Arrhythmia
/
electrophysiology
Anticoagulation in women with non-valvular atrial
fibrillation in the stroke prevention using an oral
thrombin inhibitor (SPORTIF) trials
Mardi Gomberg-Maitland
1*, Nanette K. Wenger
2, Jan Feyzi
3, Maria Lengyel
4,
Annabelle S. Volgman
5, Palle Petersen
6, Lars Frison
7, and Jonathan L. Halperin
81
Department of Medicine, Section of Cardiology, University of Chicago Hospitals, University of Chicago, 5841 S Maryland
Avenue, MC2016, Chicago, IL 60637, USA;
2Emory University School of Medicine, Atlanta, GA, USA;
3Informatics University of
Wisconsin–Madison, Madison, WI, USA;
4Hungarian Institute of Cardiology, Budapest, Hungary;
5Rush University Medical
Center, Chicago, IL, USA;
6Department of Neurology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark;
7AstraZeneca R&D Mo
¨lndal, Mo
¨lndal, Sweden; and
8Mount Sinai Medical Center, New York, NY, USA
Received 11 November 2005; revised 15 May 2006; accepted 26 May 2006; online publish-ahead-of-print 14 June 2006See page 1893 for the editorial comment on this article (doi:10.1093/eurheartj/ehl140)
AimsThe risk of stroke is greater among women with atrial fibrillation (AF) than men. Warfarin protects against stroke, but treatment-related bleeding occurs more often in women than in men.
Methods and resultsSPORTIF III (open label,n¼3410) and V (double-blind,n¼3922) included 2257 women with AF and one or more stroke risk factors randomized to warfarin [target international normal-ized ratio (INR) 2.0–3.0] or ximelagatran (36 mg twice daily). Primary outcomes were all stroke (ischae-mic/haemorrhagic) and systemic embolic event. Women were older, on average, than men, 73.4+8.0 vs. 69.8+9.0 years (P,0.0001). More women were.75-years old and women had more risk factors than men had (P,0.0001). The INR on warfarin (mean 2.5+0.7) was within target range for 67% of follow-up regardless of gender. Women more often developed primary events [2.08%/year, 95% confi-dence interval (CI) 1.60–2.56%/year vs. 1.44%/year, 95% CI 1.18–1.71%/year in men; P¼0.016). Major bleeding rates were similar (P¼0.766) but women experienced more overall (major/minor) bleeding (P,0.001). Warfarin was associated with more overall bleeding in both genders and more major bleeding in women than in men (P¼0.001).
ConclusionWhen compared with men with AF, women in these studies were older and had more stroke risk factors. Women were more prone to anticoagulant-related bleeding; the higher rate of thrombo-embolism among women was related to more frequent interruption of anticoagulant therapy.
KEYWORDS
Atrial fibrillation; Women; Anticoagulation; Thromboembolism
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac
rhythm disorder encountered in clinical practice and an
important risk factor for ischaemic stroke.
1,2Its prevalence
increases with age, affecting women slightly less often than
men.
2–6Stroke rates with AF are higher in women than in
men,
3,7–10and AF is the most common cause of stroke in
elderly women.
Despite the established efficacy of anticoagulation for
prevention of stroke in both men and women with AF,
war-farin is prescribed for less than one-third of women with
AF at hospital discharge, a lower rate than for men, and
this under-use is associated with adverse outcomes.
11Few
randomized trials have enrolled a sufficient proportion of
women to specifically evaluate differential efficacy or
safety on the basis of gender,
12,13but data from a
prospective cohort demonstrate that women have a higher
risk than men for AF-related thrombo-embolism and that
warfarin is as effective in women as in men with AF.
10The Stroke Prevention using an Oral Thrombin Inhibitor in
patients with AF(SPORTIF) programme included two clinical
trials that compared the efficacy and safety of ximelagatran
vs. warfarin in patients with AF at risk of ischaemic stroke.
The combined cohort of SPORTIF III and V includes the
largest number of women yet reported with non-valvular
AF followed prospectively on anticoagulation; 50% of the
women enrolled were over 75 years of age. The results
provide information about sex-based and age-based
differ-ences in rates of ischaemic events and bleeding among
antic-oagulated patients with AF and about the relative safety and
efficacy of warfarin and ximelagatran in women and men.
