www.elsevier.com / locate / bres
Research report
Nerve growth factor applied onto the olfactory epithelium alleviates
degenerative changes of the olfactory receptor neurons following
axotomy
a,b b a a b
Hiroki Yasuno
, Keijiro Fukazawa , Tetsuo Fukuoka , Eiji Kondo , Masafumi Sakagami ,
a ,
*
Koichi Noguchi
a
Department of Anatomy and Neuroscience, Hyogo College of Medicine, Hyogo 663-8501, Japan
b
Department of Otolaryngology, Hyogo College of Medicine, Hyogo 663-8501, Japan
Accepted 12 September 2000
Abstract
The olfactory neuroepithelium of the mammalian nervous system manifests continuous neurogenesis throughout life. Recent studies suggest that neurotrophic factors and their receptors may play a role in the regulation of development and regeneration in the olfactory system. However, there have been very few in vivo studies investigating the effect of exogenous neurotrophic factors in the olfactory system. In the present study, nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) were administered into the rat olfactory mucosa for 5 days just after the transection of the olfactory nerve. We then examined the effect of exogenous neurotrophic factors on the degenerative changes in axotomized olfactory receptor neurons (ORNs). Further, we examined the location of their receptors, Trk A and Trk B. We found that both mature and immature ORNs expressed more intense signals for olfactory marker protein and beta-tubulin mRNAs, respectively, when NGF was applied to the axotomized olfactory neuroepithelium for 5 days, compared to the ORNs of saline-treated controls. BDNF at a 10mg total dose did not show this effect. The effect of NGF applied onto the olfactory epithelium is consistent with the immunohistochemical finding that Trk A was present in the dendrites and axon bundles in normal and axotomized ORNs. These results suggest that NGF may protect the degenerative changes in mature and immature ORNs following axotomy through the binding to the Trk A receptor located on the surface of the olfactory epithelium. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Olfactory senses
Keywords: Olfactory nerve; Olfactory marker protein; Beta-tubulin; Protein gene product 9.5; NGF; BDNF
1. Introduction receptor neurons (ORNs) derived from the observation that
a developmentally heterogenous population of receptor In mammals, the olfactory neuroepithelium (OE), neurons is present in adult animals, and autoradiographic situated within the nasal cavity, has the ability to regener- studies have shown that immature neurons in the basal cell
3
ate new neurons continually throughout life from a popula- layer of the OE of adult animals incorporate H-thymidine tion of precursor cells present in the basal cell layer [5,34]. These immature neurons progressively move to [15,16]. The concept of continued turnover of olfactory more superficial layers of the epithelium to become mature
ORNs.
The process of turnover of ORNs can be modified following the injury of mature ORNs, such as physical or
*Corresponding author. Tel.: 181-798-45-6415; fax: 1
81-798-45-chemical damage to the OE proper, the axons of the ORNs
6417.
E-mail address: [email protected] (K. Noguchi). (olfactory nerve), or the olfactory bulb [8,40]. For
ple, changes in ORNs following the transection of the 2. Materials and method
olfactory nerve have been extensively examined. A few
days after transection, massive degeneration and neuronal 2.1. Animal procedures programmed cell death of the ORNs occurred and new
ORNs, derived from a stem cell population in the OE, All animal experiments conformed to the regulations of generate axons which grow along the olfactory nerve to the the Hyogo College of Medicine Committee on Animal olfactory bulb. Research and were carried out in accordance with the The normal OE is a ciliated pseudostratified columnar guidelines of the NIH on animal care. A total of 24 male epithelium. The basal cells are located at the base of the Sprague-Dawley rats weighing 200–250 g were used. All epithelium and immature ORNs are situated in the lower experimental procedures were performed on rats which layer of the epithelium. The immature ORNs express were deeply anesthetized with sodium pentobarbital (50 growth associated protein 43 (GAP-43 / B50) [39,40], mg / kg body weight, i.p.). Additional doses of anesthetics which is expressed during axonal growth and neural were given as needed.
