SJR is developed by:
Title Type SJR H
index
Total Docs. (2013)
Total Docs. (3years)
Total Refs.
Total Cites (3years)
Citable Docs. (3years)
Cites / Doc. (2years)
Ref. /
Doc. Country
1 International Journal of Energy
Economics and Policy j 0,567 6 60 44 1.745 49 44 1,11 29,08
2
Energy Education Science and Technology Part A: Energy Science and Research
j 0,269 38 432 642 3.872 270 639 0,40 8,96
ENERGY EDUCATION SCIENCE AND TECHNOLOGY PART A
ISSN: 1308-772X
Volume (Issue) 32 (1) January 2014
1. Development of municipal monitor 1-6 C. Guan,G. Wang,S. Chen,H. Wang, F. Nie,Y. Sun,L. Ming
2. Study on selective mining of coal and gangue and strip-filling technology for thin seam 7-12 L. Wan, L. Ren, Q. Zeng, L. Wang 3. Multiple biodiesel mixtures in diesel engine - performance and emission analysis 13-24 K. A. Balasubramanian, K. Srithar 4. Research to hydraulic metal structure health diagnosis based on FAHP 25-38 G. Yang,K. Yang
5. Calculation model of comprehensive filtration coefficient and its effect on fracture length of coal reservoir 39-50 L. Xu, J. Cui, J. Tang,P.Yu,J Teng1,S. Huang,Y. Chen1, L. Cai,X. Sun
6. Research on China rural land circulation legal system based on land and energy 51-56 Y. Y. Zhu
7. Sealing mechanism and experimental research of MICSE based on the theory of elastohydrodynamic lubrication 57-68 G. Zhiping, L. Guanfu, M. Shujing, W. Yanfei,G. Wei1, L. Yanzheng, H. Jianmei
8. US Fayetteville Shale Gas reservoir modeling 69-80 S. Zheng, H. Fan, S. Jiang
9. Study on capillary number curve of binary compound flooding in offshore oilfield 81-92 C. Nan, S. Kaoping, T. Engao, Z. Jian, P. Yanfu
10. Study on methods of measuring thermo-physical properties of inhomogeneous solid materials for solar thermal storage 93-106 W. Lu, F. Li, L. Jinhao
11. Leakage detection of heating network with graph theoretic approach 107-118 Y. Xianliang, J.Lianlian, H. Wenhui, W. Songling
12. Researching the law of remaining oil distribution after polymer flooding in offshore oil field 119-132 T. Engao, Y. Erlong, S. Liyan, J. Yinghua 13. Numerical computation of bend-flows using a modified 2D depth-averaged model in curvilinear coordinates 133-138 Y. Liu,S. Shao, Z Liu,W. Wei 14. The empirical study of the application of network information technology platform in the teaching of English writing 139-142 Y-J. Chu
15. The analysis of structure strength and calculation of critical speed of a new type of high-speed permanent magnet motor 143-148 X. Zhang, L. He, G. Li, Z. Zhou
16. Study on eutrophic evaluation and numerical simulation of TP content in Nansi Lake 149-154 L. F. Wang, L. Yang 17. Butterfly: the analysis of commercial housing price formation based on inflation visual 155-160 W. Liang, J. Duan 18. Simulation on the freezing process of packaged food products 161-166 G. Wang, P. Zou, M. Liu
19. Reliability analysis of key parts for differential system 167-172 X. Liu, S. Zheng, T. Chen, J. Feng 20. A construction method for dew point curves of hydrocarbon mixtures 173-180 W. Jia, C. Li, X. Wu
21. Sequence stratigraphy and paleogeography of the Late Triassic coal-measures in south China: implication for coal development 181-190 Y. Li, L. Shao, C. Zhang, C. Gao, W. Liang
22. The trend of china energy structure: Forecast natural gas consumption 191-194 Z. Cai, X. Liao
