The
11
th
International
Conference on
Membrane Science
&
Technology
"SUSTAINABLE TECHNOLOGY FOR
ENERGY, WATER
&
ENVIRONMENT"
27th - 29th Aug 2013
Seri Pacific Hotel
©
Advanced Membrane Technology Research Centre (AMTEC), Faculty of Petroluem & Renewable Energy Engineering (FPREE), Universiti Teknologi Malaysia (UTM) 2013All right reserved. No part ot trus publication may be produced, copied, stored in any retrieval system or transmitted in any form or any means - electronic, mechanical, photocopying, recording or otherwise; without prior permission in writing from the Advanced Membrane Technology Research Centre (AMTEC), Faculty of Petroleum & Renewable Energy Engineering (FPREE), Universiti Teknologi Malaysia (UTM), 8 I J
to
Skudai, Johor Darul Takzim, Malaysia.
The
11th
International Conference on Membrane Science & Technology (MST2013),
Sustainable Technology for Energy, Water & Environment, Seri Pacific Hotel, Kuala Lumpur, Malaysia,27
1h -29th
Aug
2013,
Organized by UniversitiTeknoloqi
Malaysia (UTM) Published and printed by:Advanced Membrane Technology Research Centre (AMTEC), Faculty of Petroleum & Renewable Energy Engineering (FPREE), Universiti Teknologi Malaysia (UTM),
81310
Skudai, Johor Darul Takzim, Malaysia.Tel:
+607-5535925/5581463
Fax:
+607-5535925/5535807
CONTENTS
Foreword by the Deputy Minister of Ministry of
Science, Technology and Innovation of Malaysia
(MOSTI)
i
Foreword by the Deputy Vice-Chancellor of
Universiti Teknologi Malaysia
iii
Foreword by the Chairman of MST 2013
vi
Organizing Committee
vii
Conference Program
ix
List of Poster Presentations
xxii
List of Industrial Participants
xxv
Invited Speakers
xxvi
Abstract of invited Speakers
1
Contribution papers
25
Abstract of contribution papers
27
ADVISORY COMMITTEES
Zaini Ujang Universiti Teknologi Malaysia. Malaysia
Mohd Azraai Kassim Universiti Teknologi Malaysia. Malaysia Anthony Gordon Fane Nanyang Technological University, Singapore Wirote Youravong Prince of Songkla University, Thailand
I.G. Wenten lnstitut Teknologi Bandung, Indonesia
Tutuk Djoko Kusworo Universitas Diponegoro (UNDIP), Indonesia
Takeshi Matsura University of Ottawa, Canada
H.G.L. Coster University of Sydney, Australia, Dean of College of Engineering, University of Tehran,Iran
Madzlan Aziz Universiti Teknologi Malaysia. Malaysia
Ariffin Samsuri Universiti Teknologi Malaysia. Malaysia
SCIENTIFIC COMMITTEES
Heru Susanto Universitas Diponegoro (UNDIP), Indonesia
Wang Rong Nanyang Technological University, Singapore
Neal Chung Tai-Shung National University of Singapore, Singapore Tutuk Djoko Kusworo Universitas Diponegoro (UNDIP), Indonesia Arun M lsloor National Institute of Technology Kamataka,India Mohammad M. Montazer-Rahmati University of Tehran, Iran
Mohamed Mahmoud El-Sayed
Nasef Universiti Teknologi Malaysia. Malaysia
Devaraj Veerasamy Malaysian Rubber Board (LGM), Malaysia Zularisam Bin Abd Wahid Universiti Malaysia Pahang, Malaysia Azmi Mohd Syariff Universiti Teknologi Petronas, Malaysia
Zakaria B Man Universiti Teknologi Petronas, Malaysia
Azeman Mustafa Universiti Teknologi Malaysia. Malaysia
Azman Shafawi Petronas Research Sdn. Bhd., Malaysia
Nur Awanis bt. Hashim Universiti Malaya, Malaysia Mohd lrfan Hatem bin Mohamed Dzahir Universiti Malaysia Perlis, Malaysia Rosli b. Md. lllias Universiti Teknologi Malaysia. Malaysia Wan Ramli b. Wan Daud Universiti Kebangsaan Malaysia
Kang Li Imperial College, London
Nurmin Bolong Universiti Sabah Malaysia, Malaysia
Rosalam Sarbatly Universiti Sabah Malaysia, Malaysia
SharifahAishah Syed A. Kadir UniversitiTeknologi Mara Mohamed Kheireddine b. Taieb Aroua Universiti Malaya, Malaysia
Azmi Rahmat Universiti Malaysia Perlis, Malaysia
Mohammad Reza Mehrnia University of Tehran, Iran Mojtaba Shariaty-niassa University of Tehran, Iran
Mohd. Ghazali Mohd. Nawawi Universiti Teknologi Malaysia. Malaysia
Meisam Tabatabaei Agricultural Biotechnology Research Institute of Iran Anil K. Pabby Bhabha Atomic Research Centre, Maharashtra, India
27th-29th August 2013
UNIVERSITI TEKNOLOGI MALAYSIA KUALA LUMPUR, MALAYSIA Pre-Conference
26th Aug 2013 (Monday)
1600-1800 Pre-registration
Conference Day 1 27th Aug 2013 (Tuesday)
0815-0930 Registration
0930-0945 Welcoming speech by MST2013 Conference Chairman
Prof. Dr. Ahmad Fauzi Ismail
0945-1045 MAIN HALL
Prof. Michael D. Guiver
Materials design for fuel cell ion exchange membranes
Chairperson: Prof. Abdul Latif Ahmad
1045-1130 Tea break/Poster preparation 1130 Parallel Session I
Membrane for water & wastewater application
Parallel Session II
Membrane for gas &vapor application
Parallel Session III
Membrane for fuel cell & energy, environment application 1130-1200 Dr. I. GedeWenten
Electrodeionization (EDI): State of the art
Prof. Kazukiyo Nagai
Polyimide Membranes Modified for Carbon Dioxide Separation
Prof. T. S. Chung
Molecular Design of Membranes for Clean Water and Clean Energy
Chairperson: Dr. I. GedeWenten Co-chairperson:Dr.NurAwanisHashim
Oral Session
(Pacific Ballroom A/B)
Chairperson: Prof. Kazukiyo Nagai Co-chairperson:Dr.HasrinahHasbullah
Oral Session
(Pacific Ballroom C)
Chairperson: Prof. T. S. Chung Co-chairperson:Farhana Aziz
Oral Session
(Bunga Room)
1200-1215 A022 Y. LukkaThuyavan
Clarification of sufarcane fruit juice using alginate/polyethersulfone blend ultrafiltration membranes
A005 Amir Mansorizadeh
Preparation of porous polyamide-imide Torlon membranes for CO2 absorption: Effect of glycerol in
polymer dope
A015 Evangeline Christina
Performance of polyethersulfone-cellulose acetate membrane blend on fluoride adsorption from aqueous phase
1215-1230 A024 ReshmaLakra
Novel ultrafiltration membrane technology for separation of organic acids and reducing sugars
A006 AzmanShafawi
Challenges of producing membranes from lab/R&D scale to commercial for CO2 separation from natural
A063 NadiahKhairulZaman
Novel glucamine containing adsorbent based on blended chitosan/poly(glycidyl methacrylate)
from rice husk gas electrospunnanofibrous sheet
1230-1245 A026 T. Gajendran
Concentration of orange juice using ultrafiltration employing biopolymer blend polyethersulfone membrane
A002 A. Y. Zahrim
Treatment ofdye solution via low pressure nanofiltration system
A225 R. A. Azmi
Membrane processing of crude palm oil by using crosslinkedpolyvynilidene fluoride hollow fibre membrane
1245-1300 A030 KhairulMuis B. Mohamed Yusof
Recovery of rubber from skim latex using membrane technology
A052 Kamal S. N. M.
