Fungicides cytotoxicity expressed in male gametophyte
development in
Brassica campestris
after in vitro application
of converted field doses
Milan Pavlı´k
a,*, Olga M. Jandurova´
baDepartment of Natural Products,Institute of Organic Chemistry and Biochemistry,Academy of Sciences of Czech Republic,
Flemingo6o na´m. 2,CZ-16610 Prague6, Czech Republic bInstitute of Animals Production,Pratelst
6i 850,CZ-10400Prague 10, Czech Republic Received 26 March 1999; received in revised form 27 March 2000; accepted 27 March 2000
Abstract
A simple method to determine the toxicity of fungicides on male gametophyte in Brassica campestris subsp.
oleiferaeis described. The calculation of fungicide concentration used in the test is derived from doses used in field application. The expression of regression curves and calculation of regression equations require the logarithmic transformation of fungicide concentration. The range of the sensitivity of the method is very wide. The minimal concentration detected as significantly different from control ranges from 1.7 to 7.0 pg of the active compound. Fungicides declared as non-toxic for plants in field tests were cytotoxic for male gametophyte development. The synergistic action of more than one active compound resulted in higher toxicity. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Brassica campestrissubsp.oleiferae; Carbendazim; Fungicide; Pollen germination; Toxicity; Triazole
www.elsevier.com/locate/envexpbot
1. Introduction
In the instructions for the application of pesti-cides we can find the notion of protection time, e.g. the limitation of fungicide treatment from the point of view of consumption by man or animals. The recommendation for the treatment is given in relation to the occurrence of pathogens and to
plant development stage, when the treatment is applied. There are no data related to a possible decrease of fertility due to lower pollen ger-minability. The degree of toxicity, according the WHO classification (Tomlin, 1994), is slightly hazardous for cyproconazole, flutriafol, flusila-zole, propiconazole and carbendazim. The EPA classification is similar except for carbendazim, classified in the IV degree. Cyproconazole, flutriafol, and flusilazole have no mutagenic and teratogenic effect according to the literature (Tomlin, 1994). The effect of fungicides on pollen * Corresponding author. fax: +420-2-24310177.
E-mail addresses: [email protected] (M. Pavlı´k), [email protected] (O.M. Jandurova´).
germinability and consequently on fruit-set and yield were investigated mostly on pear, apple, raspberries (Redalen, 1980; Fell et al., 1983; Mar-cucci and Filiti, 1984), as fungicides are frequently used in orchards during flowering time. The great diversity of published results is partly due to non-comparable levels of the presented data (in vitro, in vivo on stigmas, just after treatment or ex post by means of fruit crop, respectively). Taking the case of fruit trees as a model, a direct effect of fungicides on fruit crop was not detected. The study of fungicides at cellular level showed the influence of triazoles on sterol metabolism due to inhibition of ergosterol synthesis. The effect is non-specific, in that fungicides inhibit sterol syn-thesis in host plant as well as in fungus hyphae (Fuller et al., 1990; Tomlin, 1994). The accumula-tion of steroid precursors (lanosterol) after propi-conazole treatment led to cholesterol and phytosterol inhibition (Vanden et al., 1987). Tria-zole fungicides decreased the activity of peroxi-dase (Lebedev et al., 1989), while carbendazim inhibited aldehyde dehydrogenase (Wiegand-Rosi-nus et al., 1988). The quantitative estimation of the phytotoxic activity of pesticides (carbendazim) is based on specific enzyme inhibition in photo-synthetic active leaf discs from cotyledons of
Sinapis alba(Petzold et al., 1988).
Little information is available as yet concerning the effect of field applications of fungicides on pollen development, subsequent germination, and/or leaf morphology in any species. He and Wetzstein (1994) showed for the first time that the application of some early season fungicides caused degeneration of developing pollen and re-tarded growth of catkins and leaves.
Many papers have focused on testing xenobi-otics (for example fungicides) for germination of pollen grains (He et al., 1995). Studying the in vitro germination of pollen treated with fungicides showed usually a decrease in pollen grain germi-nation, deformation of pollen tubes and their bursting, but also an increase in pollen germina-tion at very low concentragermina-tions of fungicides. Authors used a great range of concentrations sometimes without relation to actual doses ap-plied in field. Papers focused on pollen in vitro tests used frequently 1 mg of testing pollen in 1 ml
of medium during experiments (Kappler and Kristen, 1987; Strube et al., 1991). Calculation of doses for tests in vitro from doses using in tests in vivo used results from tests in vitro. Therefore the aim of this work is to verify calculation of dose of fungicides for tests in vitro from doses using in field conditions and toxicity of minimum tested doses of fungicides on limited quantity of tested pollen. The evaluation of ecotoxicity of a given dose is possible when optimal conditions for pol-len germination of donor plants are known (Jan-durova´ and Pavlı´k, 1995). If the presence of xenobiotic compound is the only stress factor in the experiment, then any significant difference between control (optimal conditions) and treat-ment should be explained as a direct reaction to the stress factor.