development, and tubulin [25,32], which participates in Rats received local anesthetic (1% carbocaine, 200m1) spindle formation in dividing cells. A third type of cell, the to the site of the skin incision on the middle of the head. mature ORNs, are localized in the middle layer of the The left skull, rostral to the bone suture bregma, was epithelium and express olfactory marker protein (OMP) opened using a micro drill and the left olfactory bulb was [4,6,10,27,30,43], a 19 kDa cytoplasmic protein. Immature exposed. A microsurgery scalpel was inserted to a depth of and mature neurons express neural cell adhesion molecules 4 mm against the olfactory bulb side of the cribriform plate [28], which are important during all stages of neurode- and the fila olfactoria were transected according to the velopment and are especially important for synaptic struc- method reported by Harding et al. [19]. In a preliminary turing and protein gene product 9.5 (PGP 9.5) [23,36], experiment, we confirmed that our method could success-which is a neurospecific peptide. A fourth type of cell in fully transect the olfactory nerves by checking the thick-the OE are supporting cells, which are located in thick-the upper ness of the olfactory epithelium. Next, NGF (20 mg / ml, layer of the epithelium. Injury of ORNs also affects the recombinant rat NGF, R&D Systems, Minneapolis, MN, neurochemical expression pattern and axotomized ORNs USA), or BDNF (20 mg / ml, recombinant human BDNF, lose almost all OMP immunoreactive profiles after about a Sumitomo Pharmaceutical, Osaka, Japan) was adminis-week [31]. tered twice a day for 5 days. Rats received NGF or BDNF It is well known that several growth factors play a solution using a Hamilton syringe intermittently for 15 min critical role in the regulation of neurogenesis in the until a total volume of 50ml of solution had been injected developing central nervous system. The typical neuro- into the left nasal cavity. In control rats, saline was trophic factors, nerve growth factor (NGF) and brain- administered. The total dose of 10mg of NGF or BDNF derived neurotrophic factor (BDNF), are known to have was administered over a 5 day period. This dose was crucial roles in the differentiation, survival, morphogenesis determined according to the doses of neurotrophins, topical and regeneration of sensory neurons [2,20,24,26,37]. Re- injections of which were reported previously (6 mg / day cently, high-affinity receptors of the neurotrophic factor NGF [11,41], 5 mg / day BDNF [35], 12 mg / day BDNF tyrosine kinases (Trks) have been cloned; NGF is the [12]), and considering the very limited area of the olfac-preferred ligand of Trk A [7] and BDNF is the olfac-preferred tory epithelium.
ligand of Trk B [13]. A number of studies have suggested
that endogenous neurotrophins play a role in the regulation 2.2. In situ hybridization histochemistry of neurogenesis within normal development of the OE and
the respective cDNAs. These probes were labeled with drated in a graded ethanol series, cleared in xylene, and
35
S-deoxyadenosine triphosphate (NEN, Boston, MA, coverslipped. USA) and terminal deoxynucleotidyl transferase
(Amer-sham, Buckinghamshire, UK), giving a specific activity of 2.4. Quantitative analysis
9 9
1.0310 –1.5310 cpm /mg. Tissue sections were
hybrid-ized after thawing, without any pretreatment, overnight at Each experimental group consisted of six rats. For the in
5
