVA] = 100:5:1)
37
4.6 Aminolysis reaction of PET with polyethylenimine (PEI) at 50 °C
39
4.7 Aminolysis reaction of PET with 1,6-diaminohexane at 50 °C
39
4.8 Grafting of PS (in the solution of THF/Et3N (98/2, v/v)) and poly-LAMA (in the solution of CH3OH/Et3N (9/1, v/v)) on aminolized PET surfaces by “grafting-to” technique
xvii
LIST OF FIGURES
Figure Title Page
1.1 Chemical structure of PET 1
1.2 Representation of repelling and killing bacteria surfaces 2
1.3 Main polymer immobilization schemes 3
2.1 Chemical structure of PET 7
2.2 Measurement of contact angle 14
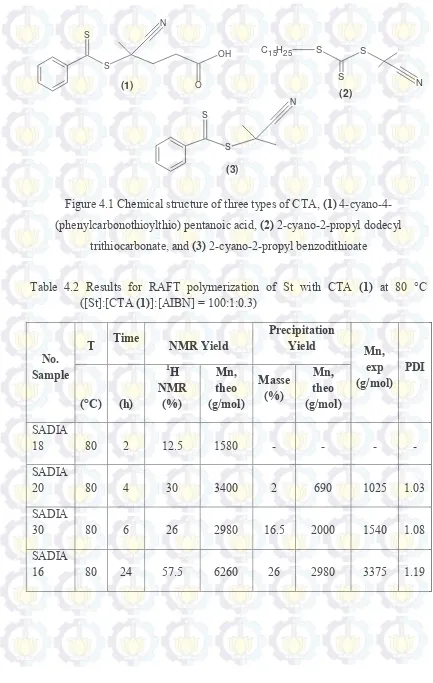
4.1 Chemical structure of three types of CTA 25
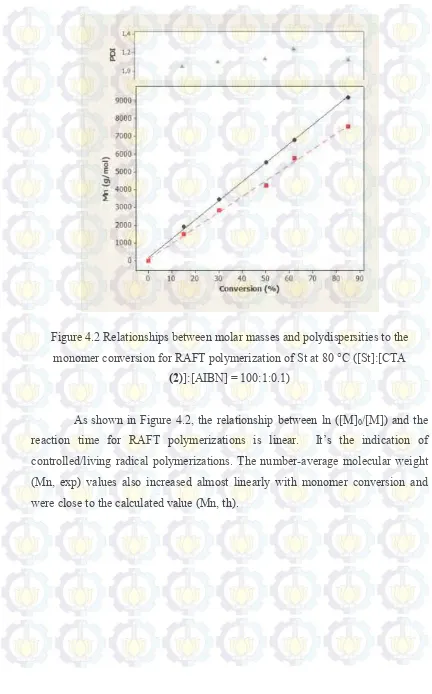
4.2 Relationships between molar masses and polydispersities to the monomer conversion for RAFT polymerization of St at 80 °C ([St]:[CTA (2)]:[AIBN] = 100:1:0.1)
29
4.3 Relationships between molar masses and polydispersities to the monomer conversion for RAFT polymerization of St using Suc-CTA at 80 °C ([St]:[Suc-Suc-CTA (5)]:[AIBN] = 100:1:0.1)
33
4.4 Relationships between ln [M]0/[M] and the polymerization time
for RAFT polymerization of DMAEMA using Suc-CTA at 80 °C ([DMAEMA]:[Suc-CTA (5)]:[AIBN] = 100:1:0.3)
35
4.5 XPS spectra of PET surfaces (a) before and (b) after aminolysis reaction with PEI
xviii
LIST OF SCHEMES
Scheme Title Page
1.1 General mechanism of RAFT Polymerization 4
1.2 General overview of surface modification of PET with end-functionalized polymers prepared from RAFT polymerizations
5
2.1 Mechanism for addition-fragmentation chain transfer 10
2.2 Equations of chain transfer rate 10
2.3 Reversible addition-fragmentation chain transfer 11 2.4 Reversible homolytic substitution chain transfer 11
2.5 Mechanism of RAFT polymerization 12
2.6 The schematic representation of aminolysis and further immobilization of biomolecules on a membrane
13
4.1 CFRP reaction of St at 70 °C ([St]:[AIBN]=100:1) 23 4.2 St Polymerization reaction with CTA (1), (2), and (3) 28
4.3 Synthesis of 2-(1-isobutyl) sulfanylthiocarbonyl-sulfanyl-2-methyl propionic acid (CTA), (4)
30
4.4 Synthesis of Succinimide based CTA (Suc-CTA), (5) 31 4.5 Synthesis of 2-Lactobionamidoethyl methacrylate (LAMA), (6) 32 4.6 RAFT polymerization reaction of St using Suc-CTA at 80 °C
([St]:[Suc-CTA (5)]:[AIBN] = 100:1:0.1)
32
4.7 RAFT polymerization reaction of DMAEMA using Suc-CTA at 80 °C ([DMAEMA]:[Suc-CTA (5)]:[AIBN] = 100:1:0.3)
4.9 Acetylation reaction of sugar compound 37
4.10 Aminolysis reactions of PET with (a) 1,6-diaminohexane, (R = (CH2)6) and (b) PEI, (R = (PEI)n)
38
4.11 Grafting of PS on aminolized PET surfaces 41
xxi
LISTS OF ABBREVIATIONS
ACVA 4,4’-azobis-(4-cyanovaleric acid) AIBN 2,2’-azobis-(isobutyronitrile)
ATRP Atom transfer radical polymerization CFRP Conventional free radical polymerization CLRP Controlled/living radical polymerization
CTA Controlled transfer agent
DCC Dicyclohexyl carbodiimide
DCM Dichloromethane
HCl Hydrochloric acid
DMAEMA N,N-diethylaminoethyl methacrylate
DMF Dimethyl formamide
DSA Drop shape analysis
FT-IR Fourier transform infra red
ICMMO “Institut de chimie moléculaire et des matériaux d'Orsay”
LAMA 2-lactobionamidoethyl methacrylate
MAM More activated monomer
Mn,exp Experimental number molecular weight Mn,th Theoritical number molecular weight
NHS N-hydrosuccinimide
NMP Nitroxide-mediated polymerization
NMR Nuclear magnetic resonance
PDI Polydispersities index
PEI Poly-ethylenimine
PET Poly-ethylene terephtalate
PMMA Poly methyl methacrylate
Poly-DMAEMA Poly(N,N-diethylaminoethyl methacrylate) Poly-LAMA Poly(2-lactobionamidoethyl methacrylate)
PS Polystyrene
RAFT Reversible addition-fragmentation chain transfer
SEC Size exclusion chromatography
St Styrene
Suc-CTA Succinimide based controlled transfer agent
TFA Trifluoroacetic acid
TMS Tetramethylsilane
UMR “Unité mixte de recherche”
1 CHAPTER 1
INTRODUCTION
1.1 Background
Polymers have became an essential thing in our daily life. They exist in many field like industry of textile, food, medicine, and also we can find it in the human body as biomacromolecules. The most widely used synthetic materials in the world is polyethylene terephtalate (PET), as shown in Figure 1.1. Its high cristallinity and high melting point are responsible for its toughness, excellent fibers and film-forming properties. PET is relatively inert and hydrophobic without functional groups. Majority, this polymer was used in packaging industry such food and drinks, cosmetics, household chemicals, toiletries, and pharmaceuticals. The other field of PET also was found in biomedical engineering as a material for artificial blood vessels, tendons, hard tissue prostheses, and surgical thread1.
