www.elsevier.com / locate / bres
Research report
Spatial distribution of semicircular canal nerve evoked monosynaptic
response components in frog vestibular nuclei
*
Hans Straka , Stefan Biesdorf, Norbert Dieringer
Physiologisches Institut, Pettenkoferstrasse 12, 80336 Munich, Germany Accepted 25 July 2000
Abstract
Most second-order vestibular neurons receive a canal-specific monosynaptic excitation, although the central projections of semicircular canal afferents overlap extensively. This remarkable canal specificity prompted us to study the spatial organization of evoked field potentials following selective stimulation of individual canal nerves. Electrically evoked responses in the vestibular nuclei were mapped systematically in vitro. Constructed activation maps were superimposed on a cytoarchitectonically defined anatomical map. The spatial activation maps for pre- and postsynaptic response components evoked by stimulation of a given canal nerve were similar. Activation maps for monosynaptic inputs from different canals tended to show a differential distribution of their peak amplitudes, although the overlap was considerable. Anterior vertical canal signals peaked in the superior vestibular nucleus, posterior vertical canal signals peaked in the descending and in the dorsal part of the lateral vestibular nucleus, whereas horizontal canal signals peaked in the descending and in the ventral part of the lateral vestibular nucleus. A similar, differential but overlapping, spatial organization of the canal inputs was described also for other vertebrates, suggesting a crude but rather conservative topographical organization of semicircular canal nerve projections within the vestibular nuclei. Differences in the precision of topological representations between vestibular and other sensory modalities are discussed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Vestibular system
Keywords: Field potential; Topographical mapping; Vestibular nerve afferent fiber; Organotopic organization
1. Introduction neurons that imply a convergence of multiple canal [3,4,6,10,14] or canal-otolith inputs [1,5]. At variance with The projection areas of afferent fibers from individual these reports, however, intracellular studies employing labyrinthine organs in the ipsilateral vestibular nuclei selective electrical stimulation of individual semicircular overlap to a large extent in non-mammalian [8,17,24– canal nerves demonstrated that monosynaptic responses of 26,30,37] as well as in mammalian species [11,31,32]. 28VN are typically evoked from only one of the ipsilateral Each of the vestibular nuclei receives inputs from most semicircular canal nerves (cat [19,29], pigeon [42], frog labyrinthine sense organs, but the density of the innerva- [34]). Therefore, 28VN select among the available afferent tion from different vestibular sense organs varies between canal inputs and the convergence of multiple canal signals different regions. A considerable convergence of inputs onto 28VN might be mediated oligosynaptically.
from different sense organs onto second-order vestibular The absence of a clear topographic order in the ana-neurons (28VN) might be expected from these overlapping tomical projection zones of afferent fibers from different projection areas. In fact, a number of single unit studies semicircular canal nerves, however, does not imply the employing natural stimuli have reported best response absence of such an order at the synaptic or at the somatic orientation vectors of second- or higher-order vestibular level. The density of synaptic contacts between afferent fibers and 28VN for instance could exhibit regional differ-ences such that zones with high synaptic densities from
*Corresponding author. Fax:149-89-5996-216.
E-mail address: [email protected] (H. Straka). one semicircular canal nerve alternate with similar zones
from other semicircular canal nerves. Since the dendrites the sylgard floor. The temperature of the bath was elec-of 28VN are long and select their appropriate semicircular tronically controlled and maintained at 1460.28C. canal input it is also conceivable that the cell bodies of 28 For electrical stimulation of individual semicircular canal nerves with an input from a particular semicircular canal nerve branches we used single constant current canal are clustered and form modular zones that represent pulses (0.2 ms; 8–12mA) across suction electrodes (inner different vestibular endorgans in a manner similar to diameter 120–150 mm; see [34]). For stimulation of the retinotopic, somatotopic or tonotopic maps of other sen- VIIIth nerve we used single constant current pulses (0.2 sory systems. ms; 5–30 mA) across a concentric bipolar electrode (tip We analyzed these possibilities first with evoked field diameter 25 mm) that was located about 2 mm more potentials recorded in vitro in the isolated frog brain. proximal than the electrodes stimulating the individual Separate electrical stimulation of individual semicircular semicircular canal nerves [34]. Constant current pulses canal nerves activated field potentials that consisted of two were produced by a stimulus isolation unit (WPI A 360) at or more negative components. The spatial distribution of a repetition rate of 0.33 Hz. For extracellular recordings vestibular nerve evoked N0 and N1 field potentials, glass microelectrodes were fabricated with a horizontal representing regional presynaptic (N ) and postsynaptic0 puller (P-87 Brown / Flaming), beveled (308, 20 mm tip (N ) response components [28], was mapped systematical-1 diameter) and filled with 2 M sodium chloride (1–3 MV). ly. We compared these results with the location of 28VN, Electrodes for intracellular recordings were filled with 2 M identified by their monosynaptic responses following elec- potassium acetate (90–120 MV) or 2 M potassium chlo-trical stimulation of the horizontal, anterior vertical or ride (80–100 MV). Vertical displacements of the recording posterior vertical semicircular canal nerve branch. The electrodes were controlled with a nanostepper. Horizontal physiological response properties of these neurons had displacements were performed with a two axes micro-been described elsewhere [34]. manipulator.