Fabrication of PDMS/PES membrane incorporated with SAPO-44 Zeolite membrane for gas separation
A224 E. Yuliwati
Polyvinylidene fluoride hollow fiber membranes for refinery wastewater treatment: Effect of air gap length on membrane morphology and performance
1300-1400 LUNCH
1400-1430
Prof. Takeshi Matsuura
Effect of surface pattern formation on membrane fouling and its control in phase
inversion process
Prof. Mohamed Mahmoud El-SayedNasef
Radiation grafted microfibrous sheets containing functionalized
poly(glycidylmethacrylate) for environmental applications
Prof. M. JavaidZaidi
Polyelectrolyte multilayer films prepared by layer by layer assembly for reverse osmosis
desalination Chairperson: Prof. Takeshi Matsuura
Co-chairperson:Dr.NurminBolong
Oral Session
(Pacific Ballroom A/B)
Chairperson: Prof. Mohamed Mahmoud El-SayedNasef
Co-chairperson: Dr.Mahesh Padaki
Oral Session
(Pacific Ballroom C)
Chairperson: Prof. M. JayaidZaidi Co-chairperson:Dr.Erna Yuliwati
Oral Session
(Bunga Room)
1430-1445 A049 N. H. Othman
The effect of membrane pre-treatment towards the stability of refined palm oil
A070 Zee Ying Yeo
Effect of synthesis duration for the formation of zeolite T membrane by secondary seeded growth and its performance in CO2/CH4 gas separation
A019 R. Balamurali
Influence of unsteady state dynamics on the rejection of cadmium (II) by ultrafiltration
1430-1500 A054 A. W. Zularisam
Effect of piper betle extract as anti-biofouling
A092 Atsushi Morisato
Development of a large membrane module for CO2
removal from hydrocarbon gas and recovery of hydrocarbon liquids
A020 R. Saranya
Development of adsorbents based mixed matrix membranes for removal of heavy metals and dissolved salts from textile industry effluent
1500-1515 A159 A. Moslehyani
Polyethersulfone ultrafiltration membrane for oil-in-water emulsion separation
A074 Mohamed Mahmoud Nasef
New CO2 adsorbent with aminated poly(glycidyl
methacrylate) radiochemically grafted onto polyethylene nonwoven sheets
A044 NorulFatiha Mohamed Noah
Selective emulsion liquid membrane extraction of palladium from simulated liquid waste solution using D2EHPA as a mobile carrier
1515-1530 A069 Feras M. Kafiaha
Preparation of
polysulfoneelectrospunnanofibers: effect of electrospinning and solution parameters.
A099 H. N. Mohammed
CO2 absorption in membrane contactor usingpiperazine, monoethaolamine and diethanolamine in membrane contactor: A mass transfer and performance study
A059 S. C. Low
Studying the role of magnetite (Fe3O4) colloids
functionality on PES membrane in removing of humic acid foulant using QCM-D
1530-1545 A076 MantakaTauntong
Acetic acid removal of fish skin collagen by ultrafiltration
A102 SinaGilassi
An experimental investigation on permeability and selectivity of PTFE membrane: A mixture of Methane and Carbon Dioxide
A061 T. M. Ting
New radiation grafted boron selective adsorbent sheets containing poly(vinyl benzyl chloride) functionalized with N-methyl-D-glucamine
1545-1600 A081 H. R. Rashidi
The application of hybrid physical pre-treatment system for treatment of simulated batik wastewater
A109 BiruhShimekit
Effect of polyphenylene oxide (PPO) concentration on the morphological, thermal, crystalline and CO2
permeation properties of flat sheet dense PPO polymeric membranes
A086 Avin J. Kajekar
Synthesis and characterization of novel PVDF/PANI nanofiber membrane and its possible applications for heavy metal rejection
1600-1615 A091 B. S. Ooi
Poly(N-isopropylacrylamide-co-acrylic acid) hydrogel and its thermo-responsive properties for polymer enhanced ultrafiltration
A111 N. Dolmat
The effect of piperazine with
N-methyldiethanolamine in emulsion liquid membrane for carbon dioxide removal
A107 AdhiKusmastuti
Emulsion liquid membrane for cadmium removal: experimental results and model prediction
1615-1630 A094 R. Saranya
A comparative study on synthesized polymer nanocomposite membranes for the treatment of paper mill effluent
A120 F. A. Ismail
Synthesis and characterization of titania-13x membrane for CO2 separation
A117 EzaliaMohd. Fauzi
Performance of ionic liquid on the extraction of mercury using hollow fiber supported liquid membrane (HFSLM)
1630-1645 A096 Rajesha Kumar
Synthesis and characterization of novel water soluble derivative of chitosan as an additive for polysulfone ultrafiltration membrane
A216 NayefGhasem
Absorption of CO2 from natural gas utilized gas-liquid
PVDF hollow fiber membrane contactors via potassium gylcinate solvent
A118 AzadehGhaee
Chitosan/polyethersulfone composite
nanofiltration membrane for removal heavy metal from wastewater
1645-1700 A097 A. Mataram
Performance of electrospunnanofibers for water filter application
A127 N. Jusoh
Removal of bulk CO2 from methane with the
presence of heavy hydrocarbon using polyimide membrane
A229Noor Hidayu Othman
Application of membrane technology for degumming and deacidification of vegetable oils refining
1700-1715 A098 N. A. Samsure
Incorporation of silicon dioxide in the preparation of PVDF hydrophilic membrane
A134 Mohammad WashimUddin
Optimization of membrane preparation conditions and effect of methanol on performance of a facilitated transport membrane for natural gas sweetening
A189 MyzairahHamdzah
Effect of sulfonation of polyethylene sheets on the adsorption of Zinc (II)
1715-1730 A213 MuhamadZainiYunos
Influence of inorganic additives on the performance of polysulfone ultrafiltration membrane
A068 W. Wongthep
Semi-interpenetrating polymer network (Semi-IPN) membranes prepared from epoxidized natural rubber (ENR-50) and crosslinkedpolydimethylsiloxane (PDMS) (ENR50-inter-cross PDMS)
A119 AzadehGhaee
Synthesis and application of chitosan/cellulose acetate composite membranes for Copper ions removal from wastewater
1730-1745 A212 MuhamadZainiYunos
Investigation on the performance of
polysulfone/zinc oxide membrane: Effect of pore forming agent
A105 AdilHatem Rashid
Separation of biobutanol from acetone-butanol-ethanol (ABE) fermentation mixtures by polymeric membrane
A214 Maisarah Mohamed Bazin
Effect of starch addition on microstructure and strength of ball clay membrane
1730-1800 Tea break/Poster session
Conference Day 2
28th Aug 2013 (Wednesday)
0830 Parallel Session I
Membrane for gas and vapor application
Parallel Session II
Membrane for fuel cell & energy, environment application
Parallel Session III
Membrane for water & wastewater application
0830-0900
Prof. Matsuyama Hideto
Cutting-edge research at the membrane center in Kobe University in Japan
Prof. WiroteYouravong
An enzymatic membrane reactor in production of bioactive peptides used as nutraceutical and
functional foods
Prof. Wang Rong
Development of high performance PRO hollow fiber membranes for salinity gradient
energy harvesting Chairperson: Prof. Matsuyama Hideto
Co-chairperson:Dr.Surya Racha
Oral Session
(Pacific Ballroom A/B)
Chairperson: Prof. WiroteYouravong Co-chairperson: Noor Maizura Ismail
Oral Session
(Pacific Ballroom C)
Chairperson: Prof. Wang Rong Co-chairperson:Dr.Mukhlis A. Rahman
Oral Session
(Bunga Room)
0900-0915 A142 HazlinaJunoh
Effect of heat treatment parameters on the performance of asymmetric polyetherimide (PEI) hollow fiber membrane for CO2/CH4 gas
separation
A051 Akhondi E.
Optimization of operating conditions and energy consumption of submerged hollow fiber membrane systems with periodic backwash
A055 A. W. Zularisam
Perspective of biofouling from batik effluent
0915-0930 A149 Suhaina M. Ibrahim
Tailoring the pore size and gas separation properties of organic/inorganic membranes using 2,4,6-tris[3(triethoxysilyl)-1-propoxyl]-1,3,5-triazine (Triazine) as a triple-silicon alkoxide and comparison with mono and di-silicon alkoxides
A028 RaisHanizamMadona
Desalination of seawater using polyamide reverse osmosis (RO) membranes
A104 A. A. Abdulkarim
Preparation and characterization of
polyethersulfone membrane containing zinc oxide nanoparticles and polyvinylpyrollidone
0930-0945 A153 Haikal Mustafa
Powder preparation effect on oxygen permeation flux of hollow fibre LSCF6428 ceramic perovskite membrane
A045 Saren Qi
Fabrication of forward osmosis membranes based on layer-by-layer assembly
A110 DzetiFarhahMohshim
Effect of operating conditions on different
membrane types for removal of monoethanilamine from wastewater
1945-1000 A154 SajidHussain Shah
Development of and highly hydrophobic composite silica membrane system for high temperature gas separation and applications under steam environment
A048 Ang Wei Lun
Integrated/hybrid membrane system as alternative process in drinking water treatment plant and pretreatment for reverse osmosis desalination plant
A112 SyazwanLiyanaSulaiman
Synthesis of PVA/Chitosan/TiO2 beads
1000-1015 A161 M. Rezaei-Darzhandi
Preparation of porous PVDF/Montmorillonite hollow fiber mixed matrix membrane contactor via a phase inversion method to capture CO2
A057 Hosam A. Shawky
Conceptual design and simulation of a small mobile PV driven RO water desalination plant to be deployed at the North West Coast of Egypt
A115 RomchatRattanaoudom
Potential removal for high TDS wastewater from petroleum-based industry
D
A
1015-1030 A169 N.H. Nordin
Preparation of asymmetric polysulfone/ZIF-8 mixed matrix membrane for CO2/CH4 separation
A058 Dalia E.