2. Material and methods
The change of pollen shape and tube emergence were microscopically observed (magn. 700×) af-ter 60 and 120 min of incubation under labora-tory conditions. Grains with a round shape with the ratio of axes approaching one were scored as hydrated; grains with tubes longer than the di-ameter of hydrated pollen were considered as germinated. The rest was aborted as non-hydrated pollen grains. Pollen grains with bursting tubes were excluded from evaluation. Thousand (10×
100) pollen grains were evaluated in every treat-ment, with three replications per treatment.
Histochemical staining methods were used to detect activities of alkaline phosphatase (APH), peroxisomal catalase (PER), succinate dehydroge-nase (SDH) and non-specific esterases (EST), after
2 h and incubation of germinated pollen with investigated fungicides. Pollen was incubated at treatments A, B and C by dropping 100 ml of pollen suspension into the slides’ chamber. The germination medium with the different fungi-cides was carefully removed and replaced with appropriate cultivation medium with specific sub-strate.
Alkaline phosphatase was detected by azocou-pling technique based on reaction between naph-thol and diazonium salt (Lojda et al., 1979). The specific substrate was naphthol AS BI phosphate, and Tris buffer was used to keep the pH between 8.2 and 9.2. The same principle was used in non-specific esterases where naphthol was liber-ated from a-naphthyl acetate and forms azodye
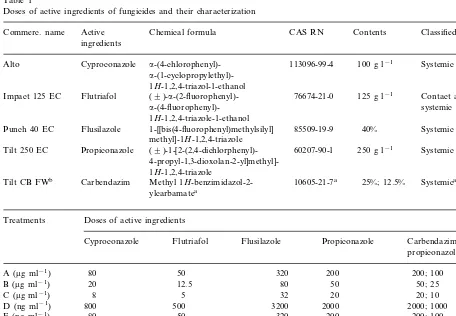
Table 1
Doses of active ingredients of fungicides and their characterization
Chemical formula Active
Commerc. name CAS RN Contents Classified
ingredients
100 g l−1 Systemic Alto Cyproconazole a-(4-chlorophenyl)- 113096-99-4
a-(1-cyclopropylethyl)-1H-1,2,4-triazol-1-ethanol
Contact and (9
)-a-(2-fluorophenyl)-Flutriafol 76674-21-0 125 g l−1
Impact 125 EC
Punch 40 EC 1-[[bis(4-fluorophenyl)methylsilyl] 40% methyl]-1H-1,2,4-triazole
250 g l−1
60207-90-1 Systemic foliar Propiconazole
Tilt 250 EC (9 )-1-[2-(2,4-dichlorphenyl)- 4-propyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole
Systemica
Carbendazim 10605-21-7a
Tilt CB FWb Methyl 1H-benzimidazol-2- 25%; 12.5%
ylcarbamatea
Doses of active ingredients Treatments
aOnly for carbendazim.
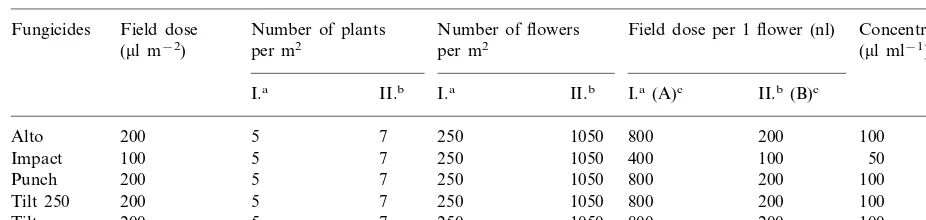
Table 2
Calculation of fungicides doses for in vitro testing (theoretical maximum doses)
Field dose per 1 flower (nl) Number of plants
Field dose
Fungicides Number of flowers Concentration
per m2
Impact 1050 400 100 50
5 800 200 100
200 1050
Punch 7 250
200 5 7 250
Tilt 250 1050 800 200 100
200
Tilt 5 7 250 1050 800 200 100
0
aMinimum number of plants (flowers) per m2for optimum yield. bMaximum number of plants (flowers) per m2for optimum yield.