Figure 1.1 Chemical structure of PET
2
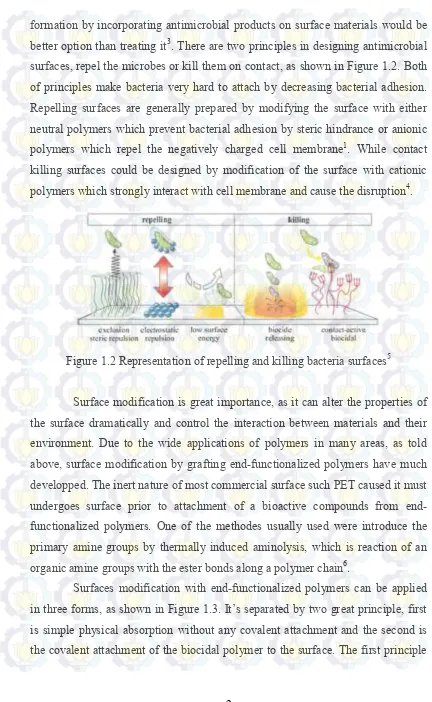
formation by incorporating antimicrobial products on surface materials would be better option than treating it3. There are two principles in designing antimicrobial surfaces, repel the microbes or kill them on contact, as shown in Figure 1.2. Both of principles make bacteria very hard to attach by decreasing bacterial adhesion. Repelling surfaces are generally prepared by modifying the surface with either neutral polymers which prevent bacterial adhesion by steric hindrance or anionic polymers which repel the negatively charged cell membrane1. While contact killing surfaces could be designed by modification of the surface with cationic polymers which strongly interact with cell membrane and cause the disruption4.
Figure 1.2 Representation of repelling and killing bacteria surfaces5
Surface modification is great importance, as it can alter the properties of the surface dramatically and control the interaction between materials and their environment. Due to the wide applications of polymers in many areas, as told above, surface modification by grafting end-functionalized polymers have much developped. The inert nature of most commercial surface such PET caused it must undergoes surface prior to attachment of a bioactive compounds from end-functionalized polymers. One of the methodes usually used were introduce the primary amine groups by thermally induced aminolysis, which is reaction of an organic amine groups with the ester bonds along a polymer chain6.
3
have a high risk with such coatings of biocide leaching out to the surrounding in some instances, which may lead to a loss of antimicrobial activity over a short time. While the second principle is classified in two technique, “grafting-to” and “grafting-from”. The antimicrobial surfaces created by this methodology do not allow the biocide to leach easily and long-term non-leachable antimicrobial coatings could be designed.
Figure 1.3 Main polymer immobilization schemes (A) Physical adsorption by non-covalent, (B) “grafting-to” methods by creating covalend bonds with the surface, and (C) “grafting-from” or surface initiated polymerization via synthesis
of antimicrobial coating from initiators7
4
most recent of the living/controlled free radical methodologies that have revolutionized the field of free radical. Compared with NMP and ATRP, the RAFT polymerization is suitable for much more monomers and in principle, all classic radical polymerization can be used with the RAFT process in the presence of efficient RAFT agents. While for NMP and ATRP, the synthesis of polymers with well-defined structures, such as some block copolymers and other complex architecture, has some limitations because the processes are not compatible with certain monomers or reaction conditions10.
The functional groups can be easily introduced into the chain ends of the polymers by adjusting the structure of the RAFT agent. Selection of the RAFT agent for the monomers and reaction conditons is crucial for the succes of a RAFT polymerization. RAFT agents, denoted Z-C(=S)SR, act as transfer agents by two steps of addition-fragmentation mechanism, as shown in Scheme 1.1. The RAFT group is typically a thiocarbonylthio group such as dithioester (Z = alkyl), trithiocarbonate (Z = S-alkyl), xanthate (Z = O-alkyl) or dithiocarbamate (Z = N(alkyl)2)11. The effectivenes of RAFT agents is determined by substituents R and Z12. The Z group should activate the C=S towards radical addition, while the R group should be a good free-radical leaving group and be capable of reinitiating free-radical polymerizations13. Fast equilibrium between propagating radicals and dormant species is needed to achieve well-defined polymers with low polydispersity.
Scheme 1.1 General mechanism of RAFT Polymerization
5
End-functionalized polymers were prepared by RAFT polymerization technique in presence of an initiator and a RAFT agent based on succinimide groups. The succinimide compounds give the ester bonds in polymer chains that will be very reactive to incorporate with amine groups on PET surfaces. Amino groups will be incorporated on PET surfaces by aminolysis reaction. After grafting, PET surfaces will be subjected in bacterial tests to study the bacteria adhesion.
Scheme 1.2 General overview of surface modification of PET with end-functionalized polymers prepared from RAFT polymerizations .