Economic analysis of a stand-alone reverse osmosis desalination unit powered by photovoltaics for possible application in the North West Coast of Egypt
A116 Nora’aini Ali
Flux decline in low-pressure ultrafiltration membrane by surimi wash water: effect of operating parameters
1030-1045 A175 N. M. Ismail
Characterization of polyethersulfone/Cloisite 15A mixed matrix membrane for CO2/CH4
separation
A072 AsifMatin
Surface modification of RO membranes used for seawater desalination
A221 Mahesh Padaki
PSF/TiO2 composite membrane for chromium
removal and effect of acidic pH
1045-1100 A164 A. K. Zulhairun
Asymmetric Polysulfone-Cloisite 15A® nanocomposite membrane for gas separation
A078 Lei Shi
Development of novel composite dual-layer nanofiltration hollow fiber membranes for water softening at low operation pressure
A129 Atiye Sadat Abednejad
Wet ability improvement of polypropylene membranes by PEG grafting
1100-1200 Tea break/Poster session (Judging session)
1200-1300 MAIN HALL
Prof. Young Moo Lee
Thermally rearranged polymer membranes for gas separation
Chairperson: Prof. HamdaniSaidi
1300-1400 LUNCH
1400-1430 Prof. XiansheFeng
Pressure-vacuum swing permeation: a novel process mode for membrane separation of
gases
Prof. MojtabaShariaty-Niassa
A comparative survey on
Cu ion membrane adsorption from aqueous solution
Ir. B. P. Chow
Greening of the palm oil milling industry
Chairperson: Prof. XiansheFeng Co-chairperson:Dr.MohdIrfanHatim
Oral Session
(Pacific Ballroom A/B)
Chairperson: Prof. MojtabaShariaty-niassa Co-chairperson:Dr.MasoudRahbariSisakht
Oral Session
(Pacific Ballroom C)
Chairperson: Dr. JuhanaJaafar Co-chairperson:MyzairahHamzah
Oral Session
(Bunga Room)
1430-1445 A190 EbrahimAbouzari-Lotf
Preparation and gas separation properties of novel polyoxadiazole containing noncoplanar
1,1’-thiobis(2-naphthoxy) groups
A082 Song Xiaoxiao
Energy recovery from the brines of seawater and wastewater using thin-film nanofiber composite pressure retarded osmosis membranes
A198 Bolong N.
Numerical estimation of hollow fiber membrane for mobile water treatment
1445-1500 A192 S. A. Hashemifard
The reliability of the conventional gas permeation testing method for characterizing the porous asymmetric membranes
A084 Chang Liu
Fabrication of high performance hollow fiber membrane using layer-by-layer deposition and crosslinking on the lumen surface for niche applications
A137 Y. H. D. Alanezi
Crossflow microfiltration of synthetic oily wastewater using multi channel ceramic membrane
1500-1515 A196 R. Naim
Effects of additives on the structure and performance of polyetherimide hollow
A095 SeemaShenvi
Study of PPEES/Chitosan composite membrane crosslinked with tripolyphosphate
A140 C. S. Ong
Preparation and characterization of PVDF-TiO2
composite membranes blended with different
D
A
fibermembrane contactor for CO2 absorption
and stripping
molecular weights of PVP for oily wastewater treatment using submerged membrane system
1515-1530 A205 Ali Kargari
Experimental study of hydrogen and methane permeation through asymmetric PEI membranes
A151 T. D. Kusworo
Effect of PEG on cellulose acetate membrane morphology and nanofiltration performance for water softening
A141 NurulNabilahAminudin
Comparison study of effect of PEG and PVP as additives on polysulfone (PSf) membrane structure and performance
1530-1545 A206 Ali Kargari
Critical concentration of PEI in NMP as a criterion for the preparation of asymmetric membranes for gas separation
A155 M. M. Zerafat
Mathematical modelling of nanofiltration-based deionization processes in aqueous media
A143 D. Novin
Effect of operating parameters on performance of ultrafiltration (UF) to fractionate catfish protein hydrolysate
1545-1600 A108 N. A. Ahmad
Synthesis and characterization of
superhydrophobic alumina membrane with different silanes chain
A160 D. Emadzadeh
Study of thin film nanocomposite (TFN) membrane morphology by microscopic image analysis
A144 MohdRiduanJamalludin
The effect of polyethylene glycol (PEG) on the performance of blend polysulfone (PSf) membrane with rice husk silica (RHS)
1600-1615 A027 A. KazemiJoujili
Separation of olefins from paraffins by membrane contactor – A review
A179 N. Misdan
Study on the properties of polysulfone support membrane and its relation to the TFC nanofiltration membrane performance
A145 MuhamadFikriShohur
Performance of Psf ultrafiltration membrane: Effect of different nonsolvent in coagulation medium
1615-1630 A170 HazlinaJunoh
The development of exfoliated
SPEEK/Cloisitenanocomposite electrolyte membrane by electrospinning
A021 T. Chidambaram
Sodium sulphate recovery using novel membranes in the presence and absence of dye molecules
A147 SitiHawaMohamad
Membrane performance: TiO2 nanoparticles coated
on polysulfone (PSf) ultrafiltration membrane surface
1630-1645 A071 SantiKhoonsap
Nanocomposite membranes from PVA filled with silica nanoparticles grafted with
Poly(2-hydroxyethyl methacrylate) (SiO2-g-PHEMA)
A166 N. A. A. Sani
Performance of polyphenylsulfone solvent resistant nanofiltration membrane: Effects of polymer concentration, membrane pre-treatment and operating pressure
A148 NazatulShimabintiAzmi
Effect of sandwich configuration of ultrafiltration membranes on POME treatment
1645-1700 A122 SiripornLarpkiattaworn
PES membrane performance for TEG removal of wastewater from natural gas separation process
A100 Ng Law Yong
Stability and performance study of polyethersulfone membranes modified using polyelectrolytes
A157 Muhammad Said
Optimization of NaOH as the cleaning agent of polyethersufone (PES) membrane fouled by palm oil mill effluent (POME)
1700-1715 A150 Serene Lock Sow Mun
Modeling of concurrent and countercurrent flow hollow fiber membrane module for multi-component system
A060 FatemehRoozbahani
Poly(ε-caprolactone) electrospunnanofibrous scaffold with protein encapsulated for tissue engineering applications
A158 SirichaiKoonaphapdalert
Effect of hydraulic retention time and bisphenol A concentration on performance of membrane bioreactor
1715-1730 A172 M. Ghanbari
CFD modelling of membrane channel: application to gas separation
A202 Lim MimMim
Electrospun biodegradable membranes for drug delivery and tissue engineering applications
A056 A. W. Zularisam
Causes and biological mitigation of biofouling in MBR
1745-1800 Tea break
2000-2300 MST2013 GRAND DINNER/VVIP LAUNCHING
Conference Day 3 29th Aug 2013 (Thursday)
0830-0930 MAIN HALL
Prof. Wang Rong
Development of high performance PRO hollow fiber membranes for salinity gradient energy harvesting
Chairperson: Prof. Ir. Abdul WahabMohamad
0930-1000 Tea break
1000 Parallel Session I
Membrane for fuel cell & energy, environment application
Parallel Session II
Membrane for gas &vapor application
Parallel Session III
Membrane for water & wastewater application
1000-1030 Prof. ArunIsloor
Chitosan and its derivatives as potential materials for membrane technology
Dr. Anil K. Pabby
Hollow fiber membrane contactor technology in chemical and nuclear industry: current status, challenges, and future perspectives
Prof. ArthanareeswaranGangasalam
Polymer modification for performance enhanced membrane separations Chairperson: Prof. ArunIsloor
Co-chairperson:Dr.SeyedAbdollatifHashemifard
Oral Session
(Pacific Ballroom A/B)
Chairperson: Dr. Anil K. Pabby Co-chairperson:Dr.GholamrezaBakeri
Oral Session
(Pacific Ballroom C)
Chairperson: Prof. ArthanareeswaranGangasalam Co-chairperson:RosmawatiNaim
Oral Session
(Bunga Room)
1030-1045 A001 Helen Morabi
Industrial wastewater treatment in textile industry-Nylon 6
A032 Abdul Hakim M. Salem
Development and characterization of sago/PVA blend membrane for recover of ethyl acetate from water
A162 N. A. MohdNazri
Improved performance of PAN-based UF
membrane with PAN-g-PVA amphiphilic copolymer
1045-1100 A050 ThiamLeng Chew
Development of Ba-SAPO-34 zeolite membrane for separation of CO2 in the binary gas mixtures
A220 R. Surya Murali
Preparation and characterization of Pebax/zeolite 13X mixed matrix membranes for propane-propylene
A174 M. A. Mohamed
The potential of cellulose-based photocatalytic membrane for wastewater treatment application: A review
1100-1115 A126 Zing-Yi Ooi
Recovery of kraft lignin from pulping wastewater by an emulsion liquid membrane process
A128 Bolong N.