23. A multi-agent architecture for the self-healing of SGs based on IEC 61499/61850 195-212 Z. Jiang, O. Mosbahi, M. Khalgui 24. Traveling-wave-based Fault-location method under low sampling rate 213-220 J. Tang, X. Yin1, Z. Zhang
25. Distribution characteristics and influencing factors of indoor particulate concentration in university functional buildings 221-234 L. Yang, F. Dong, J. Wei,G. Song, T. Qiang, A. Cheng
26. The causal relationship between economic growth, energy consumption and CO2 emissions in Hong Kong 235-248 S. L. Lai, K. C. Kuo, P. Kanyasathaporn, M. Liu
27. The construction of forest farm’s sustainable development indicators and the empirical study--Taking ming xi state-owned forestry farm as an example 249-258 T. Li, B. Cheng, J. Zhao, J. Chen
28. Numerical simulation with Lattice Boltzmann method for flow and heat transfer of the nanofluids 259-272 S. Yao, G. Wang, X. Jia, L. Duan, C. Zhou 29. Study on quality evaluation method of color reproduction of coated paper in ink-jet printing 273-282 W. Da, C. Qifeng
Energy Education Science and Technology Part A. Energy Science and Research experimentally. The results showed that both of perforated and secondary flames were formed in very lean mixture with maximum laminar flame velocity (SL) higher than that of hexadecane and almost
similar with that of ethanol flame. Slight increase of equivalent ratio (φ) causes drastic decrease of SL
of perforated and secondary flame and SL reaches minimum at φ = 0.355 where the physic of flame
change into open tip Bunsen and triple flame. Above φ = 0.365, SL of open tip Bunsen flame relatively
constant much lower than that of hexadecane flame at around stoichiometry. Small explosions occur due to ambient air intervention attributed to unsaturated fatty acid components of Jatropha curcas which reaches 55% of the overall composition. Without ambient air intervention perforated flames experienced lift off at φ = 0.355 to 0.375, perforated and secondary flame are stable at φ = 0.387 to 0.467, and from φ = 0.489 to 0.585 the flame becomes cellular in the form of island and petal. Above φ = 0.632 the flame become very unstable.
Keyword: Bunsen flame with open tip; Cellular flame; Laminar burning velocity; Perforated
flame; Triple flame
©Sila Science. All Rights Reserved.
1. Introduction
The availability of fossil fuels become the world attention as limited as non-renewable energy sources. To solve this problem, scientists have tried to make biofuels from crops as a fuel alternative to fossil fuels. The difficulty in making biofuels appears when the plant is also used as a food ingredient. In order to avoid this competition, non-food plants producing high
___________ *
Corresponding author: Tel.: +62-361-431-214; fax::+62-361-703-321.
284 I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292
oil is needed. One of the plants that produce high oil but cannot be used as food is Jatropha curcas. Jatropha curcas contains 40.70% monounsaturated fatty acids and 37.80% polyunsaturated fatty acids that could potentially cause an explosion when combustion occurs [1]. Urgency using Jatropha curcas is as a source of energy because it produces biofuel with high productivity, while jatropha curcas is non edible and growing in arid regions.