Preliminary parts of this study had been published in In order to standardize our results field potentials were abstract form [35]. recorded at the beginning of each experiment at the same standard recording site (0.4 mm caudal to the caudal end of the entry of the VIIIth nerve at a depth of 0.4 mm below
2. Material and methods the top of the brainstem). The stimulus threshold for the evoked N component of the field potential was similar for1 In vitro experiments were performed on the isolated each of the three semicircular canal nerves and ranged brains of grass frogs (Rana temporaria) and comply with usually between 2 and 3mA. Stimulus intensities are given the Principles of Animal Care of the National Institutes of as multiples of these threshold values (3T) and were Health, publication no. 86-23, revised 1985. Permission for restricted to intensities of maximally 43T.
these experiments was granted by Regierung von Ober- In order to cast our electrophysiological results into a bayern (211-2531-31 / 95). Grass frogs were deeply anes- normalized geometry of the vestibular nuclei we used the thetized (0.1% 3-aminobenzoic acid ethyl ester; MS-222) same reference frame for a three-dimensional coordinate and perfused transcardially with iced Ringer solution (75 system as in a parallel anatomical study [24]. Distances in mM NaCl; 25 mM NaHCO ; 2 mM CaCl ; 2 mM KCl;3 2 the rostro-caudal direction refer to the caudal end of the 0.5 mM MgCl ; 11 mM glucose; pH 7.4 [33]). The skull2 entry of the VIIIth nerve, in dorso-ventral direction to the and the bony labyrinth were opened by a ventral approach top of the brainstem and in medio-lateral direction to the and the three semicircular canals on either side were medial surface of the brainstem at the particular rostro-sectioned. Then, the brain was removed with the labyrin- caudal level (Fig. 1B–D). For the latter measurement the thine endorgans attached to the VIIIth nerve. The isolated midline of the brainstem was not practical because of the brain was submerged in iced Ringer and the dura, the variability in the shape of the alar plate after the choroid labyrinthine endorgans and the choroid plexus above the plexus had been removed. Measurements rostral to the IVth ventricle were removed. The forebrain was discon- caudal end of the entry of the VIIIth nerve and medial to nected and in part of the experiments also the cerebellum the top of the brainstem were denoted with a negative sign was removed. Brains were stored overnight at 68C in (see Fig. 1B,D). The mean distance between the caudal end continuously oxygenated Ringer solution with a pH of of the cerebellum and the obex was 3.7460.07 mm (n5
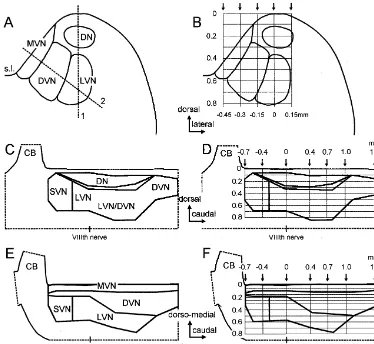
Fig. 1. Schematic presentation of the vestibular nuclei in the brainstem and how the spatial distribution of semicircular canal nerve evoked field potentials was explored. (A,C,E) Frontal (A), parasagittal (C) and oblique medio-lateral views of the vestibular nuclei as reconstructed from data published by Matesz [23] and Kuruvilla et al. [22]. Dashed lines in A indicate the orientation of the parasagittal (1) and oblique medio-lateral (2) planes shown in C and E, respectively. (B,D,F) Electrode tracks (from surface to 0.8 mm in depth) through the vestibular nuclei. Arrows indicate the laterality (B) or the rostro-caudal position (D,F) of electrode tracks. Zero laterality (B) refers to the top of the brainstem, zero rostro-caudal position (D,F) refers to the caudal end of the entry of the VIIIth nerve in the brainstem. Same calibrations for A and B and for C–F, respectively. CB, cerebellum; DN, dorsal nucleus; DVN, descending vestibular nucleus; LVN, lateral vestibular nucleus; MVN, medial vestibular nucleus; s.l., sulcus limitans; SVN, superior vestibular nucleus.
in a frontal (Fig. 1A,B), parasagittal (Fig. 1C) and in an In the latter case electrodes were advanced perpendicularly oblique medio-lateral plane (Fig. 1E) was used to specify to the surface of the medial wall of the brainstem (dashed the search area for this mapping study. line 2 in Fig. 1A). The touch point was about 20.4 mm The spatial distribution of the evoked field potentials medial to the top of the dorsal brainstem. Typically, was mapped systematically with recording tracks from the records were taken every 0.1 mm in depth. However, in the surface of the brainstem to a depth of 0.8 mm in three oblique medio-lateral plane we recorded every 0.05 mm up different planes. Electrode tracks in the frontal plane were to a depth of 0.3 mm because of the relatively thin strip positioned rostral (20.4 mm) or caudal (0.4 mm or 0.7 that represents the medial vestibular nucleus (Fig. 1A,E). mm) to the caudal end of the entry of the VIIIth nerve and Deeper measurement points were separated by 0.1 mm up consisted in each case of five depth tracks separated by to a depth of 0.8 mm (see grid in Fig. 1F).