Methods
The design, characteristics of enrolled patients, statistical analysis, and main results of the SPORTIF III and V trials have been
&
The European Society of Cardiology 2006. All rights reserved. For Permissions, please e-mail: [email protected]*
Corresponding author. Tel:þ1 773 702 5589; fax:þ1 773 834 1764.E-mail address: [email protected] doi:10.1093/eurheartj/ehl103
by guest on June 3, 2013
http://eurheartj.oxfordjournals.org/
reported.14–16Entry criteria, based on current clinical indications for antithrombotic therapy,16,17were identical for the two studies16and included persistent or paroxysmal non-valvular AF verified by ECG within 2 weeks before randomization and at least one of the following additional risk factors for stroke: hypertension, age75 years, pre-vious stroke, transient ischaemic attack (TIA) or systemic embolic event (SEE), left ventricular (LV) dysfunction [LV ejection fraction (LVEF),40% or symptomatic congestive heart failure (CHF)], age
65 years with coronary artery disease (CAD), or age65 years and diabetes mellitus. The principal exclusion criteria were: stroke, TIA, or SEE within 30 days before entry; bleeding disorders; con-ditions requiring conventional anticoagulant therapy; AF secondary to reversible disorders (e.g. thyrotoxicosis); hypertension .180/ 100 mmHg despite medication; previous disabling stroke with modi-fied Rankin score .3; acute coronary syndrome within 30 days; planned cardioversion, treatment with platelet-inhibitor drugs other than aspirin100 mg/day within 10 days; renal insufficiency
(calculated creatinine clearance,30 mL/min); active liver disease or persistent elevation of liver enzymes two or more times the upper limit of normal (ULN); childbearing potential, pregnancy, or lactation.
Randomized treatment
Patients were randomized to treatment with ximelagatran at a fixed dose of 36 mg twice daily or to warfarin dose adjusted to maintain the international normalized ratio (INR) between 2.0 and 3.0, based on blood test monitoring at maximum intervals of 4 weeks. Allocation was balanced according to aspirin therapy at entry, pre-vious stroke or TIA, and country, using an adaptive allocation algor-ithm. In SPORTIF III, treatment was administered open label at 259 sites in 23 countries in Europe, Australia, New Zealand, and Asia, with blinded endpoint event assessment. In SPORTIF V, treatment was double blind at 409 sites in North America.
Concomitant estrogen replacement therapy
Some women took estrogen replacement therapy (ERT) during the trial on the advice of their physician. For this analysis, women were considered to be on ERT when either oral or subcutaneous estrogen alone or in conjunction with progesterone therapy was recorded as a concomitant medication at randomization, but topical therapies and progesterone monotherapy were excluded.
Clinical assessments and endpoints
After randomization, patients were seen at 1, 4, and 6 weeks and then at 2, 3, 4, 5, 6, 8, 10, and 12 months, and every 3 months thereafter for detection of stroke or SEE (primary events), TIA, acute myocardial infarction (MI), or bleeding complications. Events were also detected by a standard stroke-symptom question-naire administered by telephone; positive responses prompted additional evaluation. A study-affiliated neurologist or stroke specialist blinded to treatment allocation evaluated all possible primary events and TIA. An independent, central clinical event adjudication committee also blinded to treatment reviewed clinical reports of all primary and secondary events. Primary endpoints for the trial were stroke and SEE. Secondary endpoints were acute MI, TIA, death, and major and minor bleeding.16
Statistical analysis
The trials were based on establishing non-inferiority within an absol-ute margin of 2%/year for the difference in primary event rates between the two treatment groups. The analysis reported here was planned to compare the efficacy and safety of anticoagulation in women with that in men enrolled in both trials. Comparisons were made for age 75 or younger, paroxysmal or persistent AF, and use of ERT at randomization. In addition, we performed multi-variable analysis of the pooled data for trial effect, study treatment
effect, and their interactions within these subgroups. Sensitivity analyses comparing men and women for the primary endpoint were performed on the basis of a Cox-regression using study as a factor in the model and for interaction between study and gender. The intention-to-treat (ITT) analysis for primary events and mor-tality included all randomized patients until study closure, irrespec-tive of protocol adherence, or continued participation. Remaining endpoints were recorded during the on-treatment (OT) period with exposure censored from the 31st consecutive day or 61st cumulative day off study drug. Events before first dose intake in randomized patients were included in the analysis. No patient was counted more than once for a given composite endpoint.