Zeta potential measurement of hollow fiber membranes modified by negatively charged-modifying macromolecule
A176 Norafiqah Ismail
Preparation and characterization of blends polysulfone/polyphenylsulfone ultrafiltration membranes for palm oil mill effluent treatment
1115-1130 A139 NorherdawatiKasim
Potential of nanofiltration membrane in groundwater treatment for drinking water resources
A199 Fouzia T. Minhas
Developing solvent resistant nanofiltration (SRNF) membranes for treatment of dye solutions
A180 R. Jamshidi
Adsorptive removal of arsenite from contaminated water solutions by PES/Fe-Mn binary oxide UF flat sheet mixed matrix membrane
1130-1145 A156 N. A. Jalanni
Investigation of new polyester nanofiltration(NF)
A203 RohaidaChe Man
Immobilization of recombinant Escherichia coli using
A171 N. A. M. Nor
A review study of nanofibers in
D
A
membrane fouling with humic acid solution hollow fiber membrane for improvement of extracellular enzyme expression and cell viability
photocatalyticprocess
1145-1200 A079 NurulIzzati
Separation of biobutanol using polymeric membrane: A review
A204 AlirezaKhademi
Classical design of experiments application on submerged membrane ultrafiltration system for refinery produced wastewater treatment
A183 YasamanSanayei
Modeling for biological treatment of reactive dye (Cibacron yellow FN_2R) wastewater in SBR systems
1200-1215 A167 E. Halakoo
The effect of SPEEK additive on
polyethersulfonenanofiltration membrane for dye removal
A208 D. Veerasamy
Latices concentration via utralfiltration as a cleaner processing technique
A187 S. Ismail
Fouling characteristics and cleaning of
ultrafiltration membranes in palm oil mill effluent (POME) treatment
1215-1230 A184 Zahra ShariatiNiasar
Removal of malathion from groundwater using nanoadsorbents
A209 N. Shamsinar
Lysozyme recovery from chicken egg white proteins using batch adsorption of cation exchange mixed matrix membrane chromatography
A195 Chin Boon Ong
Performance investigation of pilot study for wastewater reclamation using ferric chloride as coagulant
1230-1245 A123 Raja Norimie Raja Sulaiman
Recovery of ionized nanosilver from wash water using emulsion liquid membrane process
A210 TzeChingOng
Drying Simulation for hygroscopic membrane
A197 HeruSusanto
Membrane hydrophilization: towards low fouling polymeric membranes
1245-1300 A211 MuhamadZainiYunos
Effect of Zinc oxide on performance of ultrafiltration membrane for humic acid separation
A103 Ng Ching Yin
Fractionating the value-added product from skimmed coconut milk using membrane separation technique
1300-1400 LUNCH
1400-1430 Prof. Kang Li
Structural-controlled micro-tubular solid oxide fuel cell via phase inversion technique
Dr. Simon J. Shilton
Mass transfer and skin formation in hollow fibre membranes for gas separation Chairperson: Prof. Kang Li
Co-chairperson:Dr.Mohd Hafiz Dzarfan
Oral Session
(Pacific Ballroom A/B)
Chairperson: Dr. Simon J. Shilton Co-chairperson:Dr.HatijahBasri
Oral Session
(Pacific Ballroom C)
Chairperson: Dr. Lau WoeiJye Co-chairperson:Dr.Mukhlis A. Rahman
Oral Session
(Bunga Room)
1430-1445 A016 K. Sriram
Effects of nano particles on
sulfonatedpolyethersulfone membrane for fuel cell application
A047 Z. Khan
Effect of process parameters on electro spun polyacrylonitrile (PAN) fibers and fiber mats
A080 Zhang Jinsong
Feasibility of anaerobic membrane distillation bioreactor (AnMDBR) for wastewater treatment
1445-1500 A017 Elakkiya E
Comparison of various membrane performance in microbial fuel cell for power generation
A053 NorlisaHarruddin
Behaviour study of microporous polypropylene membrane development via thermally induced phase separation
A093 A. O. Imdakem
Simulation of heat and mass transfer in membrane distillation (MD) processes: the effect of membrane pore space description
D
A
1500-1515 A023 Arjun Ramesh
Utilizing optimize imidazolium functionalized polysulfone in a completely noble metal free alkaline membrane fuel cell
A066 S. Shahaidah
Study of binding interaction in BPA-MIP flat sheet membrane
A113 Mohammad Mahdi A. Shirazi
Characterization of PTFE membranes for membrane distillation using atomic force microscopy
1515-1530 A029 MostafaGhasemi
Preparation of sulfonated polyether ether ketone (SPEEK) and optimization of degree of sulfonation for using at microbial fuel cell (MFC)
A121 A. S. Noor Adila
A review on spinning parameters in fabricating the hollow fiber membrane
A163 N. M. Mokhtar
Effect of different additives on the performance of PVDF hollow fiber membranes for textile
wastewater treatment using DCMD
1530-1545 A152 S.E. Rosli
Study of the variation of catalyst loading in cathode for SPEEK/CSMM membrane in direct methanol fuel cell (DMFC)
A136 AlaminIdris
The grafting of γ ray pre-irradiated polytetrafluoroethylene film membrane
A185 M. N. A. Hawlader
Experimental study on a multi-stage air gap membrane distillation (AGMD) unit
1545-1600 A178 H. ilbeygi
Prediction and optimization of proton
conductivity for nanocomposite membrane using response surface methodology
A087 F. Nufaiei
(PAN)/CNT electrospunnanofiber mats: effect of concentration on the mechanical and surface properties
A186 S. O. Lai
Characteristic and performance of polyvinylidine fluoride membranes blended with lithium chloride in direct contact membrane distillation
1600-1615 A018 N. Samsudeen
Influence of operating parameter on electricity generation in microbial fuel cell during distillery wastewater treatment
A215 Chung Ying Tao
Membrane surface modification through UV-grafting
A114 Mohammad Mahdi A. Shirazi
Separation of water-glycerol mixture using sweeping gas membrane distillation
1615-1630 A025 Arjun Ramesh
Optimization of Imidazolium functionalized polysulfone for use as anion exchange membranes in fuel cell
1630-1700 CLOSING CEREMONY (ORAL AND POSTER PRESENTATION AWARDS)
LIST OF POSTER PRESENTATION
A031 RosiahRohani
Synthesis and characterization of conducting nanofiltration membranes from polyaniline coated PVDF with tuneable permeation properties
A034 A. Yoshida
Characterization and water 10ehavi sorption property of ABA-type triblock copolymer membranes derived from fluorine-containing polyimide and polymethylmethacrylate
A035 D. Tanaka
Effect of substituent groups on alcohol 10ehavi sorption property of POSS-containing polymer membranes
A036 M. Kishida
Characterization and gas separation properties of polyimide/zeolite composite membranes containing sulfolane
A037 M. Ichikawa
Preparation of ABA-type triblock copolymer membrane designed using fluorine-containing polyimide with high transparency and gas barrier properties
A038 M. Yamada
Effect of volatile organic compound on dissolved gas transport properties through poly(1-trimethylsilyl-1-propyne) membranes
A039 M. Kobayashi
Synthesis and Gas barrier properties of the crosslinked membrane with diacetylene group-containing polymer derived from ferulic acid
A040 R. Shindo
Characterization and CO2separation property of polyimide composite membranes containing ionic liquid
A041 S. Amanuma
Synthesis of UV-cured telechelic polyimide membranes with crosslinker
A042 S. Ando
Synthesis and characterization of ABA-type triblockcopolymer membranes derived from polyimide macroinitiator with 10ehaviour10-containing methacrylate
A043 Y. Hayashi
Effect of vacuum ultraviolet irradiation on dissolved gas transport properties through poly(lactic acid) membranes
A062 Farah Syuhada Abdul Halim
Preparation and characterization of 10ehaviou membrane electrode assembly
A067 ThanitpornNarkkun
Water permeability and diffusivity in natural rubber grafted with polyvinyl alcohol (NR-g-PVA)
A073 MuhammadameenHajihama
Concentration and desalination of protein derived from tuna cooking juice by nanofiltration
A075 PenpornSriniworn
Separation of oligosaccharides from UF-pretreated tofu whey by nanofiltration
A083 Nor Emma ErwinaWahab
A088 Amir-Al-Ahmeda