1.2 Objectives of Research
Generally, the objective of this study is to prepared antibacterial PET surfaces with the end-functionalized polymers by “grafting-to” technique. The objective classification of each work will be explained on the specific objectives.
1.2.1 Specific objectives
1. To synthese the controlled transfer agents (CTA) based on succinimide groups (Suc-CTA)
2. To get the end-functionalized polymers using RAFT polymerization technique.
3. To give the amine function on PET surfaces by aminolysis
7 CHAPTER 2
LITERATURE REVIEW
2.1 Polyethylene terephtalate (PET)
PET is a major polymer used in the packaging industry and is used to package both carbonated and non carbonated drinks by an injection moulding and strecth blow moulding process. It is the polymer of choice to pack a wide variety of products from food and drinks to cosmetics, household chemicals, toiletries and pharmaceuticals. Packaged drinks include soft drinks, waters, fruit juices, wine, spirits and beer. Packaged foods include edible oils, vinegars, fruit, meat and fresh pasta. PET is also used to manufacture tough, clear industrial sheet which can be thermoformed14.
The characterizations of PET are high cristallinity and high melting point. They are responsible for its toughness and its excellent fiber and film forming properties. As are most synthetic polymers, PET is relatively inert and hydrophobic without functional groups able to take part in covalent enzyme immobilization. To overcome this drawback chemical modifications have been attempted to alter the surface properties of the material1. The structure of PET was showed in Figure 2.1.
Figure 2.1 Chemical structure of PET
2.2 Functional Polymers
8
entirely on the functional group substituents on the macromolecules. The high demand on the design and the actual tailormaking of such macromolecular materials require a great deal of imagination and detailed knowledge in synthesis and structure or property relationships (macromolecular architecture and macromolecular engineering). In the last decade, research in polymer chemistry and production in the polymer-related industries have shifted from the emphasis on polymers based on raw material availability and high cost efficiency to market and use-oriented tailor-made polymeric materials.
In the design of macromolecular structures with functional groups, it is not only necessary to be concerned with the macromolecule and the functional group, but it is becoming of further importance to be concerned with the spacing of the functional groups with respect to the macromolecular backbone chain. Nature has carefully designed natural macromolecular structures and has placed functional amino acid units with spacer groups in sugar units in polysaccharides to obtain macromolecular structures with opimal biological activity. With clever structure design, sequence, and spacer arrangements, nature has designed enzymes, biologically and immunologically active macromolecular structure. Much could be done in the design of synthetic macromolecular with proper knowledge of the intricacies and interrelations of macromolecular backbone chains, functionalities, and spacer groups15.
2.3 Reversible Addition-Fragmentation Chain Transfer (RAFT)
Polymerization
9
polymerization processes. Among the other unique features of the RAFT process is high tolerance to functional monomers such as vinyl acetate and acrylic acid which can be polymerized with living characteristics with ease. The RAFT process is an equally powerful tool for the coalmost instruction of complex macrromolecular architectures via variable approaches, Z and R group designs, that allow for limitless possibilities in the synthestic protocols in terms of the low molecular weight16.
2.3.1 Addition-fragmentation chain transfer
10
Scheme 2.1 Mechanism for addition-fragmentation chain transfer
In addition-fragmentation chain transfer, the rate constant for chain transfer (ktr) is defined in terms of the rate constant for addition (kx) and a partition coefficient (Φ) which defines how the adduct is partitioned between products and startig materials, as shown in Scheme 2.2 as Eqs (1) and (2)17.
Scheme 2.2 Equations of chain transfer rate
2.3.2 Reversible addition-fragmentation chain transfer (RAFT)
11
Scheme 2.3 Reversible addition-fragmentation chain transfer
Reversible chain transfer may, in principle, involve homolytic substitution as shown in Scheme 2.4 or addition-fragmentation (RAFT) as shown in Scheme 2.5 or some other transfer mechanism20. An essential feature is that the product of chain transfer is also a chain transfer agent. The overall process has also been termed degenerate or degenerative chain transfer since the polymeric starting materials and products have equivalent properties and differ only in molecular weight (where R· and R’· are both propagating chains).
12
Scheme 2.5 Mechanism of RAFT polymerization
2.4 Surface Modification
Several surface modification techniques have been developped to improve wetting, adhesion, and printing of polymer surfaces by introducing a variety of polar groups, with little attention to functional group specificity. However, when surface modification is a precursor to attache a bioactive compound, these techniques must be tailored to introduce a specific functional group. Techniques that modify surface properties by introducing random, non-specific groups or by coating the surface are less useful in bioconjugation to polymer surfaces21.
2.4.1 Surface Modification Technique of PET via Aminolysis
13
the surface. Such action increased the hydrophility of the polymer and created the anchor functionalities for subsequent reactions. The main problem however is to find the proper parameters of these processes, parameters that do not cause high degradation or significant decrease of the mechanical properties of the sample. The same processes but in much more severe conditions are applied also for chemical recycling of PET28,29.
Primary amine groups are often introduced by thermally induced aminolyis, which is reaction of an organic amine agent with the ester bonds along a polymer chain, as shown in Scheme 2.630. Among the most often used amines are hydrazine, ethylenediamine, and 1,6-diaminohexane31.
Scheme 2.6 The schematic representation of aminolysis and further immobilization of biomolecules on a membrane
2.4.2 Surface Characterization
2.4.2.1 Water Contact Angle
14
Figure 2.2 Measurement of contact angle
By taking contact angle with a range of buffered aqueous solutions varying in pH value, one can identify the surface pKa, which can be used to identify if a surface contains acidic or basic functionalities32. Knowing surface pKa not only helps identify the nature of the surface functional groups, but it aids in determining the proper pH for a conjugation buffer in order to optimize covalent bonding. While contact angle is a simple and rapid measure of the change of a surface’s hydrophilicity, it is limited by its inability to distinguish between different hydrophilic functional groups and by many ways error can be introduced into the measurement, including the following: difference in operator measurement, inconsistent water Ph and hardness, and changes in environmental temperature and humidity33.