vestibular nuclear complex after stimulation of the VIIIth nerve or of one of its canal nerve branches. To minimize the variability of results we averaged the amplitudes recorded in a given depth track separately for each stimulated canal nerve, compared these averaged values between different parasagittal depth tracks and normalized the averaged values according to the largest values evoked by a given canal nerve branch in a particular track (see Fig. 3). This procedure facilitated a comparison of the results following stimulation of different semicircular canal nerves or of the VIIIth nerve and a comparison of data from different experiments. From the analysis of data obtained in parasagittal depth tracks we obtained evidence for a differential spatial distribution of the largest evoked field potential amplitudes following stimulation of different semicircular canal nerves (Fig. 3). In later experiments we estimated the maximal response amplitude of N or N in0 1 each series of depth tracks for a given nerve branch and normalized all other responses evoked by the same nerve branch. In order to include depth information in our analysis (field potentials are continuous functions and recording tracks were relatively close to each other in space) isopotential surface plots were calculated through linear interpolation between maximal response amplitudes (Stanford graphics, 3-D visions, Torrance, CA). Four groups of relative response amplitudes (up to 25%, 50%, 75% or 100%) were represented by different intensities of gray tones.
Statistical analyses were performed with the aid of
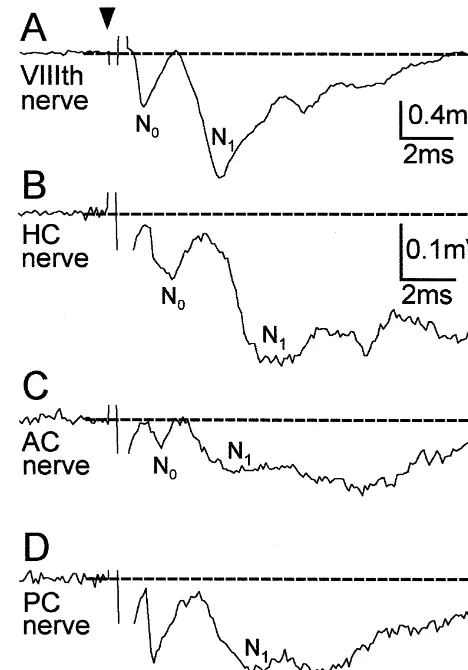
Fig. 2. Field potentials in the vestibular nuclear complex evoked by
commercially available computer software (INSTAT;
stimulation of the VIIIth nerve or its semicircular canal nerve branches on
Graphpad, San Diego, CA). Statistical differences in
the ipsilateral side. (A–D) Pre- (N ) and postsynaptic (N ) field potential0 1 latencies, amplitudes and areas were calculated according
components evoked by stimulation of the VIIIth nerve (A), the horizontal
to the Wilcoxon signed rank test (test for paired parame- canal (HC) nerve (B), the anterior canal (AC) nerve (C) or the posterior ters). Graphical presentations were performed with the aid canal (PC) nerve branch (D). Each of the responses in A–D were recorded at the same site. Dashed lines indicate baseline and arrow head
of commercially available computer software (Origin,
the onset of stimulus. Calibration bars in B apply also for C and D. Each
Microcal Software, Northampton, MA; Designer,
Microg-record represents an average of 20 responses.
rafx, Richardson, TX).
differed between nerve branches and recording sites.
3. Results Horizontal canal (HC) nerve evoked field potentials were characterized by a negativity (Fig. 2B) that lasted longer 3.1. Field potentials following stimulation of the VIIIth than that of the anterior canal (AC), posterior canal (PC) nerve or of individual semicircular canal nerve or VIIIth nerve evoked field potentials (Fig. 2A,C,D). branches. Here, areas instead of peak amplitudes of evoked N field1 potentials were compared (Table 2). This comparison Canal nerve evoked presynaptic N and postsynaptic N0 1 indicated that HC nerve evoked postsynaptic field po-field potential components recorded at the standard record- tentials were characterized by a significantly (P#0.0001) ing site had significantly longer onset latencies and smaller larger relative area than AC, PC or VIIIth nerve evoked amplitudes than the corresponding VIIIth nerve evoked field potentials recorded at the same site (Table 2). potentials (Fig. 2; Tables 1 and 2). This difference in onset
latency was expected, since the site of canal nerve 3.2. Topography of vestibular field potential components stimulation was further away from the recording site than
Table 1
Parameters of presynaptic N field potential components evoked by electrical stimulation of the VIIIth nerve or of an individual semicircular canal nerve on0 a
the ipsilateral side at the standard recording site
VIIIth nerve Horizontal semicircular canal Anterior semicircular canal Posterior semicircular canal
Latency 0.960.2 ms 1.760.6 ms* 1.860.4 ms* 1.760.4 ms*
Amplitude at 43T 5026202mV 161684mV* 109672mV* 147662mV*
a
n529 for all instances. *P#0.0001, significance of difference with respect to values evoked by VIIIth nerve stimulation (Wilcoxon signed-rank test).