cA, B, Basic treatments of doses, which were taken from basic stock solution. dZ,ml of fungicides per 1 ml of basic stock solution.
with diazonium salt (Davis and Ornstein, 1959). The reduction of different tetrazolium salts and formasan formation is routinely used for dehydro-genase detection (Stanley and Linskens, 1966). In our experiments the substrate was sodium succinate and the hydrogen acceptor was nitro blue tetra-zolium. Activities of peroxisomal catalases were detected with diaminobenzidine that produced brown coloured product after oxidation (Roels, 1976). The evaluation of enzyme activity was made after appropriate incubation microscopically. Ten times 100 pollen grains were counted and percent-age of stained grains represented the level of enzyme activity. No fixation step was needed as the samples were observed immediately after the incu-bation period.
Programs Quattro Pro 3.01 for DOS, Statgraph-ics 4.0 for DOS and Delta Graph Professional 2.0 for Windows were used for calculation. It is not possible to include extreme measured values (de-pendent variableyi=0 oryi=1) for calculating the regression equation that follows from single doses (independent variablexi). TheYis the probability of pollen germination in the interval0;1.Y=1 corresponds to probability of pollen germination for control treatment. TheXis the scale of logarith-mic value of fungicide doses in the intervalX]0. Units are determined as the minimum value of dilution of fungicide, which has a negative effect on the probability of pollen germination, for which it is valid, that logxi]0 orxi]1. Function I is given
as y=f(x) for X{0;xmin}, for which it is valid, thatY=1 if the germination of the pollen treated by fungicide was identical with the control (100%). Value xmin is the dose of fungicide, which has no effect on pollen germination. It is not significantly different from the control. Function II (y=f(x)) is by polynome nth of level of common of form:
f(z)=a0+a1z more than six orders, it was necessary to make a logarithmic transformation of value xi to log xi. Value xmax is the dose of fungicide, when pollen germination is 0.1%. Function III is function y=
f(x) for X{xmax; 100}, for which it valid, that
Y=0. Pollen treated by fungicides, does not germi-nate. The calculation was performed by thet-test.
3. Results
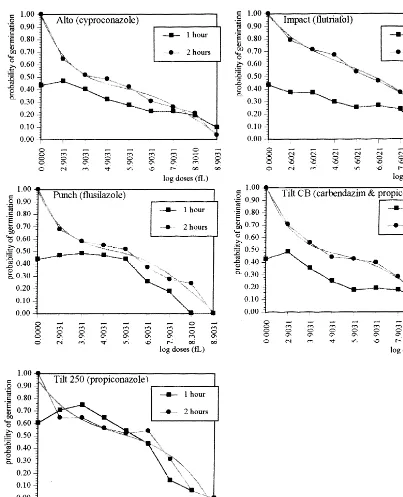
Single statistical characteristics are shown in Table 3. The method used is well reproducible. The differences in number of hydrated and non-germinated pollen grains between the first and the second hour, were non-significant (a=0.05) for all
fungicides.
case of Tilt 250 (concentrations A, H, G, F and E) and Alto (concentration A) the percentage of pollen germination decreased during the second hour of observation. The reason for this discrepancy could be found in the simultaneous in-creasing amount of pollen grains with giant club shaped or twisted tubes, or bursted tubes, after 1 h of treatment. Such pollen grains were scored as non-germinated, as they exhibited abnormal development, probably due to longer treatment of pesticide doses.
Fig. 2 presents fungicide effects on selected enzymes. Succinate dehydrogenase was inhibited considerably only by Alto. Peroxisomal catalases were inhibited considerably by Tilt 250 EC (Tilt 250) and Tilt CB FW (Tilt), again only Impact 125 EC (Impact) let increase the activity. Alkaline phosphatase was inhibited by Impact, Tilt and Tilt 250 in comparison to controls. Non-specific es-terases were inhibited by all fungicides.
Regressions describing the effect of fungicides on pollen germination are summarized in Table 4. The regression could be described by a polynome of the third and fourth order. The third order function is not suitable to be used for extreme values, which is neary=1 andy=0. The curve did not intersect the x axis for y=0 or resulted for y=0 in two different values ofx. Therefore we used polynomes of the fourth order, from which it was possible to calculated by statgraphics from valueyto valuex
(Table 5).