All patients were followed for primary events and mortality until study closure. Remaining outcomes were recorded during the OT period. Hence, composite endpoints confined to stroke, SEE, and death were analyzed according to ITT, whereas assessments of all other outcomes, composite endpoints and exploratory analyses used the OT approach.
In addition to analyses of efficacy, the safety profiles of ximelaga-tran and warfarin were compared between genders. For each treat-ment group, the proportions of patients experiencing major bleeding, minor bleeding, or bleeding causing permanent treatment cessation were calculated, accounting for duration of exposure for each patient. Other adverse events and laboratory variables are summarized using descriptive statistics.
The analysis of the difference in primary event rates between the treatment groups was made under the assumption of exponentially distributed lifetimes. This is equivalent to assuming a constant event rate over time and hence the probability of an event over a year can be estimated from the complete follow-up of all patients. The variance for the difference in event rates was estimated using the method described by Cox and Oakes.18Two-sided P-values for differences between treatments were obtained by Fisher’s exact test, using the number of patient-years as analyses units. ERT was a conceived subgroup analysis prior to completion of the trials. Because both age.75 and pattern of AF (paroxysmal or persistant) are perceived risk factors for AF, we performed substudy analyses. Also, for both SPORTIF III and V comparisons of primary event rates according to age .75 vs. ,75 was a predefined tertiary endpoint. Thus the writing group felt that examination in this cohort was important for substudy analyses.
Cox-regression analyses used the following baseline stroke risk factors as covariates for multivariable analyses after validation of the proportional hazards model assumption: gender, baseline systo-lic blood pressure and diastosysto-lic blood pressure (DBP), prior stroke/ TIA, SEE, hypertension, CAD (MI, angioplasty, or angina), diabetes mellitus, LV dysfunction (LVEF ,40% or symptomatic CHF), age (both as a continuous and as a categorical variable), smoking (past or present vs. none), body mass index (,30 vs.30), alcohol as a dichotomous variable, trial, and study treatment.
Results
Baseline characteristics
Baseline characteristics of enrolled patients are compared
by gender in
Table 1
. Of the 7329 subjects enrolled, 2257
(30.8%) were women (30.9% in SPORTIF III and 30.7% in
SPORTIF V). The mean age was 73.4
+
8 years for women
and 69.8
+
9 years for men (
P
,
0.0001). The mean blood
pressure in women was 138.5
+
18 mmHg systolic and
79.8
+
10 mmHg
diastolic
when
compared
with
134.0
+
18 mmHg (
P
,
0.0001) and 79.2
+
11 mmHg in
men (
P
¼
0.015). Ninety-three per cent of women and 92%
of
men
were
Caucasian
compared
to
other
races
(
P
,
0.0001). Only 5% of women were occasional or habitual
smokers when compared with 11% of men (
P
,
0.0001). The
onset of AF was within 1 year in 23% of women vs. 18% of
by guest on June 3, 2013
http://eurheartj.oxfordjournals.org/
men (
P
,
0.0001). A history of hypertension was more
frequent (81% vs. 75%;
P
,
0.0001), but the prevalence of
diabetes (22% vs. 24%;
P
¼
0.03), coronary heart disease
(38% vs. 48%;
P
,
0.0001), and LV systolic dysfunction (33%
vs. 38%;
P
,
0.0001) was lower among women. Women had
a larger number of associated thrombo-embolic risk factors
(43% vs. 38% with three or more risk factors in addition to
AF;
P
,
0.0001).
Prior to randomization, women were less often treated
with
cardiovascular
medications
such
as
b
-blockers,
angiotensin-converting enzyme (ACE) inhibitors, or statins
but there was no difference in use of vitamin K antagonists
or aspirin. After randomization, the INR was within the
target range (2.0–3.0) 66.6% of the time on treatment and
in the expanded range of 1.8–3.2 for 81.5% of the time
among the 1099 women assigned to warfarin therapy.
Among men on warfarin (
n
¼
2525), INR was between 2.0
and 3.0 for 67.8% and between 1.8 and 3.2 for 82.7% of
the time on treatment.