2.4.2.2 X-ray photoelectron spectroscopy (XPS)
15
measured atomic concentrations by theoritical values calculated by assuming complete conversion36.
17
CHAPTER 3 METHODOLOGY
3.1 Materials
Preliminary experiment for styrene polymerization by RAFT technique used the commercial CTA of 4-cyano-4-(phenylcarbonothioylthio) pentanoic acid
18
3.2Methods
3.2.1 Preliminary Polymerizations
3.2.1.1 Conventional Free Radical Polymerization (CFRP) of Styrene
2,2’-azo-bis-(isobutyronitrile) (AIBN, 143 mg, 0.87 mmol) dissolved in styrene (1 mL, 8.7 mmol). The mixture was stirred in different times (2, 4, and 6 h) under argon atmosphere at temperature of 70 °C. After that times, the polymer solution was cooled in ambient temperature and then precipitated with cold methanol. The white polymer solid was filtered and dried under vacum.
3.2.1.2RAFT Polymerization of Styrene
Three RAFT agents was tried in this preeliminary experiments. Each of compounds (1) (244mg, 0.09 mmol), compounds (2) (30.2 mg, 0.9 mmol), and compounds (3) (19.3 mg, 0.09 mmol) was added to 2,2’-azo-bis (isobutyronitrile)/ AIBN (1.4 mg, 0.009 mmol). Then, it was solubilized in 1 mL of styrene. The mixture was stirred under Argon atmosphere at temperature of 70 °C and will be compared in the polymerization time of 24 h. After that, the polymer solution was cooled in ambient temperature and then precipitated with methanol. The solid polymer was filtered and dried under vacum. Each of polymer solid from RAFT agents of compounds (1), (2), and (3) had white, yellow, and pink colours.
3.2.2 Synthesis of Functional Chain Transfer Agent (CTA)
3.2.2.1 Synthesis of 2-(1-isobutyl) sulfanylthiocarbonyl-sulfanyl-2-methyl propionic acid (CTA), (4)
19
concentrated HCl 37% was added in solution to check the pH range of 1-2. Acetone was removed by evaporation. Then, the solid was filtrated and washed with water 5 mL for three times. The orange solid was collected and recrystallized from 7 mL of acetone/hexane (1/10 v/v). It was filtrated again and washed with about 3 mL hexane. The yellow solid was dried in desiccator. The product was obtained as a bright yellow solid in 36% yield.
1H NMR (250 MHz, CDCl3), δ (ppm from TMS) : 1.03 (d, Ј = 6.1 Hz,
6H, CH-(CH3)2), 1.74 (s, 6H, C(CH3)2), 2 (m, 1H, CH2-CH-(CH3)2), 3.22 (d, Ј =
6.8 Hz, 2H, S-CH2-CH).
3.2.2.2 Synthesis of Succinimide based CTA (Suc-CTA), (5)
A suspension of N-hydroxysuccinimide (1 g, 8.7 mmol) in 40 mL of acetate/petroleum ether (1/2 v/v) as eluent. The product was obtained as a bright yellow solid in 31.5 %.
1H NMR (360 MHz, CDCl3), δ (ppm from TMS) : 1.03 (d, Ј = 6.8 Hz,
6H, CH-(CH3)2), 1.88 (s, 6H, C(CH3)2), 2.02 (m, 1H, CH2-CH-(CH3)2), 2.82 (s, 4H, (C=O)-CH2-CH2-(C=O)), 3.25 (d, Ј = 6.5 Hz, 2H, S-CH2-CH).
3.2.3 Synthesis of 2-Lactobionamidoethyl methacrylate (LAMA), (6)
20
sugar solution. The solution was stirred at ambient temperature under argon atmosphere overnight. The solvent was evaporated and the formed sugar was solubilized in the mixture solution of methanol/isopropanol (2/3 v/v). It was stirred for 1 h to precipitate then filtrated and dried. The product was obtained as white solid in 88 %.
1H NMR (250 MHz, D2O), δ (ppm from TMS) : 1.78 (s, 3H, C(CH3)),
3.84 (m, 26H, CH-OH of sugar), 5.85 (s, 1H, CH2=C).
FT-IR (cm-1) : 3439 (O-H), 2920 (C-H), 1734 (C=O), 1070 (C-O).
3.2.4 Preparation of End-Functionalized Polymers via RAFT
Polymerization
3.2.4.1RAFT Polymerization using Suc-CTA, (5)
Compounds (5) as RAFT agents (30.4 mg, 0.09 mmol), 2,2’-azobis-(isobutyronitrile) (AIBN, 1.4 mg, 0.009 mmol) was solubilized in styrene (1 mL, 8.7 mmol). The solution was mixed by stirring for 15 min in ambient temperature under argon atmosphere then continued stirring at T = 80 °C in different times. Polymer solution was precipitated in 100 mL cold methanol, then filtrated and dried under vacum. The yellow solid was obtained as polymers.
The similar methods was also applied in polymerization of dimethylaminoethyl methacrylate (DMAEMA, 1 mL, 5.94 mmol) [DMAEMA]:[Suc-CTA (5)]:[AIBN] = 100:1:0.3) and 2-lactobionamidoethyl methacrylate (LAMA, 469 mg, 1 mmol) in solution of H2O/DMF (5/1 v/v) with ACVA as intiator ([LAMA]:[Suc-CTA (5)]:[ACVA] = 100:5:1).
3.2.4.2RAFT Polymerization using CTA, (4)
21
cold methanol, then filtrated and dried under vacum. The white solid was obtained as polymers.
3.2.4.3Acetylation of poly-LAMA
Poly-LAMA (10 mg, 0.02 mmol) was solubilized in acetic anhydride (0.5 mL, 5.3 mmol) and pyridine (1 mL, 12.4 mmol). The solution was stirred overnight in temperature 50 °C to acetylate hidroxyl groups of glycopolymers. About 5 mL of toluene was added to the solution then evaporated to get the acetylated glycopolymers. The obtained glycopolymers need to be purified again by extraction with water/DCM then cleaned with NaCl solution and MgSO4. The solvent was evaporated and the protected glycopolymers was dried. It was preparated for SEC analysis.