Study the properties of polyethersulfone(PES) /polyetherimide(PEI) composite membranes
A090 AbdellahAmmi Said
The effect of complexing agent through the exchange membranes
A106 B. Darunee
The use of ceramic membranes for micro-filtration of Thai beverage made from Indian gooseberry fermentation broth
A124 Oh Pei Ching
Effects of aluminosilicate mineral nano-clay fillers on polysulfone mixed matrix membrane for carbon dioxide removal
A125 BanderBawareth
Membranes separation of 2-Ethyl Hexyl Amine/1-Decene
A130 M. A. IndokNurulHasyimah
Ultrafiltration 11ehaviour of organic mixtures simulating sweetwater solutions: Influence of membrane surface and feed chemistry
A131 N. Yousefimehr
Investigation on influence of volatile solvent ratio, shear stress, forced convection residence time on gas separation performance of asymmetric polysulfone membrane
A165 SitiMuniraJamil
A single step co-extrusion/co-sintering technique: A review
A168 D. Emadzadeh
Neural networks simulation of thin-film nanocomposite membrane for brackish water desalination
A173 H. Dzinun
The potential of photocatalytic membrane process in treating wastewater – A review
A181 M. Rezaei-Darzhandi
Mixed matrix membranes (MMMs) comprising PVDF polymer with dispersed montmorillonite clay particles for CO2absorption
A188 KanungnuchKeawsupsak
Poly(lactic acid)/biodegradable polymer blends for the preparation of flat-sheet membrane
A191 Muhammad Irfan
Carbon nanostructured ultra-filtration membrane
A193 ArisaJaiyu
The effect of spinning parameters on pla hollow fiber membrane formation
A194 WeerapongBootluck
Innovation of polymer coating machine for producing various pore sizes of composite polymer/ceramic membranes
A207 W. N. W. Salleh
The effect of stabilization environment on the structure and gas permeation properties of carbon membrane
A217 N. Bolong
Tongkat Ali extraction using hollow fiber membranes modified by negatively charged-modifying macromolecule
A223 H. Hasbullah
Effect of co-solvent on Mindel S-1000 gas separation asymmetric membrane
Enhancement of ceramic water filtration using synthesized rice husk particles
A227 MohdRiduanJamalludin
The effect of synthetic silica to the ultrafiltration PSF membrane
A228 NurafiqahRosman
A224-1
POLYVINYLIDENE FLUORIDE HOLLOW FIBER MEMBRANES FOR
REFINERY WASTEWATER TREATMENT: EFFECT OF AIR GAP
LENGTH ON MEMBRANE MORPHOLOGY AND PERFORMANCE
E. Yuliwatia,*, A.F. Ismailb,c , A.S. Mohrunid ,T. Matsuurac,e
a
Department of Industrial Engineering, Faculty of Engineering, University of Bina Darma, Sumatera Selatan, Indonesia
Tel. +62 (711) 515-679
bAdvanced Membrane Technology Research Centre (AMTEC),
c
Faculty of Petroleum and Renewable Energy Engineering, Universiti Teknologi Malaysia, 81310 UTM, Skudai Johor, Malaysia
Tel. +60 (7) 553-5592; Fax: +60 (7) 558-1463
dDepartment of Mechanical Engineering, Faculty of Engineering,
Sriwijaya University, South Sumatera, Indonesia Tel. +62 (711) 580-272
eDepartment of Chemical Engineering, Industrial Membrane Research Laboratory,
University of Ottawa, Ont., Canada KIN 6N5
*Corresponding author: [email protected]
Abstract
Effects of air gap length on outer surface morphology and filtration performance of hydrophilic polyvinylidene fluoride (PVDF) hollow fiber membrane have been studied. Porous asymmetric hydrophilic PVDF membranes were prepared via a phase inversion method, using N,N-dimethylacetamide (DMAc) as solvent, lithium chloride monohydrate
(LiCl.H2O) and titanium dioxide (TiO2) as inorganic additives. Submerged membrane
ultrafiltration was conducted using non-ionic solutes of different molecular weight and refinery wastewater with constant suspended solid concentration. The comparison of the performance and morphology was conducted between PVDF membranes of different air gap length at 1, 4, 11, and 15 cm. The prepared membranes were characterizedthrough observations of field emission scanning electron microscope (FESEM) and atomic force microscopy (AFM), permeate flux measurement, and tensile propertytest.Average pore size and surface porosity were calculated by the permeate flux in submerged membrane system. It is resulted that the permeate flux is mainly determined by effective porosity.Moreover, the average pore size and nodules size were increased, while outer surface membrane was being smoother with increasing of air gap length. AFM analysis reveals that the air gap introduces an elongation stress due to the gravity on the membrane surface. Under the air gap length of
11 cm, high permeate flux of 148.53 L/m2h and suspended solids removal of 99.82 % and
also relatively high mechanical strength of membrane can be simultaneously achieved. It is concluded that there exists the best range of the air gap length for relatively high performance of membrane.
A224-2
1. Introduction
Synthetic membrane technology has grown up very fast and has received significant
attention from both academia and industry, since the first mention of hollow fiber membranes
in a series of patents by Mahon in 1966 and 1966b [1,2]. Nowadays, the hollow fiber
membrane configuration via phase inversion process is the most favored membrane geometry
in the most membrane applications. Hollow fibers have much larger surface area per unit
volume of the membrane module and hence offer higher productivity per unit volume. They
have also good flexibility in operation [3,4].
However, during membrane formation, the preparation of the hollow fibers often
requires more controlling parameters than those of flat sheet membranes (i.e. structure and
dimensions of the spinneret, viscosity and possibility of the spinning dope, nature of the
internal and external coagulants, flow rate of the bore fluid, dope extrusion rate, air gap
length, take-up speed, etc) [5]. Among the studies of membrane formation by the dry-jet wet
spinning process, the effects of air gap length on final hollow fiber membrane have been
investigated by many researchers [6-9]. However, the fabrication of a hollow fiber with
desirable performance is not trivial process and the effect on hollow fiber membrane
morphology and permeation process reported in the literatures often provide conflicting
observations. For instance, in spinning polysulfone hollow fibers, Aptel et al., (1985)
reported that the permeability decreased as the air gap length increased [10]. On the other
hand, it was reported by Kim et al., (1995) that air gap length had no impact on the
permselection properties of polysulfone hollow fibers [11]. Khayet (2003) was also
investigatedthat the separation factors of polyvinylidene fluoride hollow fibers increases with
increasing the air gap distance; however, the air gap distance did not significantly affect the
permeation rate [12]. He attributed this fact to the increase of the skin layer thickness with
increasing the air gap length. In addition, air gap length also proved to be an easy and
powerful tool to tune the prem-selective properties of hollow fiber membranes during
spinning process.It has been also reported that the membrane spun from air gap length of
20-30 cm exhibited very poor permeability, higher value of permeability were obtained from air
gap length of 10-15 cm and for lower air gap length the permeability was reduced drastically
[13]. Tsai et al. (2002) observed that the both inner and outer diameters decreased with
A224-3
anneals the fiber caused the change of inner and outer diameter [14]. Khulbe et al. (2004) and
Khayet et al. (2009) have similar results when using polyetherimide (PEI) polymer and
polyvinylchloride (PVC) polymer respectively [15,16]. It was explained that stress inside the
spinneret perpendicular to the fiber axis released when dope solution comes out from
spinneret. This will expand the fiber diameter, whereas the stress parallel to the fibers will
elongate the fiber and diminishing its diameter.