Endpoint events
By ITT analysis, more women than men developed primary
events (stroke or SEE), with 72 women experiencing events
during 3465 patient-years [2.08%/year, 95% confidence
interval (CI) 1.60–2.56%/year] vs. 112 men during 7759
Table 1 Baseline characteristicsBaseline characteristics Men (n¼5072) Women (n¼2257) P-value
Age (years) 69.8+9 73.4+8 ,0.0001
SBP (mmHg) 134.0+18 138.5+18 ,0.0001
DBP (mmHg) 79.2+11 79.8+10 0.015
Race,n(%)
Caucasian 4658 (92) 2099 (93) ,0.001
African-American 75 (2) 53 (2)
Oriental 323 (6) 99 (4)
Other 16 (0) 6 (0)
Smoking,n(%)
Non-smoker 1605 (32) 1496 (66) ,0.0001
Ex-smoker 2907 (57) 643 (29)
Occasional 118 (2) 22 (1)
Habitual 442 (9) 96 (4)
AF,n(%)
Persistent 4354 (89) 1956 (87) ,0.001
Paroxysmal 537 (11) 299 (13)
Years since first AF diagnosis,n(%)
,1 850 (18) 477 (23) ,0.0001
1–2 561 (12) 245 (12)
2–5 1249 (27) 555 (27)
.5 1986 (43) 761 (38)
History of stroke,n(%) 631 (12) 286 (13) 0.74
History of TIA,n(%) 530 (10) 246 (11) 0.56
History of diabetes,n(%) 1230 (24) 495 (22) 0.03
History of CAD,n(%) 2415 (48) 864 (38) ,0.0001
High-risk factors,n(%)
Hypertension 3804 (75) 1821 (81) ,0.0001
Age75 years 1670 (33) 1134 (50) ,0.0001
Prior stroke/TIA 1048 (21) 491 (22) 0.29
SEE 224 (4) 104 (5) 0.71
LV dysfunction 1942 (38) 739 (33) ,0.0001
Age65 and diabetes 921 (18) 419 (19) 0.68
Age65 and CAD 1985 (39) 779 (35) ,0.001
Number of risk factors,n(%)
0 13 (0) 4 (0) ,0.0001
1 1520 (30) 531 (24)
2 1636 (32) 745 (33)
3 1097 (22) 582 (26)
4 565 (11) 275 (12)
.4 241 (5) 120 (5)
Concomitant medications,n(%)
ASA 878 (17) 304 (14) ,0.0001
b-Blocker 2425 (48) 1040 (46) 0.17
ACE-inhibitor 2621 (52) 945 (42) ,0.0001
Statin 1737 (34) 610 (27) ,0.0001
ERT 0 (0) 381 (17) ,0.0001
ASA, acetylsalicylic acid; SBP, systolic blood pressure; DBP, diastolic blood pressure; TIA, transient ischaemic attack; SEE, systemic embolic event; LV, left ventricular; CAD, coronary artery disease; ACE, angiotensin converting enzyme.
by guest on June 3, 2013
http://eurheartj.oxfordjournals.org/
patient-years
(1.44%/year,
95%
CI
1.18–1.71%/year;
P
¼
0.016;
Table 2
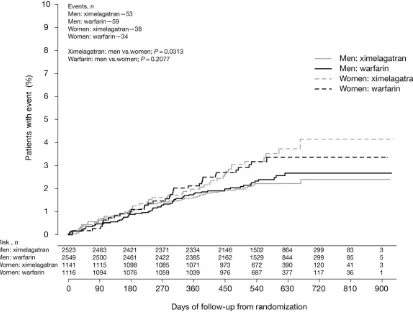
). However, this difference was limited
to the ximelagatran group (2.17%/year, 95% CI 1.48–2.87%/
year vs. 1.38%/year, 95% CI 1.00–1.75%/year;
P
¼
0.03).
There was no significant difference in primary event rates
based on gender in the warfarin group (
Figure 1
).
Comparing women in the ximelagatran and warfarin
groups, 38 patients experienced primary events during
1748 patient-years with ximelagatran (2.17%/year) vs. 34
during
1717
patient-years
for
warfarin
(1.98%/year;
P
¼
0.722). Among men, during 3854 patient-years, 53
developed primary events in the ximelagatran group
(1.38%/year) when compared with 59 in the warfarin
group during 3905 patient-years (1.51%/year;
P
¼
0.635).
In contrast to their more favourable outcome with respect
to the primary events, men suffered higher all-cause
mor-tality than women, with 301 male deaths over 7831
patient-years (3.84%/year, 95% CI 3.41–4.28%/year) when compared
with 93 deaths among women over 3507 patient-years
(2.71%/year,
95%
CI
2.16–3.25%/year;
P
¼
0.002).