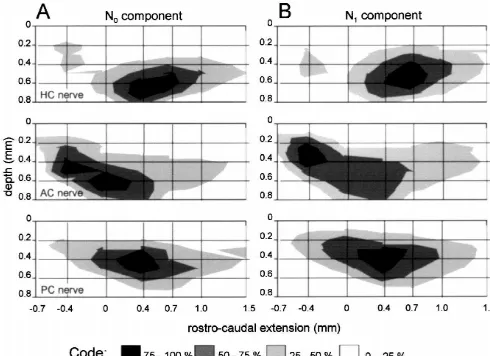
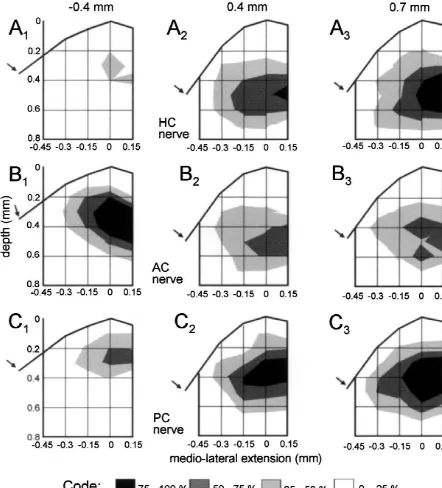
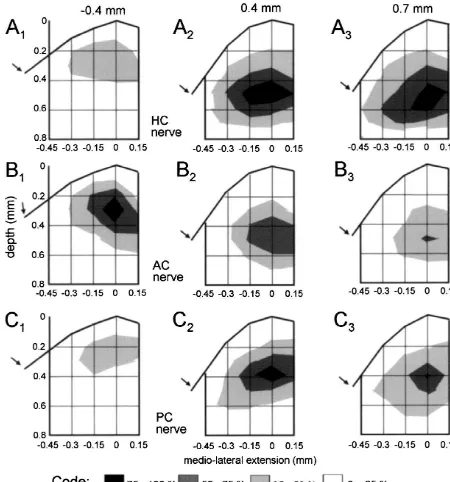
the most caudal site was at 1.5 mm with respect to the maxima evoked by stimulation of the AC nerve were caudal end of the entry of the VIIIth nerve (see Fig. 1D). located rostral with respect to the entry of the VIIIth nerve The stimulus intensity was 4-times threshold (43T) of the (0 mm; Fig. 5A,B). In the medio-lateral direction the N component recorded at the standard recording site.1 maximal amplitudes of the N and N components were0 1 The spatial distribution of the normalized average observed in the intermediate and lateral part of the amplitudes of N and of N components evoked at a given0 1 vestibular nuclear complex. Only very small field potential stimulation site was not uniform along the rostro-caudal amplitudes were recorded in the medial vestibular nucleus. search area but exhibited peaks that differed in their In frontal plane recordings the rostro-caudal location and location (Fig. 3). Stimulation of each of the different nerve the depth of the local maxima following stimulation of branches evoked potentials that peaked at a different but each of the three different canal nerves were again similar for this nerve branch characteristic site in the vestibular for N and for N components (Figs. 6 and 7). The spatial0 1 nuclear complex (Fig. 3A–D for the N and Fig. 3E–H for0 distribution of these potentials was consistent with those the N1 components). N0 and N1 response components observed in the parasagittal and in the oblique medio-evoked by a given nerve branch had rather similar spatial lateral planes (Figs. 4 and 5). The depth of the local distributions along the rostro-caudal extent of the vestibu- maxima of the N0 as of the N1 components ranged lar nuclear complex. between 0.2 and 0.6 mm. Differences in depth of the local To include depth information, the data shown in Fig. 3 maxima were observed between the rostral plane i.e. at are presented in Fig. 4 as isopotential surface plots. The 20.4 mm (AC nerve, Figs. 6B1 and 7B ) and the two1 largest relative amplitudes (75–100%) of the N and N0 1 caudal planes i.e. at 0.4 and 0.7 mm (HC nerve, Figs. field potential components following stimulation of an 6A ,A and 7A ,A ; PC nerve, Figs. 6C ,C and 7C ,C ).2 3 2 3 2 3 2 3 individual semicircular canal nerve were recorded at a Differences in the laterality of the local maxima between depth between 0.4 and 0.6 mm from the top of the N and N components were observed in the frontal planes0 1 brainstem at a laterality of 0 mm. This location corre- following stimulation of all three semicircular canal sponds to the anatomical center of the vestibular nuclear nerves. Independent of the stimulated semicircular canal complex [22–24]. Differences in the spatial distribution of nerve the maximal amplitudes of the N components were0 the peak amplitudes of N and N field potentials evoked0 1 located more laterally (Fig. 6) than the corresponding N1 by different canal nerves were now more apparent. components (Fig. 7).