Several studies have been conducted with different fuel compositions to get a diesel alternative fuel with the raw material Jatropha curcas. Jatropha curcas can be used as biodiesel fuel and environmental friendly without modification. Jatropha curcas oil meets ASTM standards and showed better performance than ordinary fuel in diesel engines. Another advantage of jatopha is as follows: (i) able to reduce the greenhouse gas, (ii) used as a raw material and (iii) do not compete with food crops [2]. Different fuel properties of jatropha curcas oil methyl ester (JMEs) including heating value, filter plugging point, density, kinematic viscosity and oxidation stability in a mixture of diesel is calculated. Recommended mixture ratio of JMEs with diesel above 40% volume is compared with the relevant specifications for biodiesel-diesel blend [3]. Jatropha curcas oil has been tested and is able to replace fossil diesel fuel in a multi-cylinder with water cooling direct injection (IDI) engine type CI. Atrophy curcas oil give 3% higher pressure at top dead center, showed 5% shorter combustion duration, the same cumulative heat release at full load, but lower heat release at lower loads when compared to fossil diesel. Thus, minor modifications in the coolant and fuel supply circuit are needed on CI IDI engine type when using jatropha curcas oil [4]. Jatropha curcas seeds shell allows the generation of heat without formed into pellets or briquettes. Thermal power generated is 2.9 kg / hour jatropha curcas seed shells of 11.1 kW with 87% efficiency furnace or 9.0 kg / h shell jatropha curcas seeds of 36.7 kW with 91% efficiency furnace. The carbon monoxide is 0.4 - 2 g/m3 lower than burning wood by legal requirements in Germany for up to 50 kW combustion units [5]. Quasi-steady gas-phase combustion of spherical particles fed from jatropha curcas bio-diesel has been carried out numerically and experimentally [6]. Jatropha curcas bio-diesel used in the experiment were mixed with convective air environment and compared with ordinary diesel fuel under the same conditions. The investigation revealed that biodiesel from jatropha curcas without purification is suitable as an alternative to diesel fuel. Improvement in jatropha curcas biodiesel mixture is required to obtain optimal performance, the characteristics of good combustion and low emissions [7]. Influence of various parameters such as acid value (AV), water content (WC), and ash content (AC) of jatropha curcas oil sediment accumulation has been investigated. It also resulted in safe operation, low maintenance, and optimal power, required acid value AV is lower than 6.00 mg KOH / g, WC moisture content less than 0.15% and ash content below 0.10% AC [8]. Experimental investigations have been conducted for the feasibility of Jatropha curcas as an alternative diesel fuel. The experiment used B0 (100% diesel), B10 (90%
diesel and 10% biodiesel jatropha curcas) and B20 (20% biodiesel jatropha curcas and 80%
diesel). The results showed that the characteristics of the B10 and B20 are almost similar with
B0. These results showed that jatropha curcas biodiesel blends (B10 and B20) can be used in
Diesel engines without major modifications [9].
I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292 285
2. Methods
The experimental study on premixed combustion of jatropha curcas oil was carried out in an experimental apparatus shown schematically in Fig.1. The jatropha curcas oil was evaporated in a boiler with steam temperature kept constant at 160oC. The oil steam from boiler was mixed with air from compressor at mixing chamber with equivalent ratio (φ) varied
from lean (φ=0.310) to rich mixture (φ=1.548). Lean mixture is range below φ=0.894 and rich is above 1.094 while nearly stoichiometric is between φ=0.894 to 1.094. The reactant then
flows into nozzle before it was ignited to form premixed flame at perforated plate installed on the top of the nozzle.
Fig.1. Experimental equipment.
The perforated plated was installed to utilize thermal contact resistant for preserving
temperature distribution which is more uniform in entire surface of the plate and ensure the uniformity flow for jatropha curcas oil with air during the combustion process. Perforated plate was made from steel and designed with geometrical matrix with 19 holes. The diameter of each hole was 2.5 mm and the distance between holes was 3.75 mm.
The flame image was captured by camera in two experimental conditions: (1) premixed flame of jatropha curcas oil in contact with surrounding ambient air, (2) premixed flame of jatropha curcas oil shielded from surrounding ambient air.
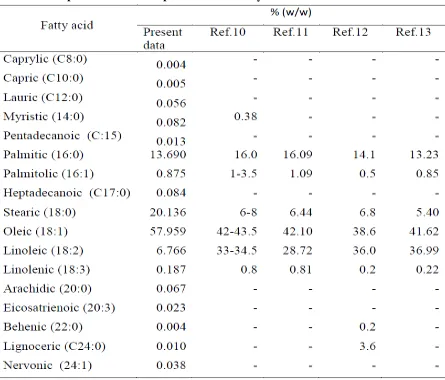
The jatropha curcas oil used in this experiment consists of 85% fatty acid and 15% glycerol. The component of fatty acids in jatropha curcas oil is listed in table 1. More than 55% jatropha curcas components are unsaturated long-chain fatty acids. The reaction of jatropha curcas oil with the oxidizer was estimated by simple molar analysis described with equation 1 and equation 2 as follows:
0.004C8H18O2+ 0.005C10H20O2+0.056C12H24O2+0.082C14H28O2+0.013C15H30O2
+13.690C16H32O2 +0.875C16H30O2 +0.084 C17H34O2+20.136C18H36O2
286 I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292
+ 0.023C20H34O2+ 0.004C22H44O2 +0.010C24H48O2+0.038 C24H46O2
+33.68(O2+3.76N2) 17.70CO2 + 33.95H2O+126.63N2 ... 1
C3H5(OH)3 + 3.5(O2+3.76N2) 3CO2 + 4H2O+13.16N2 ... 2
The equation 1 is the combustion reaction of equivalent fatty acids molecule from present data in table 1 and equation 2 is that of glycerol. From equation 1 and 2 the stoichiometric air fuel ratio (AFRstoic) of jatropha curcas oil was 14.87 g air / g fuel. The equivalent ratio (φ) was
calculated as the ratio of stoichiometric air fuel ratio to actual air fuel ratio.
