Effect of an
Alcaligenes faecalis
inoculant strain on
bacterial communities in flooded soil microcosms
planted with rice seedlings
M. Lin
a,b, K. Smalla
c, H. Heuer
c, J.D. van Elsas
a,∗aResearch Institute for Plant Protection (IPO-DLO), PO Box 9060, 6700GW Wageningen, Netherlands
bInstitute for Application of Atomic Energy, CAAS, Beijing, 100094, PR China
cFederal Biological Research Centre for Agriculture and Forestry, Institute for Biochemistry and Plant Virology,
Messeweg 11/12, 38104 Braunschweig, Germany
Received 31 May 1999; received in revised form 24 November 1999; accepted 23 March 2000
Abstract
The fate and impact ofAlcaligenes faecalisstrain A1501R, a rifampicin-resistant derivative of a rice inoculant strain, were studied in flooded silt loam microcosms planted with rice seedlings. Selective plating revealed that strain A1501R survived at high, initially stable and later slowly declining population sizes (108–106CFU per gram of dry soil), for 60 days. Inoculant
survival in the rice rhizosphere showed a similar trend, and only at one time point (15 days), the inoculant CFU numbers were significantly higher in the rhizosphere than in corresponding bulk soil.
A probe for the detection of strain A1501R in soil, based on the 16S rDNA variable region V6, was obtained via PCR amplification with specific primers. Alignment of the sequence of this V6 region with that of a range of different bacterial species indicated that it provided a tool for the detection of strain A1501R in a soil background. Dot blot hybridization of randomly isolated strains and of soil DNA confirmed the usefulness of the probe. Use of the probe in a dilution dot blot hybridization experiment with soil DNA revealed a dynamics of strain A1501R over time similar to that indicated by the CFU counts. However, the presence of background in soil did not allow the detection of inoculant numbers below an estimated 105–106cells per gram of soil.
At regular times, i.e. 3 h, 15, 30 and 40 days after introduction, bacterial community fingerprints were generated via bacterial 16S rDNA based PCR of soil DNA followed by denaturing gradient gel electrophoresis (DGGE). The introduced strain could be clearly detected by the appearance of a novel, initially strong and progressively weaker, band in the community fingerprints up to roughly 30–40 days of incubation. Indigenous organisms underlying the comigrating bands found after 30–40 days were found to react with the strain A1501R V6 probe. These isolates were identified asPseudomonasspp.
The introduction of strain A1501R did not result in substantial changes in the bacterial community fingerprints. The fingerprints obtained over time in bulk soil were >90–95% similar to each other, and there was no clear trend evidenced via cluster analysis. For the rhizosphere fingerprints, three main clusters (each of >95% similarity), representing the day-0 plus day-15, the day-30, and the day-40 bacterial communities, were found. The communities apparently changed more as a result of rice root growth than due to the presence of A1501R.
Community level physiological profiling (CLPP) based on the use of Biolog GN plates was then applied using the soil microbial communities sampled over time. The potential for utilization of substrates of the Biolog system by these microbial
∗Corresponding author.
communities remained, with a few, varying, exceptions, largely unchanged in spite of the release of strain A1501R. However, significant differences in the utilization of selected substrates were observed between the control and inoculated soils, as evidenced by the application of PCR–DGGE to the bacterial communities inhabiting selected wells of the Biolog plates. In particular, strain A1501R was found to be highly competitive in the presence of lactic acid. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Alcaligenes faecalis; Survival rice rhizosphere; PCR–DGGE Biolog
1. Introduction
The development and agricultural application of bacterial inoculants, either unmodified or genetically modified to improve their function, has attracted con-siderable interest in the last decade (You and Zhou, 1989, 1991; van Elsas et al., 1991; De Leij et al., 1995; van Overbeek et al., 1997; van Veen et al., 1997). An important area has been the application of associative diazotrophic bacteria to crops growing in soils with nitrogen deficiencies. In particular, in-tensive rice cropping regimes such as those common in Chinese agriculture have been indicated to benefit from such associations (You and Zhou, 1989, 1991; You et al., 1991; Ueda et al., 1995).