In this study, air gap length plays an essential role in the PVDF ultrafiltration
membrane preparation,meanwhile, all parameters were kept constant.The objective of this
study is to investigate the effects of air gap length on the morphological structure and
filtration efficiency of hydrophilic polyvinylidene fluoride (PVDF) membrane. Four varied
air gap lengthsof 1,4,7,and 15 cm wereused in order to produce different structure and
morphology of membrane. Based on this results obtained, it is expected to find out the
suitable PVDF membrane for preparation of hydrophilic ultrafiltration membrane for refinery
produced wastewater treatment.
2. Experimental
2.1.Materials
Ultrafiltration membranes have been prepared using Kynar®740 PVDF polymer
pellets were purchased from Arkema Inc. Philadelphia, USA. The solvent
N,N-dimethylacetamide (DMAc, Aldrich Chemical) (Synthesis Grade, Merck, >99%) was used as
polymer solvent without further purification. Lithium chloride monohydrate (LiCl.H2O) and
nanoparticles titanium dioxide (TiO2) were used as inorganic additives. Both chemical
additives were purchased from Sigma-Aldrich and used as received. Glycerol was purchased
from MERCK (Germany) and used as non-solvent for post treatment of membrane. In all
cases, tap water was used as the external coagulation bath medium in the spinning process.
A224-4
An amount of pre-dried (24 h oven dried at 50oC) PVDF pellets in ranging of 16 to
22 wt.%were weighed and poured into pre-weighed DMAc solvent. The mixtures were
stirred to ensure thorough wetting of polymer pellets, prior to the addition of appropriate
amounts of LiCl.H2O at 50 oC. TiO2 was then carefully added to the polymer dope mixtures,
which were continuously stirred for 48 h (IKA-20-W) at 500 rpm until a homogenous
solution was formed. The polymer solution was kept in a glass bottle and air bubbles formed
in the dope were removed using water aspirator for several hours.The fully dissolved polymer
solution was transferred to a glass reservoir, allowed to stand and degassed for 24 h at room
temperature prior to spinning process. Solution viscosity was measured using rheometer
(Bohlin Instrument Ltd.) at various temperatures between 25 and 50 oC.
2.3.Fabrication of hollow fiber membranes
2.3.1 Spinning parameters
In this study, hollow fiber membrane is produced through a dry-jet wet spinning
process. The membrane can be formed only in the appropriate conditions of all the spinning
parameters (i.e. dope extrusion rate, extrusion velocity from spinneret, take up speed, bore
fluid rate, and air gap length). The air gap length variable in the spinning system effects the
fiber geometry in terms of the inner diameter, outer diameter, wall thickness, and structure of
membrane sublayer. This may be attributed to the die swell of polymer macromolecules
when exiting from the spinneret due to the viscoelastic properties of the PVDF spinnning
dope. Furthermore, the gravity will influence the achievable in the spin line and introduces an
elongation stress on membrane and generally tends to decrease the membranes wall
thickness.
2.3.2 Dry-jet wet spinnning process
PVDF hollow fiber UF membranes were spun at room temperature by a dry-jet wet
spinning method. The spinning solutions were consisted of maintained PVDF concentration
of 19 wt.%, which were prepared at different air gap lengthof 1, 4, 11, and 15 cm. LiCl.H2O
and TiO2 were maintained at 5.2 wt % and 10 wt.% of the weight of PVDF, as shown in
Table 1 respectively. The hollow fiber spinning process by dry-jet wet phase inversion was
explained by Qinet al.,(2001); Ismail and Hassan (2006) [17,18]. The detailed spinning
A224-5
Table 1 Spinning conditions of the hollow fiber membranes
Spinning condition Value
Dope extrusion rate (ml/min) 4.20
Bore fluid Distilled water
Bore fluid flow rate (ml/min) 1.40
External coagulant Tap water
Air gap distance (cm) 1, 4, 11, 15
Spinneret od/id (mm) 1.10/0.55
Coagulation temperature ˚C 25
Room relative humidity (%) 70 − 75
The hollow fiber membranes were produced using the dope formulation solution
composed of PVDF/LiCl.H2O/TiO2 at different polymer concentration, as shown in Table 2.
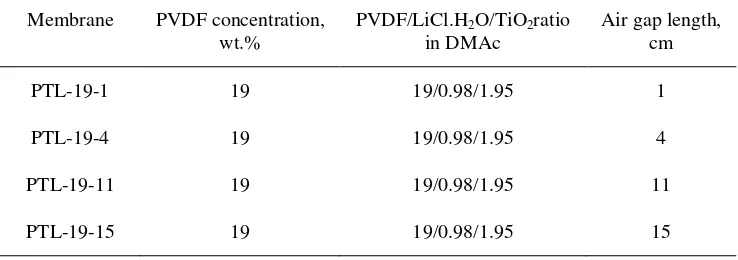
Table 2.The air gap length condition for holow fiber membrane preparation
Membrane PVDF concentration, wt.%
PVDF/LiCl.H2O/TiO2ratio in DMAc
Air gap length, cm
PTL-19-1 19 19/0.98/1.95 1
PTL-19-4 19 19/0.98/1.95 4
PTL-19-11 19 19/0.98/1.95 11
PTL-19-15 19 19/0.98/1.95 15
2.3.3 Post-treatment
The spun fibers were cut in pieces of approximately 50 cm in length and then stored
in water bath at room temperature for at 1 day to remove the residual solvent. The fibers were
then soaked in the 20 wt.% glycerol aqueous solution bath for another 1 day in order to
prevent pore collapse or change of transport properties during drying at room temperature for
4 days, they were ready for making hollow fiber test bundles as mentioned in our previous
study by Yuliwati and Ismail (2011) and Yuliwati et al., (2011) [16,17}.
A224-6 The permeation flux and rejection of PVDF hollow fiber membranes for synthetized
refinery wastewater were measured by submerged ultrafiltration experimental equipment as
shown in Fig.1 [16]. An in-house produced U-shape hollow fiber module, with a filtration
area of 11.23 dm2, was submerged in prepared suspension in membrane reservoir with
volume of 14 L. A cross-flow stream was produced by air bubbling generated by a diffuser
situated underneath the submerged membrane module for mechanical cleaning of the
membrane module. The air bubbling flow rates per unit projection membrane area was set
constantly at 2.4mL/min in order to maintain proper turbulence. The filtration pressure was
supplied by a vacuum pump and controlled by a needle valve. Permeate flow rates were
continually recorded using flow meter respectively.
In order to conduct the studies at steady state, the hollow fiber membranes have to
compacted at a transmembrane pressure (TMP) of vacuum. The desired TMP was controlled
using the pressure regulator installed at the outlet of the membrane reservoir. TMP is the
driving force for the pressure-driven membrane process and is defined as the pressure
difference across the membrane,
TMP = ���+��
2 �‒ �� (1)
where Pf is the feed pressure (bar), Pr is the retentate pressure (bar), and PP is the permeate
pressure (bar).
Membrane performance was tested with anin-house U-shape membrane bundle
having about 11.23dm2 of membrane surface area. Pure water flux was measured after the
flux was steady. The collected permeate can be recorded in terms of flux and rejection by the
expression as follows,
Jw =
At V
(2)
whereJw is the pure water flux (L/m2 h), V is the permeate volume (m3), A is the membrane
A224-7
The values of Jw obtained from the experiments were converted to corresponding unit of m.s-1
during data analysis for better understanding. The pure water permeability coefficient, Lp was
then determined from the values of Jw versus the applied pressure for all the studied
membranes with the assumption of null value of the osmotic pressure, π.
Jw = Lp. ΔP (3)
The second property of feed contained neutral solute-PEG with different MWs at a
concentration of 200 ppm. This feed solution was used for the characterization of MWCO of
a membrane. The solute rejection was determined based on the total organic carbon (TOC)
rejection determined by total organic carbon analyzer, TOC-VCSH/CSN (Shimadzu
Corporation). The third property of feed contained synthetic refinery wastewater at constant
concentration that based on mixed liquor suspended solids (MLSS) concentration of 3 g/L.