Semicircular canal nerve evoked N0 and N1 field
potential components recorded in an oblique medio-lateral 3.3. Location of the maxima of semicircular canal nerve plane (see dashed line 2 in Fig. 1A and F) and evoked by evoked N components in the vestibular nuclei1
stimulation of a given nerve branch had rather similar
topographies, but exhibited different spatial distributions The maximum of the HC nerve evoked N component1 for different canal nerves. The rostro-caudal extent of the representing the monosynaptic excitation of 28HC neurons largest relative amplitudes (75–100%) of the N and N0 1 was located in the lateral vestibular nucleus and more field potential components (Fig. 5A,B) were rather similar caudally in the descending vestibular nucleus (Figs. 8A2,3 to those observed in the parasagittal plane (Fig. 4A,B). and 9A,D). The maximum of the AC nerve evoked N1 The maxima of the field potentials evoked by the HC nerve component was observed in the central part of the superior or by the PC nerve were located caudal whereas the vestibular nucleus (Figs. 8B and 9B,E). After PC nerve1
Table 2
Parameters of N field potentials evoked by electrical stimulation of the VIIIth nerve or of an individual semicircular canal nerve on the ipsilateral side at1 a
the standard recording site
VIIIth nerve Horizontal semicircular canal Anterior semicircular canal Posterior semicircular canal
Latency 2.660.4 ms 3.660.7 ms* 3.760.6 ms* 3.560.6 ms*
Amplitude at 43T 10316379mV 2696153mV* 2086105mV* 3006247mV* Normalized area 13.164.4mV3ms 22.068.1mV3ms* 11.864.3mV3ms* 11.464.2mV3ms*
a
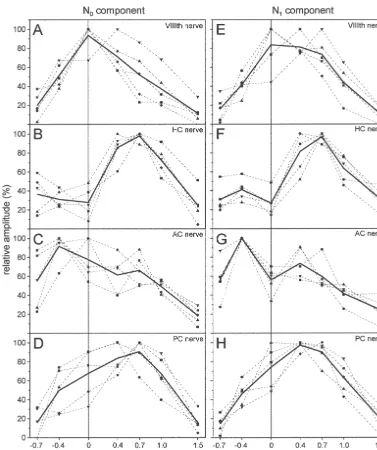
Fig. 3. Normalized average amplitudes of the VIIIth nerve or semicircular canal nerve evoked N0 and N1 field potential components recorded in rostro-caudal depth tracks along the vestibular nuclear complex at a laterality of 0 mm. Symbols connected by dashed lines represent results from four different experiments, bold lines connect mean values of these data. HC, AC and PC stand for horizontal, anterior and posterior semicircular canal, respectively. Zero in rostro-caudal extension is the caudal end of the entry of the VIIIth nerve in the brainstem.
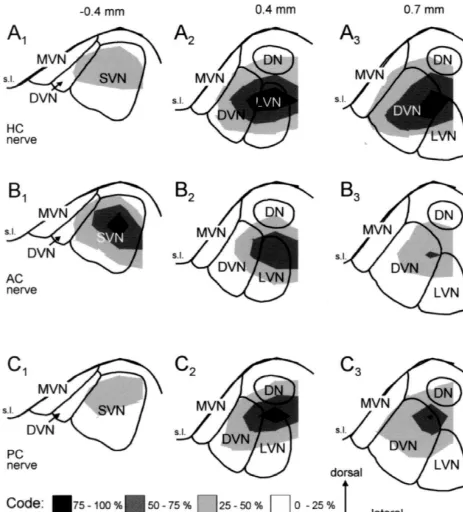
stimulation the largest amplitudes were observed in the the descending vestibular nucleus (Figs. 8 and 9). Interest-dorsal part of the lateral vestibular nucleus and to a ingly, the N component was absent or was less than 25%1 somewhat lesser degree more caudally in the descending of the maximal amplitude (Figs. 8 and 9) in the medial vestibular nucleus (Figs. 8C2,3 and 9C,F). Although, there vestibular nucleus throughout its rostro-caudal extent. were distinct areas with maximal amplitudes of the N1
components following stimulation of each one of the three 3.4. Correlation between the location of identified semicircular canal nerves areas of overlap were observed second-order canal neurons and the topography of canal as well. However, these areas of overlap consisted pre- nerve evoked N field potentials1
dominantly of areas in which the amplitudes of the N1
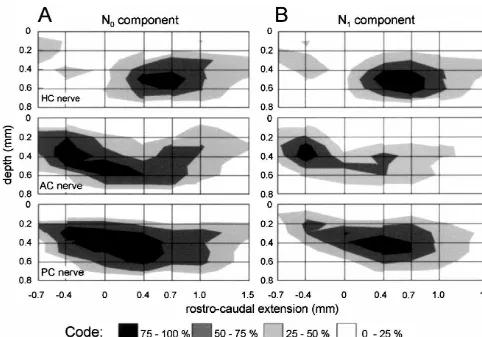
Fig. 4. Amplitude distribution plot of semicircular canal nerve evoked field potential components recorded in a parasagittal plane through the vestibular nuclear complex at a laterality of 0 mm. (A,B) Normalized amplitudes of semicircular canal nerve evoked N (A) and N (B) field potential components0 1
from four experiments. HC, AC and PC stand for horizontal, anterior and posterior semicircular canal, respectively. Zero in rostro-caudal extension refers to the caudal end of the entry of the VIIIth nerve. Relative amplitude magnitudes are represented by gray tones.