428C in humidified boxes with 5310 c.p.m. of labeled situ hybridization histochemistry, measurements of the probe per 100 ml of a mixture containing 43 SSC, 50% relative density of the areas of silver grains over selected formamide, 0.12 M phosphate buffer, 13 Denhardt’s tissue profiles were performed. OMP mRNA and beta-solution, 0.2% sodium dodecyl sulfate, 250 mg / ml yeast tubulin mRNA levels in the OE were quantified using a tRNA, 10% dextran sulfate, and 100 mM dithiothreitol. computerized image analysis system (IBAS, Zeiss, Ger-After hybridization, the sections were rinsed four times for many). At a magnification of 320 with bright-field 15 min each at 558C in 13 SSC, dipped into distilled illumination, OE were set such that only silver grains were water, transferred through 60, 80, and 95% ethanol, and accurately discriminated from the background in the then air dried. For autoradiography, the sections were lamina propria and read pixel-by-pixel by the computer. coated with NTB-3 emulsion (Kodak, Rochester, NY, We selected randomly 18 fields of view from each rat and USA; diluted 1:1 with distilled water at 458C) and exposed quantified the signal density. The signals for OMP and for 2–3 weeks in light-tight boxes at 48C. After develop- beta-tubulin were expressed as a percentage of the signal ment in D19 (Kodak) and fixation in 24% sodium thiosul- intensity of naive rats. Unpaired comparisons (t-test) were fate, the sections were rinsed in distilled water, counter- used to determine if there were significant differences stained with neutral red, dehydrated in a graded ethanol between groups. P,0.05 (two tail) was considered to be series, cleared in xylene, and coverslipped. statistically significant. All data points are expressed as the
mean6S.E.M.
For immunohistochemistry, the number of PGP 9.5-2.3. Immunohistochemistry labeled profiles per each field of view was counted and averaged. Eighteen fields of view were selected randomly The sections were immunostained using the avidin– from each rat. Unpaired comparisons (t-test) were used to biotin complex (ABC) with nickel ammonium sulfate determine if there were significant differences between intensification. The primary antibody for PGP 9.5 poly- groups. P,0.05 (two tail) was considered to be statistical-clonal antibody (Ultra Clone Limited, UK) was diluted ly significant. All data points are expressed as the 1:1500 with 0.1 M Tris–HCl buffered saline (TBS, pH mean6S.E.M.
7.5) containing 2% normal goat serum (NGS). Anti-Trk A polyclonal antibody (Chemicon International, Temecula,
CA, USA) was diluted 1:250 and anti-Trk B polyclonal 3. Results
antibody (Santa Cruz Biotechnology, Santa Cruz, CA,
USA) was diluted 1:20. Sections were incubated for 72 h In situ hybridization histochemistry and immunohisto-at 48C in this solution. The sections were then rinsed with chemistry were used to show the effect of exogenous NGF 0.1 M TBS, and then incubated in biotinylated goat anti- and BDNF on the axotomized ORNs of rats. Bright-field rabbit IgG (Vector) diluted 1:200 in TBS containing 2% and dark-field autoradiography revealed, in naive rats, that NGS for 24 h at 48C. After rinsing with TBS, the sections the hybridization signals for OMP mRNAs were concen-were incubated with ABC reagent (Vector) in TBS for 3 h trated in the middle layer of the OE, indicating that the at 48C. The sections were again rinsed in 0.1 M TBS, then mature ORNs express high levels of OMP mRNAs (Fig. reacted in 0.05% diaminobenzidine tetrahydrochloride 1A,E). Five days after transection of the central branch of (Sigma, St. Louis, MO, USA) and 0.01% H O in 0.1 M2 2 OE neurons, the OE showed significant degenerative TBS containing 0.2% nickel ammonium sulfate for 5 min. changes which resulted in a reduced thickness of the The sections were then rinsed in distilled water, dehy- epithelium (Fig. 1B,F). The signal of OMP mRNA in
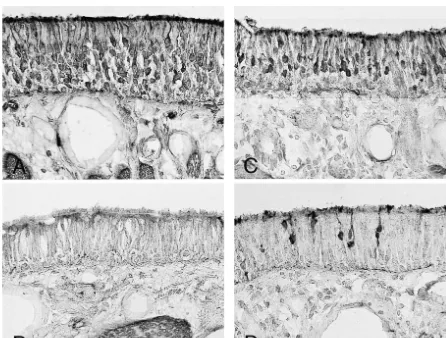
Fig. 1. (See p. 56) Bright-field (A, C, E, G) and dark-field (B, D, F, H) photomicrographs showing the expression of OMP mRNA in OE. The naive OE expressed OMP mRNA abundantly in the middle layer (A, B). The saline-treated control OE after axotomy for 5 days expressed almost no OMP mRNA with reduced height of the epithelium (C, D). The NGF-treated OE 5 days after axotomy expressed OMP mRNA moderately, but the thickness of the OE was reduced compared to the naive OE (E, F). The BDNF-treated OE after axotomy contained only a weak signal for OMP mRNA (G, H). White bars in B, D, F, and G indicate the layer of the OE. Scale bar 50mm.