The conductivity of refinery wastewater for the feed and permeate was measured using
conductivity meter (EC300, YSI Inc.). In order to determine the rejection, the effective solute
filtration efficiency, R (%) can be calculated using the following equation:
R = (1-f p C C
) x 100 (4)
WhereR is the rejection ultrafiltration process (%), Cpis the concentration of the permeate
(mg/L) and Cfis the concentration of the feed (mg/L).
2.6. Characterizations
The morphological structures of the hollow fiber membranes were studied using field
emission scanning electron microscope (JEOL JSM-6701F). The FESEM micrographs of the
cross section membranes were taken at certain magnifications. It produced photographs at the
analytical working distance of 10 nm.
Atomic force microscopy (AFM) was used to study the external and internal surfaces
A224-8 different areas of each hollow fiber membrane using a tapping mode Nanoscope III equipped
with 1553D scanner (SPA-300 HV,USA). In this study, scans were made on areas of 5 μm ×
5 μm. The AFM analysis software program allowed computation of various statistic related to
the surface roughness on predetermined scanned membrane area. To determine the pore sizes
and nodule sizes, cross-sectional line profiles were selected to traverse the obtained AFM
images and the diameter of nodules (i.e. high peaks) or pores (i.e. low valleys) were
measured by means of a pair of cursors. The sizes of the nodule aggregate are based on the
average of 15 measurements.In terms of surface roughness, the outer surface of the PVDF
hollow fiber membranes were compared using various roughness parameters such as the
mean roughness (Ra), the root mean-square roughness (Rq) and the average difference in
height between the five highest peaks anf the five lowest valleys (Rz) [16,19]. The average
pore sizes and average nodule sizes of outer surfaces were also determined. Those were
measured, as stated previously, by inspecting line profiles on the AFM images at different
locations of a membrane surface. The measured pore sizes from the line profiles on the AFM
micrographs were arranged in ascending order and the median rank (50%), χ, was calculated
using the followed equation,
100 4 . 0
3 . 0
+ − =
n i
χ (5)
wherei is the order number of the measured pore size arranged in ascending order and n is the
total number of the measured pores.
Differences in the membrane surface morphology can be expressed in terms of various
roughness parameters, such as (1) the differences between the highest and the lowest points
within the given area, Z; (2) the root mean-square of the Z data within the given area (RRMS);
(3) the mean roughness (Ra). This parameter represents the mean value of the surface relative
to the centre plane, the plane for which the volume enclosed by the image above and below
this plane is equal. It is calculated as follows
∫ ∫
= x
y L L
y x
a f x y dxdy
L L R
0 0
) , ( 1
A224-9
where f(x,y) is the surface relative to the centre plane, LxandLy are the dimensions of the
surface in the x and y directions, respectively; (4) the average difference in height, Rz,
between five highest peaks and five lowest valleys is calculated relative to the mean plane,
which a plane has a data variance. Roughness parameters obtained from AFM micrographs
should not be considered as absolute values because it depends on the treatment of the
captured surface data such as plane fitting, flattering, filtering, etc. In the present study, the
same tip was used for all experiments and all captured surfaces were treated in the same
method. The evaluation of the roughness parameters of each sample was based on micron
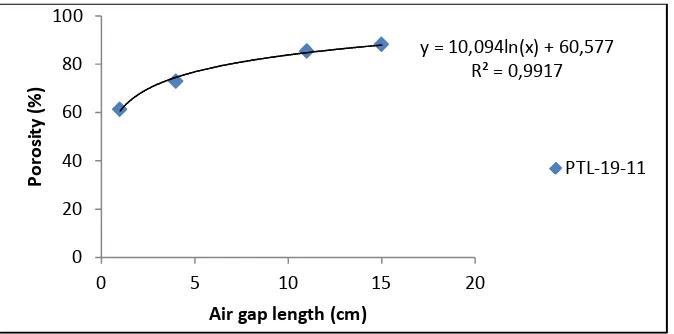
scan areas. Porosity was calculated also by the method described by Singh et al. (2008) [19].
Asymmetric porous membranes were characterized by determination of porosity and
average pore radius. The membrane porosity, ε,was defined as the volume of the pores
divided by the total volume of the porous membrane. The membrane porosity was calculated
using the following equation,
ε =
P W
W w w w
w w
ρ ρ
ρ 2 2 1
2 1
) (
) (
+ −
−
x 100 (7)
whereε is the porosity of the membrane (%), w1the weight of wet membrane (g), w2 the
weight of dry membrane (g), ρp the density of the polymer (g/cm3) and ρw is the density of
water (g/cm3).
To prepare the wet and dry membranes, five spun hollow fibers with the length of 25
cm were selected after solvent was exchanged in tap water for 3 days. The fibers were
immersed into the isopropanol for 3 days and distilled water for 3 days. The remained water
in the inner surface was removed using air flow, before weighing the membranes. The wet
membranes were dried in vacuum oven for 12 h at 40 oC and weighted.
Average pore radius, rm, was investigated by filtration velocity method, which a
measurement of the ultrafiltration flux of the wet membrane applied on pure water in limited
time (20 h) under 0.1 MPa pressure. It represents the average pore size along the membrane
thickness (), which was measured by the difference value between external radius and
internal radius of the hollow fiber membrane. The test module containing 60 fibers with the
length of 35 cm was used to determine water permeability. According to
A224-10
rm =
P xAx
Q x ∆ −
ε
η ε) 8 75 . 1 9 . 2 (
(8)
whereη is water viscosity (8.9 x 10-4 Pa s), is the membrane thickness (m), ∆P is the
operation pressure (0.1MPa), ε is the porosity of the membrane (%), Q is volume of permeate
water per unit time (m3 s-1), A is an effective area of membrane (m2).
3 Result and discussion
3.1 Effect of PVDF concentration on the membrane structure and filtration performance
3.1.1 Morphology studies by FESEM and AFM
The morphology of the membraneswas studied by FESEM to represent the
cross-sectional and surface at certain magnification.Heterogenous structure with top skin supported
by finger-like macrovoids substructure was often observed in many types of hollow fiber
fabricated via the phase inversion method due to the fast solvent and non-solvent exchange
rate [20]. Figure 1 shows FESEM micrographs of the cross-sectional morphology of the
prepared PVDF UF membranes. Increasing PVDF concentration in the range of 16 to 22
wt.% demonstrated an obvious change of morphology and suppressed both of inner and outer
finger-like macrovoids. The finger-like structure under the top layer, as this structure was
prepared from 22 wt.% PVDF in Figure 1(c), was much less than membrane structure
prepared from 19 wt.% PVDF in Figure 1(b). Furthermore, there was also a transition from
macroporous structure to asymmetric structure in membrane cross-section. A more
sponge-like substructure and thicker top layer of membranes were formed across the membrane wall.
This can be explained by the fact that higher dope viscosity decreases the solvent (DMAc)
and non-solvent (water from coagulation medium) exchange rate, thus resulted in higher
resistance of diffusion from polymer aggregation. From FESEM images, pores had not been
observed under FESEM at a magnification of 40.000. The results confirmed that the pores on
the membrane surface were in range of nanometer. Based on the previous studies, it is
reported that pore size decreases with increasing polymer concentration due to the increase in
the solution viscosity, leading to a stronger molecular orientation and tighter structure
[21,22]. Therefore, it is expected that membrane pores tend to be decreased with polymer
A224-11 means of pure water measurement and solute tranport method were carried out in the
following sections.