Furthermore,
the difference
in primary event
rates
between women and men was not apparent when assessed
by OT analysis (1.61%/year in women, 95% CI 1.16–2.07%/
year vs. 1.45%/year in men, 95% CI 1.17–1.73%/year;
P
¼
0.53). The mean number of stroke risk factors other
than AF among women who discontinued anticoagulation
during the trial was 2.54 (95% CI 2.47–2.61) when compared
with a mean of 2.21 (95% CI 2.17–2.24), among those who
were able to sustain anticoagulation (
P
,
0.0001). Primary
events included haemorrhagic stroke in four women (three
fatal), ischaemic stroke in 40 (seven fatal), and SEE in five
(one fatal). Ten men developed haemorrhagic stroke of
which five were fatal, 86 ischaemic stroke (eight fatal),
and six SEE (one fatal), using a 30-day rule.
Bleeding complications
There was no difference in major bleeding complications
between women and men overall (2.25% vs. 2.15%/year;
P
¼
0.765) or in either of the treatment groups (0.57%/
year on ximelagatran,
P
¼
0.208, and
2
0.35%/year on
war-farin,
P
¼
0.491). Among women, there was no difference in
major bleeding in the ximelagatran vs. warfarin groups.
More men developed major bleeding on warfarin (91
events; 2.57%/year) when compared with those on
ximela-gatran (58 events, 1.71%/year;
P
¼
0.016). Combined rates
of both major and minor bleeding were greater among
women
than
men
(41.29%/year
vs.
33.91%/year;
P
,
0.001) that carried across both treatment groups (a
difference in rates of 5.37%/year between women and
men on ximelagatran,
P
¼
0.001, and a difference of
8.56%/year on warfarin,
P
,
0.0001). Comparing the two
treatments, bleeding was less frequent among women in
the ximelagatran group (35.48%/year) than in the warfarin
group (44.82%/year,
P
,
0.001). The same was true among
men (30.85%/year in the ximelagatran group vs. 37.01%/
year in the warfarin group;
P
,
0.001).
Liver function abnormalities
Women in the ximelagatran group developed serum levels of
S
-alanine aminotransferase (ALT) beyond three times the
ULN more often than men (8.4% vs. 5.1%;
P
,
0.001;
Table 3
). Incidences in the warfarin group were 1.0%
among women and 0.7% among men. Incidences of ALT
Table 2 Event rates: women vs. men and women aged75 years vs. women aged,75 years
Events,n Patient-years Event rate, % per year P-value
Stroke and SEE (ITT analysis)
Women 72 3465 2.08
Men 112 7759 1.44 0.016
Women aged75 years 45 1724 2.61
Women aged,75 years 27 1742 1.55 0.032
Mortality
Women 95 3507 2.71
Men 301 7831 3.84 0.002
Women aged75 years 67 1746 3.84
Women aged,75 years 28 1760 1.59 ,0.001
Stroke and SEE (OT analysis)
Women 49 3037 1.61
Men 101 6960 1.45 0.53
MI
Women 22 3038 0.72
Men 78 6949 1.12 0.08
Major bleeding
Women 68 3022 2.25
Men 149 6924 2.15 0.766
Women aged75 years 41 1474 2.78
Women aged,75 years 27 1548 1.74 0.065
Major and minor bleeding
Women 889 2153 41.29
Men 1776 5237 33.91 ,0.001
Women aged75 years 467 1044 44.73
Women aged,75 years 422 1109 38.05 0.002
by guest on June 3, 2013
http://eurheartj.oxfordjournals.org/
beyond five times the ULN occurred more often in women
than in men (4.5% vs. 2.6%) on ximelagatran but with little
difference on warfarin (0.4% vs. 0.3%).
Differences based on age
Compared with younger women, those aged
75 years were
more often Caucasian (96 vs. 90%), non-smokers (68 vs.
65%), with higher systolic blood pressure (139.8 vs.
137.2 mmHg), taking aspirin (15 vs. 12%), with persistent
AF (89 vs. 85%) and a history of TIA (12 vs. 10%). Elderly
women had more risk factors for stroke (three or more in
63 vs. 23%) but less often had a history of hypertension (77
vs. 84%) and had a lower DBP (79.0 vs. 80.7 mmHg) or
diabetes (18 vs. 36%). Older women more often had CAD
than younger women (40 vs. 29%).