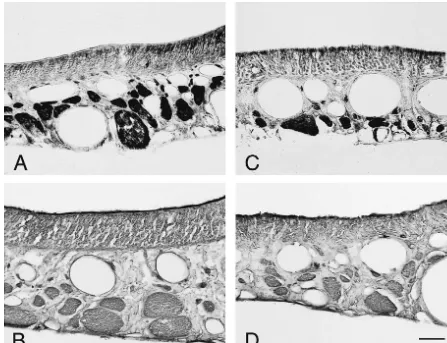
control lesioned OE (treated with saline) decreased In order to examine the effect of neurotrophic factors on dramatically compared to naive rats (Fig. 1B,F). The OE the number of neurons in the neuroepithelium, immuno-treated with NGF for 5 days after axotomy had a reduced histochemistry using anti-PGP 9.5, a neuronal cell marker, height of the epithelium, as was the case for the control was employed on the axotomized OE materials. Many lesioned OE, however the OE expressed moderate levels of neurons were labeled for PGP 9.5 at the base and in the the signal for OMP mRNA (Fig. 1C,G), which was more middle layer of the OE of naive rats (Fig. 3A). Five days dense than that of control lesioned OE that were treated after axotomy, the OE treated with saline (control lesioned with saline. In contrast, BDNF-treated OE after axotomy OE) contained very few or almost no neurons labeled for showed the same pattern of degeneration in the epithelium PGP 9.5, suggesting massive degeneration of ORNs by the as the control lesioned OE; a reduced thickness and a very axotomy (Fig. 3B). The application of NGF into the nasal weak expression of OMP mRNA (Fig. 1D,H). cavity also affected the neuronal number and resulted in We also examined the expression in beta-tubulin mRNA the detection of a moderate number of profiles labeled for on the same materials. Naive OE expressed beta-tubulin PGP 9.5 (Fig. 3C). However, very few profiles were mRNA in the lower third of the OE, suggesting its labeled for PGP 9.5 in BDNF-treated OE after axotomy presence in immature ORNs (Fig. 2A,E). Control lesioned (Fig. 3D).