The diazotrophic bacterium Alcaligenes faecalis A1501, originally obtained from paddy soil in China (You and Zhou, 1989; You et al., 1995), has been shown to be a key organism for application to rice crops, either in inoculant mixtures or as a single in-oculant (You and Zhou, 1991; You et al., 1991). This organism has been successfully used in experimental inoculant mixtures in the open field (Lin, 1997). To enhance the in situ inoculant nitrogen fixation rates, genetically modified (GM) derivatives have been pro-duced for strain 1501, as well as for other components of inoculant mixtures, i.e. nitrogen-fixing strains of
Enterobacter cloacae and Klebsiella oxytoca
(Fu-jii et al., 1991; You and Zhou, 1991, 1989; You et al., 1995). The modifications in these strains were brought about via the introduction of an exogenous, constitutively expressed, nifA regulatory gene on a plasmid or inserted into the chromosome (You and Zhou, 1989, 1991). The resulting GM strains thus displayed an enhanced capacity to fix nitrogen and even fixed nitrogen under high-ammonia conditions (You et al., 1995).
Field experiments with rice and soybean inoculated with the GM A. faecalis A1501 inoculant in mixes
have been performed in south China since 1989 (You et al., 1995). The main purpose of these field releases has been the improvement of crop productivity and economization of nitrogen fertilizer, as it is known that intensive rice cropping can cause a depletion of avail-able nitrogen (Reichardt et al., 1997). In rice, nitro-gen fixation by the modified strain was about 15–20% raised over unmodified controls, as evidenced by using the15N isotope dilution technique (Lin, pers. comm.). Yield increases of field-grown rice were about 5–12% when GM strains were used and 3–7% with parent strains (You et al., 1995). TheA. faecalis inoculant strain has further been proposed and tested for use in rice and soybean cropping in salt-affected or degraded soils, due to its beneficial characteristics, such as the production of the plant growth hormone indole acetic acid and tolerance to salt (Lin et al., 1992).
To understand the performance and putative adverse effects of theA. faecalisinoculant in soil, monitoring of its fate and impact over time is needed. Such an as-sessment is particularly crucial as a prelude to future (commercial) large-scale releases. In addition to data on inoculant persistence and potential spread, its ef-fect on indigenous microbial communities should be understood, for instance via monitoring of changes in the diversity and activity of microbial communities in soil (Wunsche et al., 1995; van Elsas et al., 1998).
the dominant members of soil microbial communities (Heuer and Smalla, 1997b; Duineveld et al., 1998). Furthermore, the metabolic potential of microbial communities can be assessed via community level physiological profiling (CLPP) using the Biolog GN microtiter plate setup (Garland and Mills, 1991; Gar-land, 1997). In spite of its obvious drawbacks (Smalla et al., 1998), this method has been widely used as a rapid and powerful community-level approach to study the potential activity of microbial populations in natural environments (Winding, 1994; Zak et al., 1994; Garland, 1997; Heuer and Smalla, 1997b; Hitzl et al., 1997; Insam, 1997; Knight et al., 1997). Combi-nation of the two methods thus allows a simultaneous functional and structural assessment of the impact of the release of inoculant strains into soils (Akkermans et al., 1995; Smalla et al., 1998).
This study aimed to assess the fate and impact
of A. faecalis strain A1501R in soil cropped with
rice in microcosms. To achieve this objective, the autecology of the inoculant strain was monitored by different methods. Furthermore, the impact of theA.
faecalisstrain on the soil and rhizosphere microbiota
was assessed by comparing the PCR–DGGE profiles and Biolog GN metabolic fingerprints of microbial communities between soil samples inoculated or not with strain A1501R.