(a) (b) (c)
Fig.1. Partial cross-section of hollow fiber membranes (a) 16 (b) 19 (c)
PVDF-22
3.1.2 Flux characteristic
Experiment were performed with distilled water to determine the pure water flux
(PWF) and PWF coefficient of PVDF membranes prepared from different polymer
concentration. The experiments were tested using submerged membrane system at vacuum
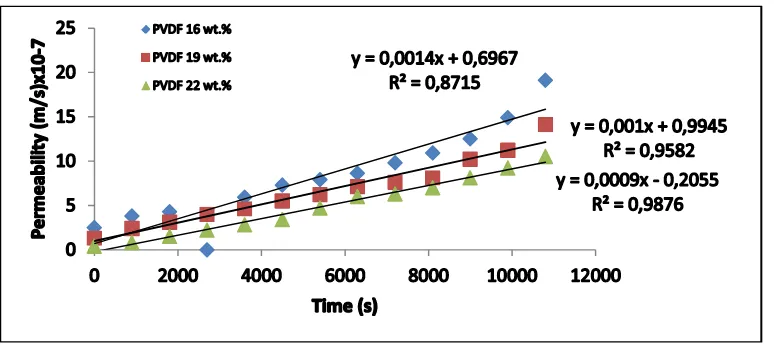
and room temperature. Figure 2 shows the evaluation of permeate flux obtained in these
experiments. A straight line correlation was obtained with reasonably high coefficient (R2 =
0.8715-0.9876) while plotting the membrane permeability on ordinate vs. trans-membrane
pressure. The example of membrane permeation data and its PWP determination is shown in
A224-12
Fig.2. The pure water flux of the hollow fiber membranes prepared from 16 to 22 wt.%
PVDF as a function of filtration time
3.1.2 Solute rejection characteristics
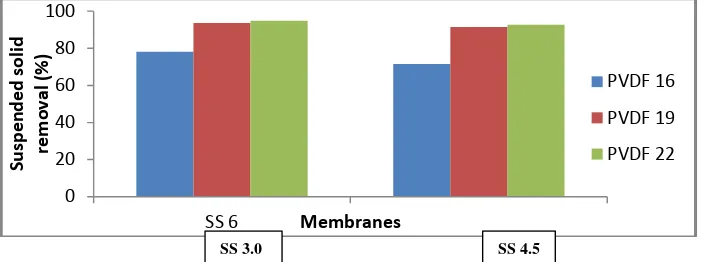
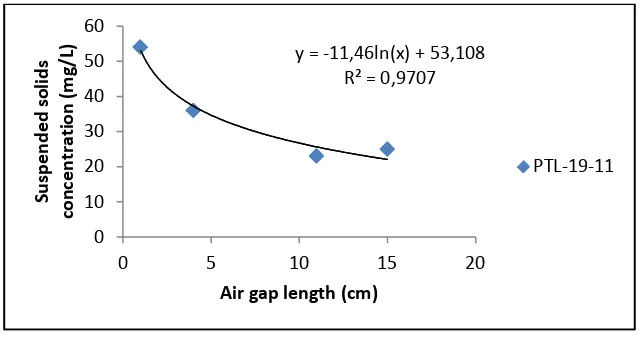
The filtration efficiencies of PVDF membranes were further demonstrated using two
different feed solutions that based on suspended solids concentration (3000 mg/L and 4500
mg/L concentration) as shown in Figure 2. Basically, the electrostatic interactions between
ions and membrane surface charge are responsible for ion filtration. Therefore, the
steric-hindrance effect is also responsible for ion retentions. As can be seen in Figure 1, an increase
in polymer concentration led to increase suspended solids removal efficiency. The results
presented that increasing suspended solids removal is due to the corresponding decrease in
average pore size of membranes as tabulated in Table 3.
Table 3 The PEG rejection and MWCO of various membranes as a function of PVDF
concentration
a
Membrane Neutral solutes, R (%) MWCO (kDa)
PVP k-30 PVP k-60 PVP k-90 PVP k-360
PVDF 16 19.20 36.70 48.45 60.30 77
(±0.23) (±0.07) (±0.35) (±0.03)
PVDF 19 35.50 78.86 91.23 98.83 155
(±0.57) (±0.21) (±0.50) (±0.04)
PVDF 22 30.20 71.45 82.45 95.20 185
(±1.01) (±0.28) (±0.09) (±0.31)
a
A224-13 Figure 4 shows the retention of suspended solids with different percentages of PVDF
content based on suspended solids of 3.0 g/L and 4.5 g/L.
Figure 4: Suspended solids retention of hollow fibers prepared from 16 to 22 wt. %of PVDF
3.2 Effect of air gap length on the membrane structural and separation performance
It is well known that in hollow fiber spinning, the pressurized viscous solution is
subjected to various stresses when it extrudes thorugh the complicated channel within a
spinneret. These stresses may influence molecular orientation and relaxation, and
subsequently fiber formation and separation performance, as well as productivity [23].
Macromolecules may experience swell and relax when exiting from spinneret, if there is an
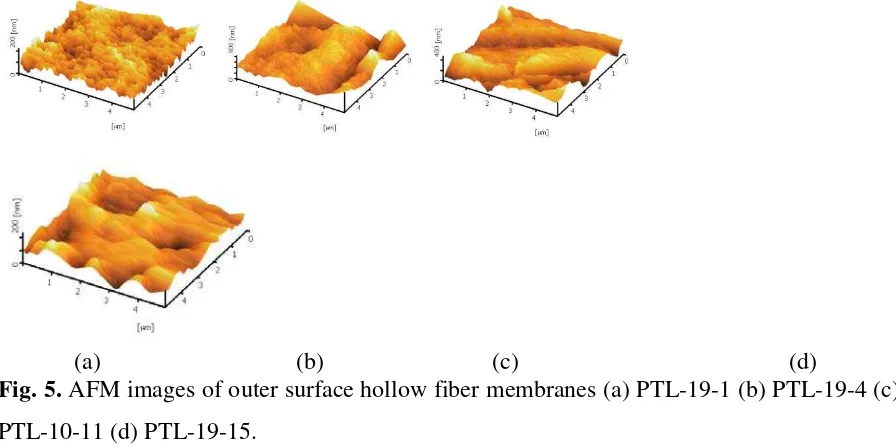
air gap before coagulation and will change their orientation.Figure 5 represents the AFM
images of the surface properties of PVDF hollow fibers. These images revealed that the
membrane surfaces were not smooth and that the nodule aggregates were formed at the
surfaces of the PVDF hollow fibers. The mean roughness parameter Ra obtained from the
AFM images showed 23.45, 15.02, 12.28, and 9.85 for the PTL-19-1, PTL-19-4, PTL-19-11,
and PTL-19-15, respectively. The high peaks seen as bright regions in the AFM images
characterize the nodules while the pores are seen as dark depression. The figures further
indicate that the surface nodule appears to be randomly arranged when the air gap is small but
forms small rows of nodule aggregates aligned in the spinning direction for high air gap
lengths. Generally, the average nodule size at the outer surface seems to increase with an
increase of the air gap length. Molecular chains that experinced higher air gap tend to align
themselves much better than those experienced lower air gap length; and this enhanced
orientation will cause the polymer molecules to be packed closer to each other resulting in a
tigher structure. The nodule size from the AFM images are listed in Table 4. Therefore, the
0 20 40 60 80 100
SS 6
S
u
sp
e
n
d
e
d
s
o
li
d
re
mo
v
a
l (
%)
Membranes
PVDF 16 PVDF 19 PVDF 22
A224-14 average nodule size (nm) and standard deviation (S.D.) for air gap length of 1 cm larger than
those of the other hollow fibers. This may be partly attributed to the fact that polymer
macromolecules may experience die swell when existing from the spinneret as stated by Qin
et al., (2001) [24]. For the dry-jet wet spun fibers, 1 cm air gap length is not enough for
molecular orientation induced by shear stress within the spinneret to relax in the air gap
region. The average nodule size of hollow fibers increases clearly with increasing the air gap
length (>4 cm). This may be due to the increase of interchain entanglement and the annealing
of polymer chain with the air gap length. The radius of gyration and the collision frequency
of polymer chains have also affected the nodule size on membrane surface.
(a) (b) (c) (d)
Fig. 5. AFM images of outer surface hollow fiber membranes (a) PTL-19-1 (b) PTL-19-4 (c)
PTL-10-11 (d) PTL-19-15.
Table 4. Results of the AFM analysis of the outer surfaces of PVDF hollow fiber membranes
Membrane Nodule size Roughness parameters
(nm) S.D. Ra (nm) Rq (nm) Rz (nm)
PTL-19-1 52.30 1.03 23.45 29.55 178.51
PTL-19-4 65.23 1.72 15.02 20.34 133.90
PTL-19-11 86.93 1.19 12.28 15.03 111.50
PTL-19-15 90.08 0.93 9.85 13.75 90.40
As can be seen from the table, it is noticed that the increase of air gap length impacted
the higher values of pure water flux. Because the permeation flux is significantly influenced
by the membrane thickness as given by the Hagen-Poisseuille equation, it is difficult to