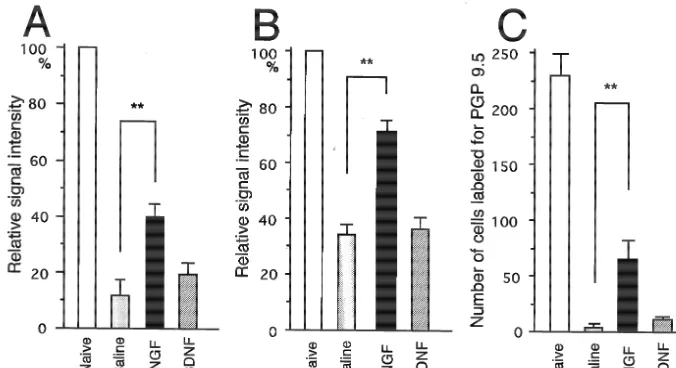
OE that were treated with saline and axotomized 5 days Fig. 4 shows the quantification of the relative signals for prior had very few beta-tubulin mRNA signals (Fig. 2B,F). OMP mRNA (A), beta-tubulin mRNA (B) and the number In NGF-treated OE, axotomized 5 days prior, dense signals of PGP 9.5-labeled profiles (C) in each OE. The level of were observed at the same level as naive OE, despite the OMP mRNA in NGF-treated OE was significantly higher decreased thickness of the epithelium (Fig. 2C,G). How- than that in saline-treated OE (P,0.01), however BDNF ever, BDNF-treated OE expressed only weak signals for treatment did not have any ameliorable effect on OMP beta-tubulin mRNA (Fig. 2D,H). mRNA expression. The same tendency appeared when the
Fig. 4. Effect of NGF and BDNF on OMP (A) and beta-tubulin (B) mRNA expression and the number of profiles labeled for PGP 9.5 (C) in ORNs. The signals for OMP and beta-tubulin mRNA were semi-quantified using a computerized image analysis system (IBAS, Zeiss) and are expressed as a percentage of the signal intensity of naive rats. The number of profiles labeled for PGP 9.5 per unit area was counted. The NGF-treated OE had a significantly different level of OMP mRNA compared to saline-treated OE, but BDNF-treated OE was not significantly different from the saline-treated OE (A). The NGF-treated OE also had a significantly different level of beta-tubulin mRNA (B) and number of labeled profiles for PGP 9.5 compared to saline-treated OE (C). **P,0.01.
effect of neurotrophins on the beta-tubulin mRNA was nerve was studied. We demonstrated that NGF has protec-compared, i.e. NGF could reverse the down-regulation of tive effects on the expression of mRNA expression in both beta-tubulin mRNA by axotomy, but BDNF could not mature and immature ORNs at the fifth day when NGF reverse this effect. The NGF-treated OE had 75% of the was applied on axotomized OE for 5 days, but BDNF had level of the beta-tubulin signal as compared with the naive no effect at a total dose of 10mg. These data are consistent OE, which suggests that the immature neurons in the lower with data that show that Trk A-IR was present in dendrites epithelium were very responsive to NGF. In contrast, the and the axon bundle of ORNs in the olfactory mucosa in number of NGF-treated OE neurons with PGP 9.5 labeling the naive rat and in the axotomized OE 5 days after injury. was only 30% of the number of similarly labeled naive OE An estimate of the average lifetime of ORNs is 1 month neurons; nevertheless, there were significantly more PGP in the rodent [21]. After transection of the olfactory nerve, 9.5-labeled NGF-treated OE neurons than in the saline- ORNs in the OE can fully mature and project afferent treated OE group. axons directly back into the olfactory bulb after 1 month in To help determine the mechanism underlying the re- the mouse. The day after transection, severe degeneration sponsiveness to neurotrophins in axotomized ORNs, we of the ORNs was found, and there was an almost complete examined the location of the receptors of NGF and BDNF, loss of OMP immunoreactive profiles for 6 days in pigeons i.e. Trk A and Trk B, using immunohistochemistry. Naive [31]. This finding is consistent with our data that saline-OE contained Trk A immunoreactive (IR) dendrites in the treated OE after axotomy for 5 days expressed almost no top layer of the epithelium and in the axon bundle of OMP mRNA and beta-tubulin mRNA (Fig. 1B,F). Two ORNs in the lamina propria (Fig. 5A). Trk B-IR was also weeks after transection, immature regenerating profiles observed both in the axon bundle in the lamina propria and appear in the OE, and become mature in morphology after in ORNs in the epithelium (Fig. 5B). Five days after 4 weeks [18,30]. Further, olfactory-related behavior recov-axotomy, Trk A-IR was similar to the naive OE in ers in 4 weeks [42].