to the caudal end of the VIIIth nerve in tracks that were Rather, the amplitudes of these potentials were prominent separated by 0.1 mm. These neurons were identified by in distinct subregions of the vestibular nuclear complex. their monosynaptic excitation from one of the three The spatial differences between the maxima of pre- and ipsilateral semicircular canal nerves. Second-order canal postsynaptic components of field potentials evoked by neurons were searched systematically in depth tracks that stimulation of a particular semicircular canal nerve were were regularly spaced over the entire rostro-caudal exten- only minor when compared with the spatial differences sion of the vestibular nuclear complex. The rostro-caudal between the maxima of field potentials evoked by different and dorso-lateral location of these second-order semicircu- semicircular canal nerves. However, the overlap of these lar canal neurons is shown in Fig. 10A–C. Regional potentials representing the afferent inputs from different differences in the distribution of these second-order canal semicircular canals in the ipsilateral labyrinth was exten-neurons are expressed by the histograms in Fig. 10D–F sive. Large field potentials following stimulation of each of (gray tone). Comparison of these distributions with those the three semicircular canal nerves were encountered in all of the canal nerve evoked N1 field potential amplitudes vestibular nuclei with the notable exception of the medial (dashed lines in Fig. 10D–F) revealed compatible local vestibular nucleus.
maxima. Anatomical tracer studies with selective labeling of individual vestibular nerve branches demonstrated in vari-ous species that the central projections of afferent fibers
Fig. 5. Amplitude distribution plot of semicircular canal nerve evoked field potential components recorded in an oblique medio-lateral plane through the vestibular nuclear complex. (A,B) Normalized amplitudes of semicircular canal nerve evoked N (A) and N (B) field potential components from four0 1
experiments. HC, AC and PC stand for horizontal, anterior and posterior semicircular canal, respectively. Zero in rostro-caudal extension refers to the caudal end of the entry of the VIIIth nerve.
achieve-Fig. 6. Amplitude distribution plot of semicircular canal nerve evoked field potential components recorded in a frontal plane through the vestibular nuclear complex at three different rostro-caudal locations. (A–C) Normalized amplitudes of semicircular canal nerve evoked N0 field potential components following stimulation of the horizontal canal (HC), anterior canal (AC) or posterior semicircular canal (PC) nerve in four animals. The rostral (20.4 mm) and the caudal (0.4 or 0.7 mm) locations of the frontal planes with respect to the caudal end of the entry of the VIIIth nerve are indicated on top. Arrows point towards the sulcus limitans in the medial wall of the brainstem.
com-Fig. 7. Amplitude distribution plot of semicircular canal nerve evoked field potential components recorded in a frontal plane through the vestibular nuclear complex at three different rostro-caudal locations. (A–C) Normalized amplitudes of semicircular canal nerve evoked N1 field potential components following stimulation of the horizontal canal (HC), anterior canal (AC) or posterior semicircular canal (PC) nerve in four animals. The rostral (20.4 mm) and the caudal (0.4 or 0.7 mm) locations of the frontal planes with respect to the caudal end of the entry of the VIIIth nerve are indicated on top. Arrows point towards the sulcus limitans in the medial wall of the brainstem.
Fig. 8. Distribution of the semicircular canal nerve evoked N components with respect to the vestibular nuclei in frontal planes at three different1
rostro-caudal levels as indicated on top. The amplitude distribution plots shown in Fig. 7 for four animals were superimposed on frontal sections showing the outlines of the vestibular nuclei as in Fig. 1. (A1 – 3) Distribution of N1 amplitudes following horizontal canal (HC) nerve stimulation. (B1 – 3) Distribution of N amplitudes following anterior canal (AC) nerve stimulation. (C1 1 – 3) Distribution of N amplitudes following posterior canal (PC) nerve1
stimulation. The length of the calibration arrows on the bottom corresponds to 0.3 mm. DN, dorsal nucleus; DVN, descending vestibular nucleus; LVN, lateral vestibular nucleus; MVN, medial vestibular nucleus; s.l., sulcus limitans; SVN, superior vestibular nucleus.
the spatial response characteristics nor for a contrast less than for other sensory systems and the encountered enhancement of the dynamic response characteristics of crude representation could be a relict, more related to second-order vestibular neurons. In essence, the demands development than to function.
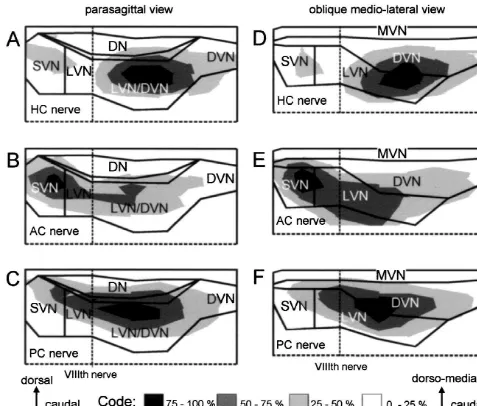
Fig. 9. Distribution of the semicircular canal nerve evoked N components with respect to the vestibular nuclei in parasagittal or oblique medio-lateral1
planes. The amplitude distribution plots of the N field potential components shown in Figs. 4 and 5 for four animals were superimposed on the outlines of1
the vestibular nuclei. HC, AC and PC stand for horizontal, anterior and posterior semicircular canal, respectively. The length of the calibration arrows on the bottom corresponds to 0.3 mm. CB, cerebellum; DN, dorsal nucleus; DVN, descending vestibular nucleus; LVN, lateral vestibular nucleus; MVN, medial vestibular nucleus; SVN, superior vestibular nucleus.