2. Materials and methods
2.1. Bacterial strain and selection of a rifampicin-resistant mutant
The A. faecalis inoculant strain, denoted A1501,
was isolated from paddy soils in south China in 1980 (You et al., 1995). It was identified asA. faecalis us-ing traditional taxonomic tests (You et al., 1995), but recent molecular evidence (based on the 16S riboso-mal RNA gene sequence) obtained in our laboratories (Genbank accession number AF143245) suggests it might in fact be closely related to fluorescent pseu-domonads (closest relative Pseudomonas stutzeri). Spontaneous rifampicin-resistant mutants of A.
fae-calisA1501 were isolated on LB medium containing
100mg of rifampicin per milliliter. One mutant clone of A1501, denoted strain A1501R, that showed a growth rate similar to that of the wild-type strain, was
selected. The mutation in A1501R was stable without reversion to wild-type after more than 30 genera-tions in LB medium, as well as in soil during 60-day microcosm experiments. Strain A1501R was grown overnight at 30◦C in LB medium supplemented with 50mg of rifampicin per milliliter, washed twice and resuspended in sterile demineralized water to obtain a cell density of the order 1010–1011 cells per milliliter for further microcosm experiments.
2.2. Soil microcosm experiment
A microcosm experiment was carried out to inves-tigate the fate and effect ofA. faecalisstrain A1501R in soil. Flevo silt loam (FSL) soil was taken from a field microplot at the Institute for Plant Protection IPO-DLO (Wageningen, the Netherlands). Washed bacterial cells in sterile demineralized water, or ster-ile demineralized water (control), were thoroughly mixed through portions of the FSL soil, which were subsequently used to fill replicate plastic pots (100 g of treated soil per pot). The initial inoculum density was about 107 CFU per gram of dry soil. Seeds of rice (Oryza sativaL.japonicaZhongzuo 9037) were germinated at 30◦C. Three-day-old seedlings were transferred to the pots (two seedlings per pot), and pots were flooded using sterile distilled water. The pots were placed in a growth chamber (80% relative humidity) with 16 h of light (26◦C) and 8 h of dark-ness (18◦C). The height of the water layer on top of the soil was adjusted with distilled water based on the growth of the rice seedlings. The water content in the soil was, thus, at 100% of the saturation level.
Replicate soil microcosms were sampled at regular times, i.e. 3 h (time 0) and 2, 5, 15, 23, 30, 40 and 60 days following incubation. Bulk and rhizosphere soils were separated as described (van Overbeek et al., 1997). Samples were processed for the assessment of total bacterial and strain A1501R CFU counts, for total community DNA extraction and for CLPP, as outlined below.
2.3. Enumeration of bacterial populations
rifampicin, which was sufficient to inhibit the growth of indigenous microorganisms; no colonies were found on rifampicin-containing plates when samples from uninoculated soil were studied. Total bacterial counts were obtained on 10% strength tryptic soy broth agar (0.1×TSA). For enumeration of bacterial populations in soil, 10 g soil samples were suspended in 95 ml of sterile 0.1% sodium pyrophoshate (PPi) solution and 10 g of gravel, in 250 ml flasks. The flasks were shaken for 10 min at 180 rpm. The sus-pensions were serially diluted in 0.1% sodium PPi, after which aliquots were plated onto LB medium containing 50mg of rifampicin plus 100mg of cyclo-heximide per milliliter, and on 0.1×TSA containing cycloheximide (100mg ml−1). The plates were incu-bated for 48 h at 28◦C prior to colony enumerations. Counts were expressed as CFU per gram of dry soil (De Leij et al., 1995; van Overbeek et al., 1997).
2.4. Soil DNA extraction and PCR amplification
Total soil community DNA was extracted and puri-fied from duplicate 2 g bulk or rhizosphere soil sam-ples as described in protocols developed in our labora-tories (Smalla et al., 1993; van Elsas and Smalla, 1995; van Elsas et al., 1997). This included cell lysis via bead beating with 100–110mm diameter glass beads, phenol extraction and further purification by CsCl and potassium acetate precipitation and Wizard spin col-umn clean-up. The purified soil DNA was dissolved in a final volume of 100ml of 10 mM Tris–EDTA buffer (pH, 8.0).