4. Discussion
The main aim of the work presented here was to create an in vitro system for estimation of toxicity
of agrochemicals. The first step was to transform actual concentrations used in field treatments to experimental doses in the germination medium. Here we found probably a promising compromise in our concentration range, where the lowest con-centrations corresponded to the situation where pollen grains were not directly attached to fungicide dose, thus no-effect level (NOEL), but they are influenced by the penetration of molecules into the tissues and acropetal transport through the vascu-lar system to the upper part of the plant. The highest concentration represented the case when the full doses were applied into the open flower. As fungicides may be often used several times during a growing period, we should take account of residual concentrations and metabolic changes in-duced by the applied chemicals in plant tissues. In spite of that our lowest concentrations were under the detection limit of analytical methods, but we found in all cases after 1 h of treatment a decrease of pollen germinability. There was also an apparent delay in pollen germination, but the number of hydrated grains did not significantly differ from the control. This means that the uptake of water was not influenced by the fungicides, but further neces-sary steps, such as the active synthesis of pollen wall compounds or RNA synthesis, were probably re-tarded. Moreover, the 2 h of treatment of the highest concentrations of carbamide and propi-conazole led to pollen wall deformation and burst-ing of tubes. This effect was probably a result of the inhibition of ergosterol synthesis and mem-brane disturbing caused by fungicides.
The activities of investigated enzymes (SDH, PER, ADP, EST) demonstrated clearly, that the influence of fungicides was not enzyme specific and
Table 3
Some factors involved in the statistical characteristics of the methods
Range
Factorsa Average Variance Std. deviation Std. error Coeffic. of variation (%)
26.2
Fig. 1. Influence of fungicides on probability of pollen germination.
we cannot explain the decrease of pollen germina-tion by enzyme inhibigermina-tion alone. The number of germinated pollen grains was in every case lower
slight stimulation of activity. This has formerly been reported by Lebedev et al. (1989). In all four enzymes we cannot detect any correlation between pollen germination and enzyme activity after fun-gicide treatment. It seems that the in vitro test of germination was more suitable for the detection of fungicide toxicity at a cellular level. We have to analyze both effects, e.g. germinability and pollen
wall permeability. As we choose B. campestris
pollen as a model, we have a good chance to retain the gametic segregation of high het-erozygotic and allogamous genotypes in our re-sults. Pollen grains represent a population of segregating gametes and haploid male gameto-phytes bearing dominant or recessive alleles, which express different sensitivity to the
environ-Fig. 2. Influence of fungicides on the activity of individual enzymes in relationship to germination of pollen grains (2 h control=100%) in 2ml of the stock solution (B variant).
Table 4
Regressions describing the effect of fungicides on pollen germination (time of germination — 2 h)
R32 R22
Fungicides f(x) R42 R12 R02
0.7016
Alto 0.0007707x4−0.02009x3+0.1804x2−0.7261x+1.553 0.6283 0.7969 0.9073 0.9888 0.9940 0.9729
0.9507 0.8942
Impact 0.0001087x4−0.005102x3+0.05562x2−0.2999x+1.238 0.8351 0.7378
0.0008318x4−0.02116x3+0.1807x2−0.6844x+1.511 0.7996 0.8696 0.9310 0.9823 Punch
−0.004542x3+0.06391x2−0.3522x+1.236 0.8490
Tilt 250 0.8876 0.8966 0.9419
0.7680
0.6997 0.9949
0.0008784x4−0.02241x3+0.1945x2−0.7528x+1.587
Tilt 0.8507 0.9345
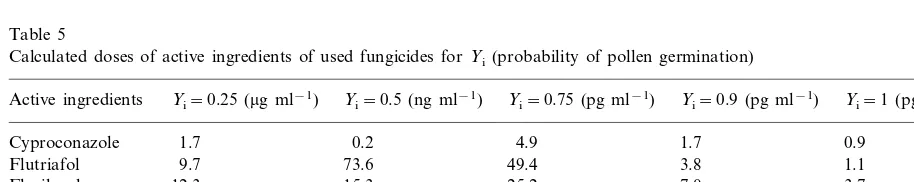
Table 5
Calculated doses of active ingredients of used fungicides forYi(probability of pollen germination)
Yi=0.75 (pg ml−1) Yi=0.9 (pg ml−1) Yi=1 (pg ml−1) Active ingredients Yi=0.25 (mg ml−1) Yi=0.5 (ng ml−1)
1.7 0.2 4.9 1.7 0.9
Cyproconazole
1.1 3.8
Flutriafol 9.7 73.6 49.4
25.2 7.0 3.7
15.3 12.3
Flusilazole
25.3
30.9 3.9
11.0
Propiconazolea 1.5
2.6
3.5 0.9 14.4 4.7
Carbendazimb
1.7 0.5 2.3 1.3
Propiconazoleb 7.2
mental stress factor. As a part of pollen grains is capable to germinate in given stress condition we can assume that segregation of tolerant genotypes exists in crop populations. We recommended to test all produced agrochemicals for pollen toxicity and to set limits by the use of these data.