Table 1. Jatropha curcas oil composition from many references
Gas Chromatography is used to determine the percentage of fatty acids in jatropha curcas oil collected from Malang, East Java-Indonesia. Fatty acids are formed by long carbon chain where the carbon is bound by a single or a double bond. Methyl group is present at one end and a carboxyl group at the other end. The absence or presence of a double bond between carbons defines saturated and unsaturated fatty acids [14].
3. Results and discussion
Premixed combustion of jatropha curcas oil is shown in Fig.2 at equivalence ratio (φ) from
I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292 287
Mixture with φ = 0.355 to 1.548 formed open tip Bunsen and triple flame. More than 55% jantropha curcas contain unsaturated fatty acids which are easily oxidized. The more it has double bonds the more susceptible to oxidation which causes unstable molecular bonds.
Fig. 2. Jatropha curcas oil flame
As shown in Fig. 3 when Jatropha curcas oil flame was isolated from surrounding ambient
air perforated flame and secondary Bunsen flame still appears at φ = 0.355 to 0.467 and φ=0.414 to 0.467 respectively. At φ = 0.355 to 0.375 perforated flame is lifted off. The
Bunsen open tip and triple flame are disappeared, however, cellular flame takes place at φ = 0.489 to 0.585 and flame become unstable at φ = 0.632 to 1.548. This phenomenon indicates
that the stability of jatropha curcas oil combustion is strongly influenced by ambient air.
288 I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292
3.1.Laminar flame speed
The reactant velocity, v is estimated as in equation 3
b
area. Laminar burning velocity of flame (SL) can be estimated by using equation (4) as
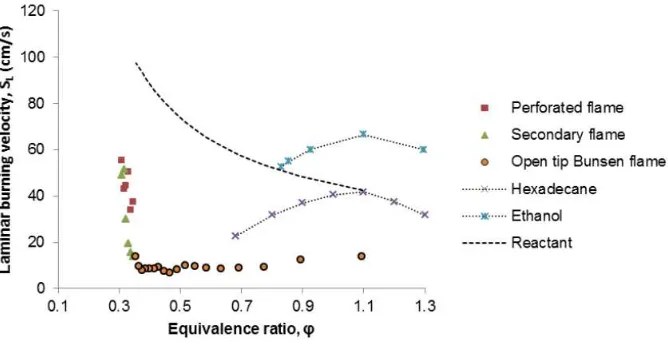
Fig. 4. Laminar flame speed of jatropha curcas oil versus equivalence ratio.
The stability of perforated and secondary flame were presented by Fig. 4 even both maximum laminar burning velocity SL is almost the same as ethanol [15] and higher than
hexadecane [16] on very lean mixture. Slight increase of φ causes abrupt decrease of SL of
perforated and secondary flame from 55.24 cm/sec to 37.53 cm/sec and from 48.93 cm/sec to 13.77 cm/sec, respectively. Enriched mixture formed open tip Bunsen flame with nearly constant SL. The flame is still stable even in the form of open tip Bunsen flame until the
mixture is very rich. This shows that flammability limit of jatropha curcas oil is much wider than that of the conventional fossil fuel.