More women over the age of 75 years developed
primary events (2.61 vs. 1.55%/year;
P
¼
0.032; ITT
analysis). Older women also displayed greater all-cause
mortality (
P
,
0.001;). Rates of major bleeding were not
significantly different between older and younger women
(2.78 vs. 1.74%;
P
¼
0.065), but the elder group developed
more overall (major and minor) bleeding events (44.73
vs. 38.05%/year;
P
¼
0.002). There were no differences
in major bleeding based on treatment group
assign-ment among either older women (2.97%/year in the
ximela-gatran group vs. 2.60%/year in the warfarin group;
P
¼
0.752)
or
younger
women
(1.66%/year
in
the
ximelagatran group vs. 1.83%/year in the warfarin group;
P
¼
0.848).
Differences based on ERT
In both trials combined, 381 women (17%) took ERT at
ran-domization and for more than half the follow-up period,
and 413 (18%) used ERT at some point during the trial. Of
those taking ERT at entry, 14 developed ischaemic stroke,
SEE, or TIA and 27 had ischaemic stroke, SEE, TIA, MI, or
death. The small number of women using ERT and the
infre-quency of events left the results of univariate and
multivari-able analyses inconclusive.
Differences based on pattern of AF
Most women (87%) had persistent AF. There were no
differences in baseline demographics between those with
Table 3 Liver function tests: number of patients by gendermaximalS-ALT multiple of ULN during study (ITT population)
ULN Ximelagatran Warfarin Total
Women,n(%)
ALT.ULN1 338 (29.6) 104 (9.3) 442 (20.0) ALT.ULN2 152 (13.3) 21 (1.9) 173 (8.0) ALT.ULN3 96 (8.4) 11 (1.0) 107 (5.0) ALT.ULN5 51 (4.5) 5 (0.4) 56 (2.0) ALT.ULN10 9 (0.8) 1 (0.0) 9 (0.0) Men,n(%)
ALT.ULN1 591 (23.4) 326 (12.8) 917 (18.0) ALT.ULN2 218 (8.6) 51 (2.0) 269 (5.0) ALT.ULN3 128 (5.1) 18 (0.7) 146 (3.0) ALT.ULN5 65 (2.6) 7 (0.3) 72 (1.0) ALT.ULN10 22 (0.9) 1 (0.0) 23 (0.0)
Figure 1 Primary event rates over time: women vs. men on ximelagatran and women vs. men on warfarin.
by guest on June 3, 2013
http://eurheartj.oxfordjournals.org/
persistent vs. paroxysmal AF except for age (42% with
paroxysmal AF were
75 years vs. 52% with persistent
AF;
P
¼
0.002) and LV dysfunction (25% with paroxysmal
AF vs. 34% with persistent AF;
P
¼
0.0025). Women with
persistent AF had more stroke risk factors (45 vs. 35%
with more than three risk factors in addition to AF;
P
,
0.0001).
Multivariable analyses
There were no significant interactions between gender and
either trial or treatment; hence, data were pooled over
trial and treatment for all gender-based analyses. When
included as the only variable in the model, gender was
sig-nificantly related to the primary event rate [hazard ratio
(HR) 1.44; 95% CI 1.07–1.93;
P
¼
0.0161). After adjusting
for the covariates above, however, this difference was no
longer significant (HR 1.27; 95% CI 0.91–1.76;
P
¼
0.16).
On the basis of Cox-regression analyses, the
P
-value for
the gender comparison for the primary endpoint remained
unchanged with (
P
¼
0.016) or without (
P
¼
0.016) study
present as a factor in the model. There was no interaction
between study and gender (
P
¼
0.92).
Discussion
This cohort of 2257 women with AF on anticoagulation is the
largest described to date; even so, as only 31% of
random-ized patients were women, statistical power of conclusions
specifically involving women remains limited. Compared
with men in these trials, women were typically older than
75 years, had a history of hypertension, and had more risk
factors for stroke though they were less often diabetic and
had normal LV function. By ITT analysis, women had a
higher rate of primary events while there was no difference
by OT analysis. Although one interpretation is that the
higher rate of thrombo-embolism among women with AF
might have been related to interruption of anticoagulant
therapy, it was not possible to verify whether the number
of primary events following drug interruption differed
between genders. The data do, however, support the
con-clusion that those who discontinued therapy were at
higher intrinsic risk, similar to the recent report from the
ATRIA investigators.