3.2.5 Surface Modification of PET 3.2.5.1 Aminolysis Reaction
A pair of washed PET films were added to tubes containing of 5 mL solution of 1,6-diaminohexane (5.8 g, 50 mmol) in 50 mL methanol. Then, it was thermostated at T = 50 °C in different times (1, 3, 5, 7, and 24 h). The solution was removed from the films by washing with methanol in 3-4 times then dried in vacum at ambient temperature for at least 8 h. The films were analysed by contact angle measurements of water.
The similar methods was also applied in surface modification of PET using polyethyleneimine (PEI) 11.6 % in methanol.
3.2.5.2“grafting-to” of End-functionalized Polymers in Aminolized PET Surfaces
22
The similar methods was also applied in “grafting-to” of poly-LAMA using the solvent of water/triethylamine (9/1, v/v) at 40 °C for 2 days.
3.2.6 Characterization
The molar masses and polydispersities of all formed polymers were determined by Size Exclusion Chromatography (SEC). THF/Et3N (98/2, v/v) was used as the eluent at a flow rate of 1.0 mL min-1 operated at 30 °C. Special treatment just for poly-LAMA that needs to be protected with acetyl groups before solubilize in THF. PS standard was used for sample measurements.
Determination of structure were recorded by 1H NMR spectra of the polymers were recorded on 350 MHz nuclear magnetic resonance intrument, using TMS as the internal standard.
Surface modification of PET was characterized by X-ray photoelectron spectroscopy (XPS) and drop shape analysis (DSA). The water dropped was 3 μL. An Kα X-ray source was used. In DSA, a drop of water was used to measure the contact angle in aminolized PET surfaces.
23
CHAPTER 4
RESULTS AND DISCUSSION
4.1 Preliminary Polymerizations
4.1.1 Conventional Free Radical Polymerization (CFRP) of Styrene
CFRP of styrene using AIBN as an intiator at 70 °C were prepared with the reaction ratios [St]:[AIBN] = 100:1 and in the bulk conditions. Polymerizations were done with different reaction time (2, 4, and 6 h) until the monomer conversion reach the highest value. The results are shown in Table 4.1 and polymerization reaction can be seen in Scheme 4.1.
Table 4.1. Results for CFRP of St at 70 °C ([St]:[AIBN]=100:1)
No. 1H NMR Precipitation
SADIA
Scheme 4.1. CFRP reaction of St at 70 °C ([St]:[AIBN]=100:1)
AIBN, 70 o C
Ar (g)
24
As shown in Table 4.1, Mn,exp was decreased in the increasing on polymerization time, for example 26300 g/mol for polymerization time of 2 h and only 8500 g/mol for polymerization time of 6 h. It showed that CFRP without an inhibitor in bulk condition cause the reaction hard to control. It produces premature radicals with high reactivity which will fastly initiate polymerization of vinyl monomer, as styrene. It usually called autoacceleration or Tromsdorff effects. This effect accelerate the initiation reaction so the number-average molecular weight (Mn) decrease in the increasing of conversion or polymerization time. Beside that, the uncontrolled process in CFRP of styrene also produce polydispersities index (PDI) broad as 3.14 for monomer conversion of 100 %.
4.1.2 RAFT Polymerization of Styrene
25
Figure 4.1 Chemical structure of three types of CTA, (1) 4-cyano-4-(phenylcarbonothioylthio) pentanoic acid, (2) 2-cyano-2-propyl dodecyl
trithiocarbonate, and (3) 2-cyano-2-propyl benzodithioate
26
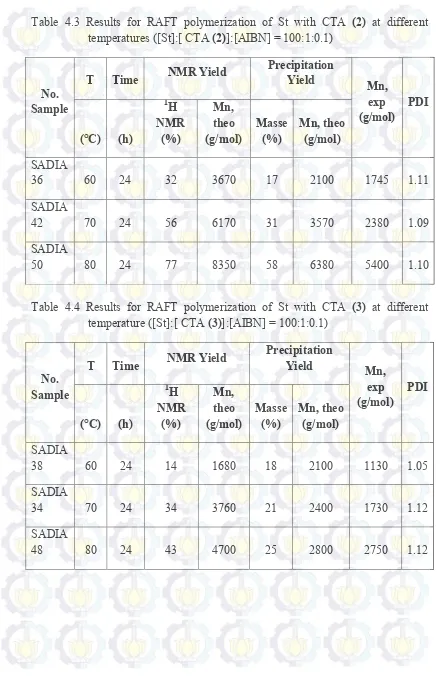
Table 4.3 Results for RAFT polymerization of St with CTA (2) at different temperatures ([St]:[ CTA (2)]:[AIBN] = 100:1:0.1)
No. temperature ([St]:[ CTA (3)]:[AIBN] = 100:1:0.1)
27
As shown in Table 4.2 up to 4.3, the highest conversion in the same time (24 h) and temperature (80 °C) of RAFT polymerization for St reached by CTA
28
Scheme 4.2. St Polymerization reaction with CTA (1), (2), and (3)
29
Figure 4.2 Relationships between molar masses and polydispersities to the monomer conversion for RAFT polymerization of St at 80 °C ([St]:[CTA
(2)]:[AIBN] = 100:1:0.1)
30
4.2 Synthesis of Functional Chain Transfer Agent (CTA)
Functionalized chain transfer agents (CTA) was needed for grafting polymers on aminated PET surfaces. Succinimide based CTA (Suc-CTA) was choosen as functional CTA in this work because it will give the ester groups in the polymers chain which be very reactive with amine agents on PET surfaces.
4.2.1 Synthesis of 2-(1-isobutyl) sulfanylthiocarbonyl-sulfanyl-2-methyl propionic acid (CTA), (4)
This method was first step to synthesize the functional CTA based on succinimide (will be discussed later). The CTA was chosen from trithiocarbonate type because in the preeliminary experiments, it was very suitable to RAFT polymerization of St that had given the high monomer conversion. CTA was conveniently prepared from 2-methyl-1-propanethiol with carbon disulfide in acetone then continued by oxidation process to be carboxylic acid groups, as shown in scheme 4.3. The product was obtained as a bright yellow solid in 36% yield.