motoneurons. In the monkey vestibulo-ocular neurons survive to some extent in frogs into adulthood (Straka, initiating eye movements of distinct directions were more unpublished results). The group of vestibulo-spinal neu-or less separated in different parts of the vestibular nuclei rons, for instance, has about the same location with respect [39]. The location of these premotor neurons for upward to external landmarks in tadpoles [36] as in adult frogs and downward rotatory and horizontal eye movements was [7,9,27,40]. A more detailed motor representation for the consistent with the distribution of semicircular canal nerve innervation of, e.g., cervical, brachial or lumbar activated second-order vestibular neurons reported here for motoneurons or for the innervation of synergistic motor the frog or with results obtained in the pigeon [42]. It is pools is so far unknown.
interesting to note such a similarity in distribution over such a wide range of vertebrate species. During
develop-ment second-order vestibular neurons form pools confined Acknowledgements
to distinct rhombomeric segments and are more (zebrafish
Fig. 10. Location of identified second-order semicircular canal neurons and comparison with the distribution of N field potential amplitudes evoked by the1
same semicircular canal nerve branch. (A–C) Parasagittal view of the location of neurons that received a monosynaptic EPSP from the horizontal canal (HC) nerve (A), from the anterior canal (AC) nerve (B) or from the posterior semicircular canal (PC) nerve (C). Neurons were recorded between 0.5 mm rostral and 1.0 mm caudal to the entry of the VIIIth nerve in depth tracks 0.1 mm apart from each other. (D–F) comparison of the locations of recorded single neurons (histogram bars) with the distributions of N field potential amplitudes evoked by the same semicircular canal nerve (dashed lines). Data for1
N field potentials were taken from Fig. 3F–H.1
[4] J. Baker, J. Goldberg, G. Hermann, B. Peterson, Spatial and
Sonderforschungsbereich 462 (Sensomotorik) der
temporal response properties of secondary neurons that receive
Deutschen Forschungsgemeinschaft and by the
Friedrich-convergent input in vestibular nuclei of alert cats, Brain Res. 294
Baur-Stiftung 44 / 95. (1984) 138–143.
[5] G.A. Bush, A.A. Perachio, D.E. Angelaki, Encoding of head acceleration in vestibular neurons. I. Spatiotemporal response prop-erties to linear acceleration, J. Neurophysiol. 69 (1993) 2039–2055.
References [6] I.S. Curthoys, C.H. Markham, Convergence of labyrinthine
in-fluences on units in the vestibular nuclei of the cat. I. Natural [1] D.E. Angelaki, G.A. Bush, A.A. Perachio, Two-dimensional stimulation, Brain Res. 35 (1971) 469–490.
spatiotemporal coding of linear acceleration in vestibular neurons, J. [7] P. D’Asciano, N. Corvaja, Spinal projections from the rhombence-Neurosci. 13 (1993) 1403–1417. phalon in the toad, Arch. Ital. Biol. 119 (1981) 139–150.
´
[2] R.A. Baird, G. Desmadryl, C. Fernandez, J.M. Goldberg, The [8] J.D. Dickman, Q. Fang, Differential central projections of vestibular vestibular nerve of the chinchilla: II. Relation between afferent afferents in pigeons, J. Comp. Neurol. 367 (1996) 110–131. response properties and peripheral innervation patterns in the [9] V.V. Fanardjian, L.R. Manvelyan, V.L. Zakarian, V.I. Pogossian, A.M. semicircular canals, J. Neurophysiol. 60 (1988) 182–203. Nasoyan, Electrophysiological properties of the somatotopic organi-[3] J. Baker, J. Goldberg, G. Hermann, B. Peterson, Optimal response zation of the vestibulospinal system in the frog, Neuroscience 94
planes and canal convergence in secondary neurons in vestibular (1999) 845–857.
properties of second-order vestibulo-ocular relay neurons in the alert [28] W. Precht, A. Richter, S. Ozawa, H. Shimazu, Intracellular study of cat, Exp. Brain Res. 81 (1990) 462–478. frog’s vestibular neurons in relation to the labyrinth and spinal cord, [11] R.R. Gacek, The course and central termination of first order Exp. Brain Res. 19 (1974) 377–393.
ˆ
neurons supplying vestibular endorgans in the cat, Acta. [29] A. Sans, J. Raymond, R. Marty, Projections des cretes ampullaires et ´ Otolaryngol. Suppl. 254 (1969) 1–66. de l’utricle dans les noyaux vestibulaires primaires. Etude
mi-´
[12] R.M. Gaze, The representation of the retina on the optic lobe of the crophysiologique et correlations anatomo-fonctionelles, Brain Res. frog, Q. J. Exp. Physiol. 43 (1958) 209–214. 44 (1972) 337–355.
[13] J.C. Glover, G. Petursdottir, Regional specificity of developing [30] D.W.F. Schwarz, I.E. Schwarz, Projection of afferents from in-reticulospinal, vestibulospinal, and vestibulo-ocular projections in dividual vestibular sense organs to the vestibular nuclei in the the chicken embryo, J. Neurobiol. 22 (1991) 353–376. pigeon, Acta Otolaryngol. 102 (1986) 463–473.