10Although the SPORTIF III trial was conducted open label
and SPORTIF V was double blind, we consider it valid to
combine data from both because of the measures taken to
minimize bias. The ‘hard’ primary endpoints (all strokes
and SEE) and multiple levels of blinded adjudication were
the same for both trials. A specific symptom questionnaire
was uniformly employed to ensure the detection of
rela-tively minor events, and all suspected endpoint events
(including TIA) were assessed at each study centre by an
independent neurologist or stroke physician unaware of
ran-domized treatment assignment and adjudicated by a single,
independent, clinical events adjudication committee, also
blinded to treatment. Major haemorrhage and MI were
simi-larly adjudicated. Other potential sources of bias included
differences in concomitant medication or choice of stroke
prevention strategy upon cessation of study medication,
but these were relatively minor.
Half the female cohort was
75 years of age, a larger
pro-portion than men, and these older women had a higher
primary event rate than younger women. Although the
results of this subgroup analysis should be interpreted
cau-tiously, they are consistent with reports emerging from
other prospectively followed cohorts of patients of both
genders with AF. Despite a lower prevalence of diagnosed
hypertension, these elderly women had higher systolic
blood pressure than men of comparable age, consistent
with previous reports.
19Although elderly women had more
stroke risk factors, there were only small differences in
the incidence of prior thrombo-embolism. Despite
compar-able or greater comorbidities, women were less likely to
have taken antithrombotic medication prior to entry,
perhaps reflecting fear of anticoagulant-related bleeding
among elderly women.
20,21In addition, fewer women than
men received such concurrent medications as
b
-blockers,
ACE-inhibitors, or lipid-lowering agents at entry or during
the course of the studies, yet the all-cause mortality rate
during follow-up was higher among men than women.
Some of the discrepancy might reflect the less frequent
diagnosis of coronary heart disease among women.
22Women had a slightly higher rate of major and minor
bleeding than men, but gender did not influence rates of
major bleeding and this argues in favour of anticoagulation
for women with AF. Compared with younger women, the
elderly had similar rates of major bleeding but slightly
more overall bleeding than younger women, arguing for
anticoagulation of all age groups. The quality of warfarin
control was as good among women as in men, with INR
values within the INR range of 2–3 about two-thirds of the
time on treatment. Warfarin-naı¨ve patients may have
more events than patients who had taken warfarin
pre-viously, as reported in another prospective trial of
anticoa-gulation in patients with AF.
23The safety and efficacy we
observed during warfarin anticoagulation reflect
high-quality management in patients of both genders, and the
higher bleeding rates in elderly women cannot be explained
by lax INR control.
Few women took ERT during the study periods, possibly
because of the reported risks of MI and death in women
with CAD taking hormonal therapy,
24although more North
American women than those in other countries were using
ERT. The limited use of ERT among enrolled women makes
it difficult to evaluate its impact on the risk of stroke, but
we observed no difference in event rates.
The higher primary event rates among women than men
were independent of treatment assignment. When
exam-ined separately on the basis of ITT, both women and men
responded as well to ximelagatran as to well-controlled
war-farin for prevention of thrombo-embolism. The efficacy of
ximelagatran was also consistent across genders when
eval-uated by OT analysis. Men had lower rates of major bleeding
on ximelagatran than on warfarin, but there were no
signifi-cant differences in rates of intracerebral haemorrhage,
which was infrequent in both genders and in both treatment
groups.
Elevations of serum transaminase enzymes occurred more
often in women, but rates in both men and women fell
within the range reported in other trials involving patients
with venous thrombo-embolism and acute coronary
syn-dromes treated with ximelagatran. The mechanism of this
reaction is not known, but concerns about potential
hepato-toxicity led to withdrawal of ximelagatran from the
marketplace.
25by guest on June 3, 2013
http://eurheartj.oxfordjournals.org/
The SPORTIF trials represent the largest randomized
cohort of women with AF on anticoagulation yet reported,
with a high representation of elderly women. As a group,
women in the SPORTIF studies were older and had greater
comorbidity than in earlier trials of anticoagulation and
thus contributed more ischaemic and fatal events for
analy-sis. Although less total bleeding occurred in women on
xime-lagatran when compared with warfarin, the elevation of
liver enzymes offset this benefit. Their clinical features
may reflect practice patterns at the time the studies were
carried out, yet the differences they exhibited compared
with men with AF appear robust across time.
Acknowledgements
The SPORTIF studies were sponsored by AstraZeneca, Mo¨lndal, Sweden. Drs G.-M., N.K.W., A.S.V., J.L.H., and Ms Fenzi have received grant support from AstraZeneca; Drs P.P. and J.L.H. have served as consultants for AstraZeneca; and Dr L.F. is a full-time employee of AstraZeneca. Dr M.L. has no financial disclosure.