dendrites and the axon bundle (Fig. 5C). In the axotomized In the olfactory system, several studies have been OE, the same pattern of labeling of Trk B-IR was observed carried out related to NGF. NGF is produced in the as in the naive OE (Fig. 5D). olfactory bulb [9,17], and is retrogradely transported to the OE [29]. NGF present in the OE may modulate neuronal turnover [1]. The NGF receptor, Trk A, was found in mature and regenerating ORNs [22,31]. Similarly, the low
4. Discussion affinity NGF receptor has been found in Schwann cells of
Fig. 5. Location of Trk A (A, C) and Trk B (B, D) immunoreactivity in the OE. The naive OE showed dendrite (arrow head) and axon bundle (arrow) of ORNs immunoreactive for Trk A-IR (A). Trk B-IR was seen weakly in the axon bundle and dendrites (B). The NGF-treated olfactory mucosa 5 days after axotomy contained the same pattern of immunolabeling for Trk A as observed in the naive rats (C). The axotomized olfactory mucosa was also weakly labeled for Trk B immunoreactivity (D). Scale bar 50mm.
dendrites in the OE, in which the olfactory receptors face olfactory bulb [17]. One possible reason for the ineffec-the nasal cavity. Exogenous NGF given in ineffec-the nasal cavity tiveness of BDNF is that the total dose of BDNF used in may bind to the receptors in the dendritic knob and work the present study (10mg for 5 days) may have been too as alleviative agents against degenerative changes, such as small to produce an effect. A larger dose and a more a decrease in neuronal number, mRNA expression of OMP effective application method for BDNF onto the olfactory and beta-tubulin. If neurotrophic factors are absorbed in epithelium should be explored in the future.
tion in the olfactory system of mammals. I. Morphological aspects
Acknowledgements
of differentiation and structural organization of the olfactory sensory neurons, J. Neurocytol. 8 (1979) 1–18.
We thank Dr. M. Furukawa and Dr. T. Miwa (Kanazawa [16] P.P. Graziadei, G.A. Monti-Graziadei, The olfactory system: a model University, Japan) for technical advice. We thank Dr. D.A. for study of neurogenesis and axon regeneration in mammals, in:
Thomas for correcting the English of this manuscript. This C.W. Cotman (Ed.), Neuronal Plasticity, Raven, New York, 1978, pp. 131–153.
study was supported by Grants-in-Aid for Science
Re-[17] K.M. Guthrie, C.M. Gall, Differential expression of mRNAs for the
search from the Japanese Ministry of Education, Science
NGF family of neurotrophic factors in the adult rat central olfactory
and Culture. system, J. Comp. Neurol. 313 (1991) 95–102.
[18] J. Harding, P.P. Graziadei, G.A. Monti-Graziadei, F.L. Margolis, Denervation in the primary olfactory pathway of mice. IV. Bio-chemical and morphological evidence for neuronal replacement
References following nerve section, Brain Res. 132 (1977) 11–28.
[19] J.W. Harding, J.W. Wright, Reversible effects of olfactory nerve section on behavior and biochemistry in mice, Brain Res. Bull. 4 [1] T. Aiba, J. Mori, Y. Nakai, Nerve growth factor (NGF) and its
(1979) 17–22. receptor in rat olfactory epithelium, Acta Oto-Laryngol. Suppl.
[20] H. Hatanaka, I. Nihonmatsu, H. Tsukui, Nerve growth factor (Stockh.) 506 (1993) 37–40.
promotes survival of cultured magnocellular cholinergic neurons [2] R.F. Alderson, A.L. Alterman, Y.A. Barden, R.M. Lindsay,
Brain-from nucleus basalis of Meynert in postnatal rats, Neurosci. Lett. 90 derived neurotrophic factor increases survival and differentiated
(1988) 63–68. functions of rat septal cholinergic neurons in culture, Neuron 5
[21] J.W. Hinds, P.L. Hinds, N.A. McNelly, An autoradiographic study of (1990) 297–306.
the mouse olfactory epithelium: evidence for long-lived receptors, [3] M.E. Buckland, A.M. Cunningham, Alterations in expression of the
Anat. Rec. 210 (1984) 375–383. neurotrophic factors glial cell line-derived neurotrophic factor,
[22] I. Horikawa, T. Miwa, T. lshimaru, M. Furukawa, T. Kato, T. ciliary neurotrophic factor and brain-derived neurotrophic factor, in
Moriizumi, Trk A expression in olfactory epithelium and bulb the target-deprived olfactory neuroepithelium, Neuroscience 90
during development, Neuroreport 10 (1999) 2205–2208. (1999) 333–347.