[14] W. Graf, J. Baker, B.W. Peterson, Sensorimotor transformation in the [31] J. Siegborn, K. Yingcharoen, G. Grant, Brainstem projections of cats vestibuloocular reflex system. I. Neuronal signals coding spatial different branches of the vestibular nerve: An experimental study by coordination of compensatory eye movements, J. Neurophysiol. 70 transganglionic transport of horseradish peroxidase in the cat, Anat.
(1993) 2425–2441. Embryol. 184 (1991) 291–299.
[15] W. Heiligenberg, Electrosensory maps form a substrate for the [32] B.M. Stein, M.B. Carpenter, Central projections of portions of the distributed and parallel control of behavioral responses in weakly vestibular ganglia innervating specific parts of the labyrinth in the electric fish, Brain Behav. Evol. 31 (1988) 6–16. rhesus monkey, Am. J. Anat. 120 (1967) 281–317.
´
[16] S.M. Highstein, J.M. Goldberg, A.K. Moschovakis, C. Fernandez, [33] H. Straka, N. Dieringer, Electrophysiological and pharmacological Inputs from regularly and irregularly discharging vestibular nerve characterization of vestibular inputs to identified frog abducens afferents to secondary neurons in the vestibular nuclei of the motoneurons and internuclear neurons in vitro, Eur. J. Neurosci. 5 Squirrel monkey: II. Correlation with output pathways of secondary (1993) 251–260.
neurons, J. Neurophysiol. 58 (1987) 714–739. [34] H. Straka, S. Biesdorf, N. Dieringer, Canal-specific excitation and [17] S.M. Highstein, R. Kitch, J. Carey, R. Baker, Anatomical organiza- inhibition of frog second order vestibular neurons, J. Neurophysiol.
tion of the brainstem octavolateralis area of the oyster toadfish, 78 (1997) 1363–1372.
Opsanus tau, J. Comp. Neurol. 319 (1992) 501–518. [35] H. Straka, S. Biesdorf, A. Birinyi, N. Dieringer N, Spatial organiza-[18] J.H. Kaas, Topographic maps are fundamental to sensory process- tion of semicircular canal inputs in the frog, Soc. Neurosci. Abstr.
ing, Brain Res. Bull. 44 (1997) 107–112. 23 (1997) 508 / 2.
[19] M. Kashara, Y. Uchino, Bilateral semicircular canal inputs to [36] H. Straka, E. Gilland, R. Baker, Rhombomeric organization of neurons in cat vestibular nuclei, Exp. Brain Res. 20 (1974) 285– hindbrain vestibular neurons in larval frog, Soc. Neurosci. Abstr. 25
296. (1999) 266 / 2.
[20] E.I. Knudsen, Auditory and visual maps of space in the optic tectum [37] C. Suarez, A. Kuruvilla, S. Sitko, I. Schwartz, V. Honrubia, Central of the owl, J. Neurosci. 2 (1982) 1177–1194. projections of primary vestibular fibers in the bullfrog. II. Nerve [21] A.W. Kunkel, N. Dieringer, Morphological and electrophysiological branches from individual receptors, Larygoscope 95 (1985) 1238–
consequences of unilateral pre- versus postganglionic vestibular 1250.
lesions in the frog, J. Comp. Physiol. A 174 (1994) 621–632. [38] H. Suwa, E. Gilland, R. Baker, Otolith ocular reflex function of the [22] A. Kuruvilla, S. Sitko, I.R. Schwartz, V. Honrubia, Central projec- tangential nucleus in teleost fish, Ann. N. Y. Acad. Sci. 871 (1999)
tions of primary vestibular fibers in the bullfrog: I. The vestibular 1–14.
nuclei, Laryngoscope 95 (1985) 692–707. [39] K. Tokumasu, K. Goto, B. Cohen, Eye movements from vestibular [23] C. Matesz, Central projection of the VIIIth cranial nerve in the frog, nuclei stimulation in monkeys, Ann. Otol. Rhinol. Larnygol. 78
Neuroscience 4 (1979) 2061–2071. (1969) 1105–1119.
´ ´ ´
[24] C. Matesz, A. Birinyi, H. Straka, N. Dieringer, Location of dye- [40] P. Toth, G. Csank, G. Lazar, Morphology of the cells of origin of coupled second order canal and otolith neurons and of efferent descending pathways to the spinal cord in Rana esculenta. A tracing vestibular neurons in the frog, Neurobiology 6 (1998) 226–227. study using cobaltic-lysine complex, J. Hirnforsch. 26 (1985) 365– [25] C.A. McCormick, M.R. Braford Jr, Organization of inner ear 383.
endorgan projections in the goldfish, Carassius auratus, Brain [41] R.J. Weinberg, Are topographic maps fundamental to sensory Behav. Evol. 43 (1994) 189–205. processing?, Brain Res. Bull. 44 (1997) 113–116.
[26] G.E. Meredith, B. Butler, Organization of eighth nerve afferent [42] V.J. Wilson, L.P. Felpel, Specificity of semicircular canal input to projections from individual endorgans of the inner ear of the teleost, neurons in the pigeon vestibular nuclei, J. Neurophysiol. 35 (1972)
Astonotus ocellatus, J. Comp. Neurol. 220 (1983) 44–62. 253–254. [27] N.M. Montgomery, Projections of the vestibular and cerebellar