Fig. 5 shows laminar burning velocity of jatropha curcas oil estimated from Fig. 3. It can be seen at Fig. 5 that when the flame is isolated from ambient air the SL of perforated and
secondary flames is almost the same as that of ethanol [15] and higher than that of
hexadecane [16] on very lean mixture. By increasing φ slightly causes drastic decrease of SL
of perforated and secondary flame from 64.08 cm/sec to 30.81 cm/sec and from 58.52 cm/sec to 17.91 cm/sec, respectively. Above φ = 0.467, SL cannot be estimated since the flame is
I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292 289
Fig. 5. Laminar flame speed of jatropha curcas oil versus equivalence ratio isolated from ambient air.
3.2.Bunsen flame with open tip
At lean mixture, the amount of air is sufficient for complete combustion so that SL is high
and the Bunsen flame occurs on each hole of perforated nozzle. As φ is increased the fraction of fuel in the mixture increases which absorb more energy for igniting the mixture. Consequently SL of longer-chain saturated fatty acids and glycerol is so low that results in
secondary Bunsen flame in the downstream of perforted flame. For SL is drop dramatically
Fig. 6. Bunsen flame with open tip at φ=0.632.
the secondary Bunsen flame tends to stretch which cause open tip. At higher φ longer-chain fatty acids and glycerol become difficult to burn and eventually escaped towardproduct zone to produce a yellow sooty diffusion flame in the tip of the flame [17]. It is clearly seen from Fig.6 that the open tip formation in the flame of jatropha curcas oil at φ = 0.632 is mainly caused by unburned long-chain fatty acids and glycerol that escapes into product zone. The open tip Bunsen flame mechanism made by both of escaped unburn fuel and pollutant is based on the Damkohler number concept [18].
3. 3. Cellular flame
290 I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292
Fig.7. (a) Islands cellular flame φ =0.516, (b) Petals cellular flame φ =0.547.
the combustion process of jatropha curcas oil at lean mixture (φ = 0.516). At this mixture the fuel number in the mixture is small so that the heat energy produced by the flame is not enough for glycerol to burn. Therefore, glycerol escapes to product zone becoming diffusion flame. Radiant heat from diffusion flame of glycerol serve heat energy for fast burning of medium-chain fatty acids (see table 1) but it is not enough to burn the long-chain of fatty acid in the multi-component that compose the oil. This process results in island cellular flame on the perforated plate. This phenomenon is similar to that reported by [19). When fuel number
in the mixture was increased to φ = 0.547, glycerol flame radiant heat disappears, so that medium and long-chain fatty acids unburned produce petals cellular flame as shown in the picture 7b. These results are similar to those discussed by [20].
3. 4. Triple flame
Fig. 8. Shape flame jatropha curcas oil with glycerol at φ = 0.547.
In Fig. 2 triple flame of jatropha curcas oil formed at mixture from φ = 0.355 to 1.548. Fig.
8 shows detail structure of jatropha curcas oil triple flame at φ = 0.547. The flame formed by
I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292 291
of hot gas free convection in lean premixed area or unsteady lean premixed flame occurs when the hot gas moves downstream as found by [21].
4. Conclusion
Jatropha curcas oil premixed combustion behavior has been observed experimentally on
perforated burner with φ varied from very lean to very rich. The results showed that at very lean mixture Bunsen flame is performed on every hole of perforated plate and their average laminar burning velocity is almost the same as ethanol. Slight increase of φ causes drastic decrease of laminar burning velocity of perforated and secondary flame and at the end of
decreasing flame speed perforated and secondary flame disappears at φ = 0.355. The equivalence ratio enhanced produces Bunsen open tip and triple flame with relatively constant burning velocity lower than hexadecane flame.
When the flame was isolated from ambient air, at very lean mixture Bunsen flame is sill formed on every hole of perforated plate with secondary Bunsen flame in the downstream of perforted flame but open tip Bunsen and triple flame disappears. Increasing φ produces island and petal cellular flame due to oxidizer leak for burning of glycerol and fatty acid. When the mixture is further enriched the flame becomes very unstable.