Conflict of interest: The SPORTIF studies were sponsored by Astra-Zeneca, Mo¨lndal, Sweden. Drs Gomberg-Maitland, Wenger, Volgmar, and Halperin and Ms Fenzi have received grant support from Astra-Zeneca; Drs Petersen and Halperin have served as consultants for AstraZeneca as members of the Executive Steering Committee; and Dr Frison is a full time employee of AstraZeneca. Dr Lengyel has no financial disclosure.
References
1. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications.Arch Intern Med1995;155:469–473.
2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 2001;285: 2370–2375.
3. Friberg J, Scharling H, Gadsboll N, Truelsen T, Jensen GB. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women vs men (The Copenhagen City Heart Study). Am J Cardiol2004;94:889–894.
4. Lake FR, Cullen KJ, de Klerk NH, McCall MG, Rosman DL. Atrial fibrillation and mortality in an elderly population.Aust N Z J Med1989;19:321–326. 5. Phillips SJ, Whisnant JP, O’Fallon WM, Frye RL. Prevalence of cardiovas-cular disease and diabetes mellitus in residents of Rochester, Minnesota. Mayo Clin Proc1990;65:344–359.
6. Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).Am J Cardiol1994;74:236–241.
7. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study.Circulation1998;98:946–952.
8. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med1998;158:229–234.
9. Wang TJ, Massaro JM, Levy Det al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study.JAMA2003;290:1049–1056.
10. Fang MC, Singer DE, Chang Y, Hylek EM, Henault LE, Jensvold NG, Go AS. Gender differences in the risk of ischaemic stroke and peripheral embo-lism in atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study.Circulation2005;112:1687–1691.
11. Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrom-botic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke2000;31:822–827.
12. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis.Ann Intern Med1999;131:492–501.
13. Risk factors for stroke, efficacy of antithrombotic therapy in atrial fibril-lation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med1994;154:1449–1457.
14. Executive Steering Committee; SPORTIF III Investigators. Stroke preven-tion with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): a randomized trial.Lancet2003;362:1691–1698.
15. SPORTIF Executive Steering Committee; SPORTIF V Investigators. Ximelagatran vs. warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA 2005; 293:690–698.
16. Halperin JL. Ximelagatran compared with warfarin for prevention of thromboembolism in patients with nonvalvular atrial fibrillation: ration-ale, objectives, and design of a pair of clinical studies and baseline patient characteristics (SPORTIF III and V). Am Heart J 2003;146:431–438.
17. Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(Suppl. 3):429S–456S.
18. Cox D, Oakes D. Analysis of survival data. New York, NY/London, England: Chapman and Hall; 1984.
19. Perry HM Jr, Smith WM, McDonald RH, Black D, Cutler JA, Furberg CD, Greenlick MR, Kuller LH, Schnaper HW, Schoenberger JA. Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) pilot study.Stroke1989;20:4–13.
20. Go AS, Hylek EM, Phillips KAet al. Implications of stroke risk criteria on the anticoagulation decision in nonvalvular atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Circulation2000;102:11–13.
21. Vasishta S, Toor F, Johansen A, Hasan M. Stroke prevention in atrial fibril-lation: physicians’ attitudes to anticoagulation in older people. Arch Gerontol Geriatr2001;33:219–226.
22. Bhatt DL, Roe MT, Peterson ED, Li Y, Chen AY, Cohen DJ, Gibson CM, Saucedo JF, Kleiman NS, Hochman JS, Boden WE, Brindis RG, Peacock WF, Smith SC Jr, Pollack CV Jr, Gibler WB, Ohman EM. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE quality improvement initiative.JAMA2004;292:2096–2104. 23. Connolly SJ. ACTIVE W. Presented at American Heart Association
Meeting, Dallas, TX, 2005, November 14.
24. Herrington DM. The HERS trial results: paradigms lost? Heart and estrogen/progestin replacement study. Ann Intern Med 1999; 131:463–466.
25. Lee WM, Larrey D, Olsson R, Lewis JH, Keisu M, Aucleit L, Sheth S. Hepatic findings in long-term clinical trials of ximelagatran.Drug Saf 2005;28:351–370.
by guest on June 3, 2013
http://eurheartj.oxfordjournals.org/