[23] E.W. Johnson, P.M. Eller, B.W. Jafek, Distribution of OMP-, PGP [4] O.I. Buiakova, H. Baker, J.W. Scott, A. Farbman, R. Kream, M.
9.5- and CaBP-like immunoreactive chemoreceptor neurons in the Grillo, L. Franzen, M. Richman, L.M. Davis, S. Abbondanzo, C.L.
developing human olfactory epithelium, Anat. Embryol. 191 (1995) Stewart, F.L. Margolis, Olfactory marker protein (OMP) gene
311–317. deletion causes altered physiological activity of olfactory sensory
neurons, Proc. Natl. Acad. Sci. USA 93 (1996) 9858–9863. [24] C. Kalcheim, Y.A. Barde, H. Thoenen, Le. Douarin, In vivo effect of brain-derived neurotrophic factor on the survival of developing [5] A.L. Calof, D.M. Chikaraishi, Analysis of neurogenesis in a
dorsal root ganglion cells, EMBO J. 6 (1987) 2871–2873. mammalian neuroepithelium: proliferation and differentiation of an
olfactory neuron precursor in vitro, Neuron 3 (1989) 115–127. [25] V.M. Lee, S.K. Pixley, Age and differentiation-related differences in [6] V.M. Carr, E. Walters, F.L. Margolis, A.I. Farbman, An enhanced neuron-specific tubulin immunostaining of olfactory sensory
neu-olfactory marker protein immunoreactivity in individual neu-olfactory rons, Brain Res. Dev. Brain Res. 83 (1994) 209–215.
receptor neurons following olfactory bulbectomy may be related to [26] J. Leibrock, F. Lottspeich, A. Hohn, M. Hofer, B. Hengerer, P. increased neurogenesis, J. Neurobiol. 34 (1998) 377–390. Masiakowski, H. Thoenen, Y.A. Barde, Molecular cloning and [7] C.C. Cordon, P. Tapley, S.Q. Jing, V. Nanduri, E. O’Rourke, F. expression of brain-derived neurotrophic factor, Nature 341 (1989)
Lamballe, K. Kovary, R. Klein, K.R. Jones, L.F. Reichard, The trk 149–152.
tyrosine protein kinase mediates the mitogenic properties of nerve [27] F. Miragall, G.G. Monti, Experimental studies on the olfactory growth factor and neurotrophin-3, Cell 66 (1991) 173–183. marker protein. II. Appearance of the olfactory marker protein [8] R.M. Costanzo, P.P. Graziadei, A quantitative analysis of changes in during differentiation of the olfactory sensory neurons of mouse: an the olfactory epithelium following bulbectomy in hamster, J. Comp. immunohistochemical and autoradiographic study, Brain Res. 239
Neurol. 215 (1983) 370–381. (1982) 245–250.
[9] T. Ebendal, Function and evolution in the NGF family and its [28] F. Miragall, G. Kadmon, M. Husmann, M. Schachner, Expression of receptors, J. Neurosci. Res. 32 (1992) 461–470. cell adhesion molecules in the olfactory system of the adult mouse: presence of the embryonic form of N-CAM, Dev. Biol. 129 (1988) [10] A.I. Farbman, J.A. Buchholz, E. Walters, F.L. Margolis, Does
516–531. olfactory marker protein participate in olfactory neurogenesis?, Ann.