It is interesting to make a comparison of detec-tion limits for a single active ingredient of fungi-cides with the sensitivity of the methods (Table 5). Fungicides can be separated on direct phase by HPTLC (high pressure thin layer chromatogra-phy) with UV detection at wavelengths from 190 to 261 nm. The minimal amount, which effected pollen germination, was below 2 pg for cypro-conazole. The detection limit was 10 ng for flutriafol, 25 ng flusilazole, 80 ng propiconazole and 20 ng carbendazim (Butz and Stan, 1995). However minimal concentrations, which affect pollen germination were calculated to be below the detection limit, for cyproconazole below 2 pg ml−1, for flutriafol below 4 pg ml−1, for flusila-zole below 7 pg ml−1, for propiconazole below 4 pg ml−1 and for carbendazim below 5 pg ml−1. The higher sensitivity of pollen to toxic com-pounds is given by its biological properties and function. Pollen grains of higher plants were uni-cellular haploid, male gametophytes, having one specific function to fertilize gametophyte. The content of their metabolites and energy metabolism is adapted to rapid growth after con-tact with the stigma. Transport of metabolites between tissues of style and tube is possible by the thin cytoplasmic membrane of pollen tube. There-fore tested fungicides or other toxic compounds can penetrate the pollen tube during in vitro cultivation more easily than for example the leaves. During transport across membranes there is a minimal probability that the applied com-pound is metabolized before its effect on the cytoplasm. Accumulation of toxic compounds in male gametophyte cytoplasm in our experiment is limited, due to the unicellular structure of the pollen tube and due to a short period of cultiva-tion, comparing to field tests. The enzyme inhibi-tion by xenobiotics can influence strongly viability of pollen grains during cultivation in vivo. The agricultural triazole fungicides showed a much greater affinity for microsomal cytochrome P 450
isoenzymes of fungi than for cytochrome P 450 in microsomal fraction prepared from higher plants. It is known, that interaction of triazole derivatives with cytochrome P 450 isoenzymes affects biosyn-thesis of steroids in plant cells (Vanden et al., 1987). Triazole antifungals inhibit ergosterol syn-thesis at nanomolar concentrations whereas al-most micromolar concentrations are needed to obtained a similar inhibition of cholesterol or phytosterol synthesis. This inhibition coincides with the accumulation of 14a-methylfecosterol, lanosterol, etc. It is speculated, that by making the 14a-methylsterols less lipophilic the cells are trying to eliminate these membrane-disturbing compounds.
plants grown under field conditions. Our results confirm this finding. The haploid number of chro-mosomes of pollen is a specific feature of this model. Heterogamous plants produce different types of pollen with regard to fungicides toxicity. It is possible to assume the existence of clearly different phenotypes (tolerance or sensitivity) ac-cording to the presence of dominant or recessive alleles in the genotype of pollen grains. It is possible to test with advantage the probability of the occurrence of resistant or hypersusceptible individuals in this test because gametic segrega-tion is analogous to segregasegrega-tion in the populasegrega-tion. Therefore pollen grains are very sensitive to toxic substances.
Acknowledgements
The authors thank Professor Dr P.H. Williams and Dr C.B. Hill (Department of Plant Pathol-ogy, University Wisconsin-Madis, Madison) for supplying seeds of turnip rape.
References
Bromilow, R.H., Rigitano, R.L.O., Briggs, G.G., Chamber-lain, K., 1987. Phloem translocation of non-ionised chemicals inRicinus communis. Pestic. Sci. 19, 85 – 99. Butz, S., Stan, H.J., 1995. Screening of 265 pesticides in
water by thin-layer chromatography with automated multiple development. Anal. Chem. 67, 620 – 630. Chamberlain, K., Burrell, M.M., Butcher, D.N., White, J.C.,
1984. Phloem transport of xenobiotics inRicinus commu
-nisvar.gibsonii. Pestic. Sci. 15, 1 – 8.