PCR amplification was performed with 1ml of soil DNA in a 50ml reaction mixture by touch-down PCR (3 min at 94◦C; 1 min at 94◦C, 1 min at 64◦C, and 3 min at 72◦C [two cycles; repeating this cy-cle with decreasing annealing temperatures of 2◦C every two cycles down to 56◦C]; 1 min at 94◦C, 1 min at 54◦C, and 3 min at 72◦C [30 cycles]; and 10 min at 72◦C). This protocol is similar to those de-scribed previously (van Elsas and Wolters, 1995). The touch-down temperature cycling scheme improved the quality of PCR products as compared to a pre-viously used fixed thermal cycling scheme (Rosado, pers. comm.). The nucleotide sequences of the 16S ribosomal RNA based conserved bacterial primers used were as follows (Heuer and Smalla, 1997a; Heuer et al., 1999): forward primer (968f)
GC-Clamp-5′-AACGCGAAGAACCTTAC-3′and reverse primer (1401r) 5′-CGGTGTGTACAAGGCCC-3′. The for-ward primer had a 40-nucleotide GC-rich sequence (GC clamp) at its 5′-end. The primer pair amplified the 16S rDNA region corresponding to positions 968–1401 (E. coli numbering) of the majority of sequences present in the ribosomal database.
2.5. Construction of a specific probe based on the 16S rDNA variable region V6
The V6 variable region of the 16S rDNA of
A. faecalis A1501 was amplified by PCR with
primers to the conserved regions around positions 971–1057 of E. coli. The nucleotide sequences of these primers were as follows: forward primer (971f) 5′-GCGAAGAACCTTACC-3′, and reverse primer (1057r) 5′-CATGCAGCACCTGT-3′ (Heuer et al., 1999). The around 86-bp PCR product (about 57 variable bases) was cloned and sequenced and the sequence obtained compared to database sequences using a BLAST homology search via the Internet (NCBI, Altschul et al., 1990). With the exception of sequences from two organisms described as
Pseu-domonas spp., the sequence revealed to be specific
for strain A1501. It was directly used as a probe in dilution dot blot assays with soil DNA, as well as on blots of DGGE profiles.
2.6. Hybridizations with the DIG labeled specific V6 probe
The strain A1501 specific V6 probe was first la-beled with the random primer DNA labeling mix (Boehringer Mannheim, Germany), using the proto-col of the manufacturer. It was kept frozen until used in hybridization experiments.
was dotted onto membranes in a threefold dilution scheme. Finally, selected DGGE gels were denatured, fixed and blotted onto nylon membranes, and the re-sulting filters used for hybridization analysis accord-ing to standard procedures (Sambrook et al., 1989).
Hybridizations, washes and detection with the DIG-labeled V6 probe were carried out at high strin-gency using the protocol described in the Boehringer (Mannheim) protocol.
2.7. Denaturing gradient gel electrophoresis analysis
DGGE was performed with a PhorU gradient sys-tem (Ingeny, Leiden, the Netherlands). The PCR products were applied directly onto 6% (wt./vol) poly-acrylamide gels in 0.5×TEA (20 mM Tris–acetate pH 7.4, 10 mM acetate, 0.5 mM Na2EDTA) with gradients from 45 to 65% denaturants. Hundred per-cent of denaturants is 7 M urea plus 40% formamide (Muyzer et al., 1996). The gels were run at 60◦C (100 V) for 16 h. After electrophoresis, the gels were incubated for 1 h in 10 ml SYBR Green I nucleic acid gel staining solution (Molecular Probes, Leiden, the Netherlands), after which they were photographed using a 302 nm UV transilluminator.
The molecular profiles obtained were analyzed using the Molecular Analyst fingerprinting software (BioRad, Veenendaal, Netherlands) as well as manu-ally.