Scheme 4.3 Synthesis of 2-(1-isobutyl) sulfanylthiocarbonyl-sulfanyl-2-methyl propionic acid (CTA), (4)41
31
4.2.2 Synthesis of Succinimide based CTA (Suc-CTA), (5)
The Suc-CTA was designed to activate the carboxyl group required in aminolyzed PET surface grafting, that will be discussed later. Suc-CTA was conveniently prepared from N-hydroxysuccinimide (NHS) and activated ester of CTA, (4), as shown in scheme 4.4. The product was obtained as a bright yellow solid in 32 %.
Scheme 4.4 Synthesis of Succinimide based CTA (Suc-CTA), (5)42
The structure of Suc-CTA was confirmed by 1H NMR recorded in CDCl3 at 25 °C. The spectrum revealed the presence of the characteristic signals of the succinimide unit (2,8 ppm) and the other spectrum shown the structure of CTA,
(4).
4.3 Synthesis of 2-Lactobionamidoethyl methacrylate (LAMA), (6)
32
Scheme 4.5 Synthesis of 2-Lactobionamidoethyl methacrylate (LAMA), (6)43 The structure of LAMA was confirmed by1H NMR. The spectrum revealed the presence of the characteristic signals of the carbohydrate groups (3.2 – 4.4 ppm) and vinyl of methacrylates groups (5.6 and 6 ppm).
4.4 End-Functionalized Polymers via RAFT Polymerization 4.4.1 RAFT Polymerization of Styrene using Suc-CTA (5)
RAFT polymerization of St was promoted using Suc-CTA (5). The polymerizations was prepared in bulk conditions same as preliminary experiments. It was initiated by AIBN in the presence of Suc-CTA (5) at 80 °C in the different time ([St]:[Suc-CTA (5)]:[AIBN] = 100:1:0.1), as shown in Scheme 4.6.
33
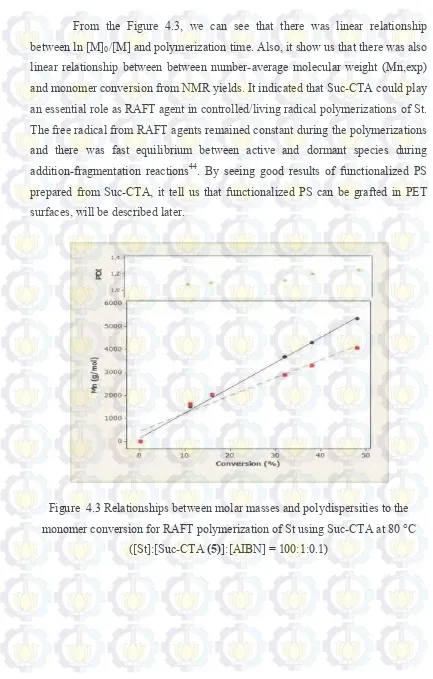
From the Figure 4.3, we can see that there was linear relationship between ln [M]0/[M] and polymerization time. Also, it show us that there was also linear relationship between between number-average molecular weight (Mn,exp) and monomer conversion from NMR yields. It indicated that Suc-CTA could play an essential role as RAFT agent in controlled/living radical polymerizations of St. The free radical from RAFT agents remained constant during the polymerizations and there was fast equilibrium between active and dormant species during addition-fragmentation reactions44. By seeing good results of functionalized PS prepared from Suc-CTA, it tell us that functionalized PS can be grafted in PET surfaces, will be described later.
Figure 4.3 Relationships between molar masses and polydispersities to the monomer conversion for RAFT polymerization of St using Suc-CTA at 80 °C
34
4.4.2 RAFT Polymerization of DMAEMA using Suc-CTA (5)
DMAEMA also was promoted by RAFT polymerization using Suc-CTA
(5) as RAFT agents. This polymerization was prepared in bulk conditions with AIBN act as initiator. DMAEMA was polymerized at 80 °C in the different time [DMAEMA]:[Suc-CTA (5)]:[AIBN] = 100:1:0.3), as shown in Scheme 4.7.
Scheme 4.7 RAFT polymerization reaction of DMAEMA using Suc-CTA at 80 °C ([DMAEMA]:[Suc-CTA (5)]:[AIBN] = 100:1:0.3)
35
Figure 4.4 Relationships between ln [M]0/[M] and the polymerization time for RAFT polymerization of DMAEMA using Suc-CTA at 80 °C
([DMAEMA]:[Suc-CTA (5)]:[AIBN] = 100:1:0.3)
4.4.3 RAFT Polymerization of LAMA using CTA (4) and Suc-CTA (5)
RAFT polymerizations using CTA (4) and Suc-CTA (5) was specially prepared for glycomonomers of LAMA. Glycomonomers of LAMA contained two reactive groups, carbohydrate and methacrylates, so they have two type of properties in their structure. Many hydroxyls in carbohydrate groups give hydrophilic properties while methacrylate groups give hydrophobic properties. Based on two different properties in LAMA glycomonomers, it needs to be tried doing RAFT polymerization using CTA (4) that is more suitable with hydrophilic properties and Suc-CTA (5) that is more suitable with hydrophobic properties.
RAFT polymerization of LAMA couldn’t be prepared in bulk conditions. Due to the presence of the unprotected hydroxyl groups of the carbohydrate moieties, it would be more interesting to investigate the RAFT polymerization in water. However, due to the low solubility of the chain transfer agents in pure water, the polymerizations was conducted in mixtures of water and N,N’ -dimethylformamide (DMF), (H2O/DMF = 5/1)45. It was initiated by ACVA in the
36
presence of CTA at 80 °C ([LAMA]:[CTA (4)]:[ACVA] = 100:5:1 and ([LAMA]:[Suc-CTA (5)]:[ACVA] = 100:5:1, as shown in Scheme 4.8.
Scheme 4.8 RAFT polymerization reaction of LAMA using CTA (4) and Suc-CTA (5) at 80 °C ([LAMA]:[CTA (4)]:[ACVA] = 100:5:1 and
[LAMA]:[Suc-CTA (5)]:[ACVA] = 100:5:1)
37
Scheme 4.9. Acetylation reaction of sugar compound
Table 4.5. Results for RAFT polymerizations of LAMA using CTA (4) and Suc-CTA (5) at 80 °C ([LAMA]:[CTA (4)]:[ACVA] = 100:5:1 and [LAMA]:[Suc-CTA (5)]:[ACVA] = 100:5:1)
No.