New York Acad. Sci. 30 (1998) 248–251. [29] T. Miwa, N. Uramoto, T. lshimaru, M. Furukawa, K. Shiba, T. [11] M. Fitzgerald, P.D. Wall, M. Goedert, P.C. Emson, Nerve growth Morjizumi, Retrograde transport of nerve growth factor from factor counteracts the neurophysiological and neurochemical effects olfactory bulb to olfactory epithelium, Neuroreport 9 (1998) 153– of chronic sciatic nerve section, Brain Res. 332 (1985) 131–141. 155.
[12] B. Friedman, D. Kleinfeld, V.M. Verge, R. Moulton, P. Boland, E. [30] G.G. Monti, P.P. Graziadei, Experimental studies on the olfactory Zlotchenko, R.M. Lindsay, L. Liu, BDNF and NT-4 / 5 exert marker protein. I. Presence of the olfactory marker protein in tufted neurotrophic influences on injured adult spinal motor neurons, J. and mitral cells, Brain Res. 209 (1981) 405–410.
Neurosci. 15 (1995) 1044–1056. [31] N. Oley, R.S. DeHan, D. Tucker, J.C. Smith, P.P. Graziadei, [13] D.J. Glass, S.H. Nye, P. Hantzopoulos, M.J. Macchi, S.P. Squinto, Recovery of structure and function following transection of the M. Goldfarb, G.D. Yancopoulos, Trk B mediates BDNF / NT-3- primary olfactory nerves in pigeons, J. Comp. Physiol. Psychol. 88 dependent survival and proliferation in fibroblasts lacking the low (1975) 477–495.
affinity NGF receptor, Cell 66 (1991) 405–413. [32] S.K. Pixley, CNS glial cells support in vitro survival, division, and [14] Q. Gong, M.S. Bailey, S.K. Pixley, M. Ennis, W. Liu, M.T. Shipley, differentiation of dissociated olfactory neuronal progenitor cells,
Localization and regulation of low affinity nerve growth factor Neuron 8 (1992) 1191–1204.
[34] L.M. Schwartz, D.M. Chikaraishi, J.S. Kauer, Characterization of expression of the growth associated protein B50 / GAP43 in the potential precursor populations in the mouse olfactory epithelium olfactory system of neonatal and adult rats, J. Neurosci. 9 (1989) using immunocytochemistry and autoradiography, J. Neurosci. 11 683–691.
(1991) 3556–3564. [40] J. Verhaagen, A.B. Oestreicher, M. Grillo, Y.S. Khew-Goodall, W.H. [35] M. Sendtner, B. Holtmann, R. Kolbeck, H. Thoenen, Y.A. Barde, Gispen, F.L. Margolis, Neuroplasticity in the olfactory system: Brain-derived neurotrophic factor prevents the death of motoneurons differential effects of central and peripheral lesions of the primary in newborn rats after nerve section, Nature 360 (1992) 757–759. olfactory pathway on the expression of B50 / GAP43 and the [36] K. Taniguchi, H. Saito, M. Okamura, K. Ogawa, Immunohisto- olfactory marker protein, J. Neurosci. Res. 26 (1990) 31–44.
chemical demonstration of protein gene product 9.5 (PGP 9.5) in the [41] J. Wong, M.M. Oblinger, NGF rescues substance P expression but primary olfactory system of the rat, Neurosci. Lett. 156 (1993) not neurofilament or tubulin gene expression in axotomized sensory
24–26. neurons, J. Neurosci. 11 (1991) 543–552.
[37] H. Thoenen, The changing scene of neurotrophic factors, Trends [42] K.K. Yee, R.M. Costanzo, Restoration of olfactory mediated be-Neurosci. 14 (1991) 165–170. havior after olfactory bulb deafferentation, Physiol. Behav. 58 [38] N. Uramoto, T. Miwa, T. Donjyo, T. lshimaru, M. Furukawa, Study (1995) 959–968.