Davis, B.J., Ornstein, L., 1959. High resolution enzyme lo-calization with a new diazo reagent, ‘hexazonium pararosaniline’. J. Histochem. Cytochem. 7, 297 – 298. Fell, R.D., Rajotte, E.G., Yoder, K.S., 1983. Effects of
fun-gicides sprays during apple bloom on pollen viability and honey bee foraging. Environ. Entomol. 12, 1572 – 1575. Fuller, M.S., Roberson, R.W., Gisi, U., 1990. Effects of the
sterol demethylase inhibitor cyproconazole on hyphal tip cells of Sclerotinium rolfsii. III. Cell wall cytochemistry. Pestic. Biochem. Physiol. 36, 115 – 126.
He, Y., Wetzstein, H.Y., 1994. Pollen degeneration and re-tarded leaf development from fungicidal sprays applied during microspore development and shoot expansion. J. Hortic. Sci. 69, 975 – 983.
He, Y., Wetzstein, H.Y., Palevitz, B.A., 1995. The effects of a triazole fungicide, propiconazole, on pollen germina-tion, tube growth and cytoskeletal distribution inTrades
-cantia6irginiana. Sex. Plant Reprod. 8, 210 – 216. Jacob, F., Neumann, S., 1987. Chapter 1. Principles of
up-take and systemic transport of fungicides within the plant. In: Lyr, H., Fisher, G. (Eds.), Modern Selective Fungicides — Properties, Applications, Mechanisms of Action. Press Verlag, Jena, pp. 13 – 29.
Jandurova´, O.M., Pavlı´k, M., 1995. In vitro pollen germina-tion of species and two interspecific hybrids from the genusBrassica. Sex. Plant Reprod. 8, 33 – 36.
Kappler, R., Kristen, U., 1987. Photometric quantification of in vitro pollen tube growth: of various environmental substances. Environ. Exp. Bot. 27, 305 – 309.
Lebedev, V.B., Suslova, T.A., Khorosheva, T.M., Gryazeva, O.A., 1989. Brown rust and fungicides effect on the ac-tivity and isoenzymes of foliar peroxidase of wheat. (in Russian) Dokl. Vses. Akad. S-kh. Nauk im. V. I. Lenina, 11 – 13.
Lojda, Z., Gossrau, R., Schiebler, T.H., 1979. Enzyme His-tochemistry. A Laboratory Manual. Springer Verlag, Berlin, p. 339.
Marcucci, M.C., Filiti, N., 1984. Germination of pear and apple pollen as influenced by fungicides. Gartenbauwiss ecschaft. 49, 28 – 32.
Petzold, U., Wichmann, R., Dobe, H., 1988. A rapid bioassay for detection of the phytotoxicity of active in-gredients in pesticides, using the leaf disk buoyany of
Sinapis alba L. cotyledons. Arch. Phytopathol. Pflanzen-schutz 24, 445 – 447.
Redalen, G., 1980. Effect of fungicides on pollen germina-tion and fruit set in raspberries. Gartenbauwiss enschsaft. 45, 248 – 251.
Roberts, I.N., Gaude, T.C., Harrod, G., Dickinson, H.G., 1983. Pollen-stigma interaction inBrassica oleracea; new pollen germination medium and its use in elucidating the mechanism of self incompatibility. Theor. Appl. Genet. 65, 231 – 238.
Roels, F., 1976. Cytochemical demonstration of extraperox-isomal. I. Sheep liver. J. Histochem. Cytochem. 24, 713 – 724.
Stanley, R.G., Linskens, H.F., 1966. Pollen. Biologie, Bio-chemie, Gewinnung und Verwendung. Urs Freund Ver-lag, Greifenberg, p. 308.
Strube, K., Janke, D., Kappler, R., Kristen, U., 1991. Toxi-city of some herbicides to in vitro growing tobacco pol-len tubes (the polpol-len test). Environ. Exp. Bot. 31, 217 – 222.
Tomlin, C., 1994. The Pesticide Manual. Incorporating the Agrochemicals Handbook. The British Crop Protection Council, Surrey and The Royal Society of Chemistry, Cambridge, p. 1341.
1987. Interaction of azole derivatives with cytochrome P-450 isozymes in yeast, fungi, plants and mammalian cells. Pestic. Sci. 21, 289 – 306.
Wiegand-Rosinus, M., Obst, U., Haberer, K., Wild, A., 1988.
Pesticides are inhibitors of aldehyde dehydrogenase in vitro. Vom Wasser 71, 225 – 229.
Williams, P.H., Hill, C.B., 1986. Rapid-cycling populations of
Brassica. Science 232, 1385 – 1389.