Sample RAFT agents Time (h)
NMR Yield Precipitation Yield
38
polymerization time of 3 h, poly-LAMA using CTA (4) had the molar masses higher than poly-LAMA using Suc-CTA (5) (13840 > 9300, g/mol). In otherwise, poly-LAMA using CTA (4) had the polydispersities lower than poly-LAMA using Suc-CTA (5) (1.54 < 1.88). It means that RAFT agents of CTA (4) was more suitable for RAFT polymerization of LAMA.
4.5 Surface Modification of PET 4.5.1 Aminolysis Reaction
PET surfaces were prepared by aminolysis reactions with 1,6-diaminohexane and polyethylenimine (PEI). This treatment was proposed to give the amine functions on PET surfaces. The aminolized PET surfaces will be very reactive to incorporate covalently the ester bonds of end-functionalized polymers in grafting process. By thermally induced aminolysis at 50 °C, the amine groups from 1,6-diaminohexane and polyethylenimine (PEI) in methanol solution will form the covalent bonds with carbonyl groups on PET surfaces, as shown in Scheme 4.10.
39
PET surfaces were characterized by water contact angle measurements and X-ray photoelectron spectroscopy (XPS). Both of measurements were performed on PET surfaces before and after aminolysis reaction, as shown in Table 4.6 and 4.7.
Table 4.6 Aminolysis reaction of PET with polyethylenimine (PEI) at 50 °C
Time (h) ƟH2O (°)
0 64.4 ± 6.0 1 50.4 ± 0.5
4 47.9 ± 3.8 24 48.4 ± 1.9
Table 4.7 Aminolysis reaction of PET with 1,6-diaminohexane at 50 °C
40
shown in Figure 4.5 Although, there was degradation on aminolized PET surfaces prepared from 1,6-diaminohexanes for aminolysis time of 24 h. Consequently, it couldn’t be grafted by end-functionalized polymers. The surface degradation didn’t occur in aminolized PET surfaces prepared from 1,6-diaminohexane for aminolysis time of 24 h.
Figure 4.5 XPS spectra of PET surfaces (a) before and (b) after aminolysis reaction with PEI
4.5.2 Grafting “to” of End-functionalized Polymers on Aminolized PET Surfaces
41
64 % was prepared in the solution of THF/Et3N (98/2, v/v) at ambient temperature for 48 h, as shown in Scheme 4.11. While grafting of poly-LAMA from the monomers conversion 100 % was prepared in the solution of CH3OH/Et3N (9/1, v/v) at 40 °C for 24 h, as shown in Scheme 4.12. Triethylamine (Et3N) was used as base functions to prepare the formation of amide groups from end-functionalized polymers on aminolized PET surfaces.
Scheme 4.11 Grafting of PS on aminolized PET surfaces
42
Grafting of end-functionalized polymers on aminolized PET surfaces was characterized by contact angle measurements, as shown in Table 4.8. Both of PET surfaces either were grafted by PS or poly-LAMA show the difference of contact angle with aminolized PET surfaces without grafting (PET reference, Ɵ = 48°). Grafting of PS on aminolized PET surfaces obtained the higher contact angle than PET reference because of the hydrophobic properties of PS. While grafting of poly-LAMA on aminolized PET surfaces obtained the lower contact angle than PET reference because of the hydrophobic properties of poly-LAMA. Although, grafting of PS and poly-LAMA on aminolized PET surfaces still didn’t reach the angle contact of standard hydrophobic and hydrophilic materials (Ɵhydrophobic = 110°)47 and (Ɵhydrophilic = 14°)48. Preliminary results of “grafting-to” obtained the conditions which need to be optimized.
Table 4.8 Grafting of PS (in the solution of THF/Et3N (98/2, v/v)) and poly-LAMA (in the solution of CH3OH/Et3N (9/1, v/v)) on aminolized PET surfaces by “grafting-to” technique
Grafting of Time (h) Temperature (°C) ƟH2O (°)
PS 48 ambient 63.3 ± 2.9
43 CHAPTER 5
CONCLUSIONS AND RECOMMENDATIONS
5.1 Conclusions
End-functionalized polymers have been prepared by RAFT polymerization technique. A new RAFT agent prepared from succinimide compounds was designed and used to mediate homopolymerizations of styrene, DMAEMA, and LAMA. Controlled molar masses and narrow polydispersities were observed by SEC.
Surface modification of PET also have been prepared by aminolysis reactions. PET surfaces was incorporated with amine groups from 1,6-diaminohexane and PEI. The hydrophilic properties of aminolized PET surfaces was observed by contact angle and XPS.
Grafting end-functionalized polymers on aminolized PET surfaces was prepared by “grafting-to” technique. Only PS and poly-LAMA could be grafted on aminolized PET surfaces by “grafting-to” technique because of nothing formed precipitate of poly-LAMA. The properties of PET surfaces after grafting process were characterized by water contact angle measurements. Grafting of PS on aminolized PET surfaces obtained the increasing of contact angle (Ɵ = 63°) because of their hydrophobic properties. In otherwise, grafting of poly-LAMA on aminolized PET surfaces obtained the decreasing of contact angle (Ɵ = 39°) because of their hydrophilic properties. As the comparison, the water contact angle with aminolized PET surfaces without grafting is equal to 48°. By this results, modification of PET surfaces with end-functionalized polymers prepared from RAFT agents can be proposed to antibacterial tests.
44
removed such as unreacted monomers, in contrast to preformed polymer for the “grafting-to” approach.
5.2 Recommendations
1. Optimize grafting end-functionalized polymers by “Grafting-to” method. 2. Grafting end-functionalized polymers on aminolized PET surfaces by
“Grafting-from” method.
49
NMR Yield Precipitation Yield
50
Table 3. Results for RAFT polymerization using Suc-CTA of DMAEMA at 80
45
REFERENCES
(1) Irena, G., Jolanta, B., Karolina, Z. ,Applied Surface Science 2009, 255, 8293-8298.