[1] Berchmans HJ, Morishita K, Takarada T. Kinetic study of hydroxide-catalyzed methanolysis of jatropha curcas–waste food oil mixture for biodiesel production. Fuel 2010; xxx: xxx-xxx. [2] Mofijur M, Masjuki HH, Kalam MA, Hazrat MA, Liaquat AM, Shahabuddin M, Varman, M. Prospects of biodiesel from jatropha curcas in Malaysia. Renew Sustain Energy Rev
2012;16:5007-5020.
[3] Chen LY, Chen YH, Hung YS, Chiang TH, Tsai CH. Fuel properties and combustion
characteristics of jatropha curcas oil biodiesel–diesel blend. J Taiwan Inst Chem Eng 2013;44:214-220.
[4] Hossain AK, Davies PA. Performance, emission and combustion characteristics of an indirect injection (IDI) multi-cylinder compression ignition (CI) engine operating on neat jatropha curcas and karanj oils preheated by jacket water. Biomass Bioenergy 2012;46:332-342.
[5] Kratzeisen M, Müller J. Suitability of Jatropha curcas seed shells as fuel for small-scale combustion units. Renew Energy 2013;51:46-52.
[6] Rajesh S, Raghavan V, Shet USP, Sundararajan T. Analysis of quasi-steady combustion of jatropha curcas bio-diesel. Int Commun Heat Mass Transfer 2008;35:1079-1083.
[7] Sahoo PK, Das LM. Combustion analysis of jatropha curcas, karanja and polanga based biodiesel as fuel in a diesel engine. Fuel 2009;88:994-999.
[8] Kratzeisen M, Muller J. Prediction of deposit formation during combustion of jatropha curcas oil from standard quality parameters. Fuel 2010;89:2769-2774.
292 I. K. G. Wirawan et al. / EEST Part A: Energy Science and Research 31 (2014) 283-292
[10] Verma KC, Gaur AK. Jatropha curcas L.: substitute for conventional energy. World J Agric Sci 2009;5:552-556.
[11] Deng X, Fang Z, Liu, Y. Ultrasonic transesterification of Jatropha curcas L. oil to biodiesel by a two-step process. Energy Convers Manage 2010;51:2802-2807.
[12] Jain S, Sharma MP. Biodiesel production from Jatropha curcas oil. Renew Sustain Energy Rev 2010;14:3140-3147.
[13] Wang R, Hanna MA, Zhou WW, Bhadury PS, Chen Q, Song BA, Yang S. Production and
selected fuel properties of biodiesel from promising non-edible oils: euphorbia lathyris L., sapium sebiferum L. and jatropha curcas L. Biores Technol 2011;102:1194-1199.
[14] Calviello G, Serini S. Diet and cancer 1. dietary omega-3 polyunsaturated fatty acids and cancer. Springer Italy, pp. ix-xxi, 2010
[15] Broustail G, Seers P, Halter F, Moréac G, Mounaim RC. experimental determination of laminar burning velocity for butanol and ethanol iso-octane blends. Fuel 2011;90:1–6.
[16] Chaos M, Kazakov A, Dryer FL, Zhao Z, Zeppieri SP. High Temperature compact mechanism development for large alkanes: n-hexadecane. 6th International Conference on Chemical Kinetics 2005.
[17] Schönborn A, Ladommatos N, Williams J, Allan R, Rogerson J. The influence of molecular tructure of fatty acid monoalkyl esters on diesel combustion. Combust Flame
2009;156:1396-1412.
[18] Ishizuka S, Sakai Y. Structure and tip-opening of laminar diffusion flames. Twenty-first Symposium (International) on Combustion/The Combustion Institute 1986:1821-1828. [19] Kadowaki S, Takahashi H, Kobayashi H. The effects of radiation on the dynamic behavior of cellular premixed flames generated by intrinsic instability. Proceedings Combustion Institute 2011;33:1153-1162.
[20] Wang Y, Hu S, Pitz RW. Extinction and cellular instability of premixed tubular flames. Proceedings of the Combustion Institute 2009;32:1141-1147.