(2) Droumaguet, B.L., Nicolas, J., Polym. Chem. 2010, 1, 563-598.
(3) Benedicte, L., Wang, X., Baltaze, J., Liu, H., Hery, J., Bellon-Flntaine, M., Roger, P., European Polymer Journal 2011, 47, 1842-1851.
(4) Ferreira, L., Zumbuehl, A., J. Mater. Chem. 2009, 19, 7796.
(5) Gour, N., Ngo, K.X., Vebert-Nardin, C., Macromol. Mater. Eng. 2014, 299, 648-668.
(6) Nikles, D.E., Farahat, M.S., Macromol. Mater. Eng. 2005, 290, 13.
(7) Tiller, J.C., Liao, C.J., Lewis, K., Klibanov, A.M., Proc. Natl. Acad. Sci.
2001, 98, 5981.
(8) Goddard, J.M., Hotchkiss, J.H., Prog. Polym. Sci. 2007, 32, 698-725. (9) Lai, J.T., Filla, D., Shea, R., Macromolecules 2002, 35, 6754-6756.
(10) Fu, J., Cheng, Z., Zhou, N., Zhu, J., Zhang, W., Zhu, X., Polymer 2008, 49, 5431-5438.
(11) Goddard, J.M., Hotchkiss, J.H., Prog. Polym. Sci. 2007, 32, 698-725. (12) Moad, G., Rizzardo, E., Thang, S.H., Polymer 2008, 49, 1079. (13) Banerjee, I., Pangule, R.C., Kane, R.S., Adv. Mater. 2011, 23, 690. (14) Petcore, 2010, PET Profile, Issue, Belgium.
(15) Bergbreiter, D.E., and Martin, C.R., 1989, Functional Polymers, Texas A&M University, Texas.
(16) Barner, C., and Kowollik, 2008, Handbook of RAFT Polymerization, WILEY-VCH Verlag GmbH & Co.KGaA, Sydney.
(17) Moad, C.L., Moad, G., Rizzardo, E., Thang, S.H., Macromolecules 1996, 29, 7717-7726.
(18) Krstina, J., Moad, G., Rizzardo, E., Winzor, C.L., Berge C.T., Fryd, M.,
Macromolecules 1995, 28, 5381-5835.
46
(20) Moad, G., Solomon, D.H., The chemistry of radical polymerization. 2nd ed. Oxford: Elsavier, 2006, 498-525.
(21) Goddard, J.M., Hotchkiss, J.H., Prog. Polym. Sci. 2007, 32, 698-725.
(22) Brueckner, T., Eber, A., Heumann, S., J. Polym. Sci. A: Polym. Chem. 2008, 46, 6435.
(23) Zohdy, M.H., Rad. Phys. Chem. 2005, 73, 101.
(24) Chen, K.S., Ku, Y.A., J. Appl. Polym. Sci. 2005, 245, 223.
(25) Zhu, A.P., Zhao, F., Fang, N., J. Biomed. Mater. Res. A, 2008, 86A, 467. (26) Laskarakis, A., Logotheditis, S., Thin solid films 2008, 516, 1443.
(27) Kurihara, Y., Ohata, H., Kawaguchi, M., J. Appl. Polym. Sci. 2008, 108, 85. (28) Nikles, D.E., Farahat,M.S., Macromol. Mater. Eng. 2005, 290,13.
(29) Lorenzetti, C., Manaresi, P., J. Polym. Environ. 2006, 14, 89.
(30) Zhu, Y., Gao, C.E, Liu, X., Shen, J., Biomacromolecules 2002, 3, 1312-1319. (31) Lopes, A.A.B., Peranovich, T.M.S., Maeda, N.Y., Bydowski, S., P.
Thhromb. Res. 2001, 101, 291.
(32) Holmes –Farley, S.R., Reamey, R.H., McCarthy T.J., Deuthch, J., Whitesides, G.M., Langmuir 1985, 1, 725-740.
(33) Goddard, J.M., Hotchkiss, J.H., Prog. Polym. Sci. 2007, 32, 698-725. (34) Sabbatini, L., Zambonin, P.G., Surface characterization of advanced
polymers. New York: VCH Publishers, Inc., 1993.
(35) Kiss, E., Golander, C.G., Eriksson, J.C., Prog Coll Pol Sci 1987, 73, 113-119.
(36) Crombez, M., Chevallier, P., Gaudreault, R.C., Peticlerc, E., Mantovani, D., Laroche, G., Biomaterials 2005, 26, 7402-7409.
(37) Briggs, D., Brewis, D.M., Dahm, R.H., Fletcher, I.W., Surf. Interface Anal. 2003, 35, 156-167.
(38) Terlingen, J.G.A., Gerritsen, H.F.C., Hoffman, A.S., Jen, F.J., J. Appl. Polym. Sci. 1995, 57, 969-982.
(39) Boshmann, D.; Edam, R.; Schoenmakers, P.J.; Vana, P. Polymer 2008, 49, 5199-5208.
47
(42) Nikles, D.E.; Farahat, M.S. Macromol. Mater. Eng. 2005, 290, 13. (43) Narain, R.; Armes, S.P. Macromolecules 2003, 36, 4675-4678.
(44) Zobrist, C.; Sobocinski, J.; Lyskawa, J.; Fournier, D.; Miri, V.; Traisnel, M.; Jimenez, M.; Woisel, P. Macromolecules 2011, 44, 5883-5892.
(45) Housni, A.; Cai, H.; Liu, S.; Pun, S.H.; Narain, R. Langmuir 2007, 23, 5056-5061.
(46) Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. J. Polym. Sci. 2007, 45, 2172-2183.
(47) Adamson, A.W. Physical chemistry of surfaces (3rd edition) wiley, 1976, New York.
51
BIOGRAPHY



![Table 4.1. Results for CFRP of St at 70 °C ([St]:[AIBN]=100:1)](https://thumb-ap.123doks.com/thumbv2/123dok/1818409.2100750/46.595.84.514.86.775/table-results-cfrp-st-c-st-aibn.webp)