2.8. Biolog GN substrate utilization patterns
On days 0, 2, 5, 15, 23, 30 and 40, CLPP patterns were determined using the Biolog system, which de-tects the utilization of 95 specific carbon sources by bacterial communities in microplates (Garland and Mills, 1991; Garland, 1997). A modified protocol was used, as follows: 3 g (dry weight) of soil was added to 30 ml of sterile one-quarter strength Ringer’s solution in a 50 ml plastic tube containing 2 g of gravel. This slurry was shaken at 200 rpm for 2 h, then kept for 3 min. Five milliliter sample was removed and added to 45 ml of sterile one-quarter-strength Ringer’s so-lution and centrifuged for 15 min (3500g). The pellet was washed once with 0.85% NaCl and resuspended in 50 ml of 0.85% NaCl. The numbers of cultur-able bacteria in suspension were determined using
unselective or selective (containing 50mg of ri-fampicin per milliliter) LB agar. The suspension was placed in a sterile tray with a magnetic stirrer, which kept it homogeneous during inoculation of the mi-crotiter plates. For each assessment, three replicate plates were inoculated. After inoculation with 150ml of bacterial suspension per well (containing about 104CFU), the plates were shaken at 120 rpm (25◦C). The plates were checked at regular times during 65 h of incubation for color development, using the Biolog microplate reader. In addition, selected wells were analyzed for the number of total and strain A1501R CFU as described above. The measured extinction values were automatically corrected by incorporating the extinction value of the substrate-free reference well (E0) according to the Biolog instruction manual (Biolog, Hayward, CA). The readings obtained at set times, i.e. 37–48 h, when average well color devel-opment (AWCD) was maximal (Heuer and Smalla, 1997b) — were corrected for AWCD (Garland and Mills, 1991; Garland, 1997), and corrected values were used in comparative analyses. The data obtained with a selection of 32 discriminative substrates were used in the full analyses.
2.9. Statistics and cluster analyses
All experiments were performed in triplicate, whereas samples from duplicate pots were subjected to DNA extraction and PCR–DGGE analysis. Data were analyzed by analysis of variance and differences were considered significant at p<0.05. The DGGE
banding patterns were compared using phenetic meth-ods with the Dice coefficient of similarity (NT-SYS program). Dendrograms were constructed using the neighbor joining unweighted pair group method with mathematical averages (UPGMA).
3. Results
3.1. Fate of culturable A. faecalis A1501R and total bacterial counts in soil planted with rice seedlings
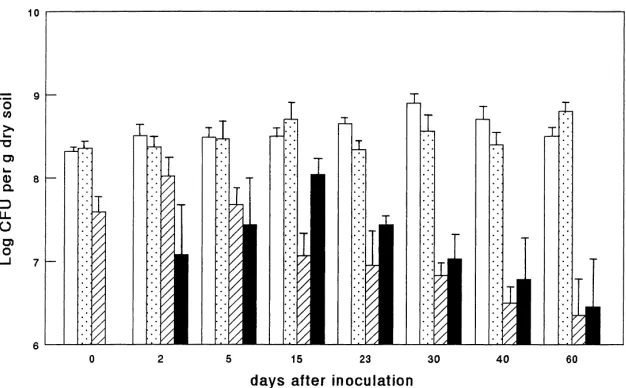
Fig. 1. Survival of strain A1501R following introduction into FSL soil microcosms: (h) total bacterial numbers (CFU) in uninoculated (control) soils; total number of bacteria (CFU) in inoculated soils; A1501R CFU counts in bulk soil; (j) A1501R CFU counts in rhizosphere soil. Bars indicate standard deviations of triplicate systems.
soil after 2 days in bulk soil or after 15 days in rhizo-sphere soil, and then showed a gradual decline. The inoculant finally kept a roughly stable population size in bulk soil, of between 106and 107CFU per gram of dry soil, during the later part of the 60 day incubation period. The numbers of inoculant CFU in rhizosphere soil were largely similar to those in corresponding bulk soil samples. They were significantly higher only at one time point, i.e. 15 days after inoculation (Fig. 1). Total counts of culturable bacteria in bulk and rhi-zosphere soil samples of the FSL soil were between 108and 109CFU per gram of dry soil, and changed very little over time (Fig. 1). In addition, there was no clear rhizosphere effect on the total bacterial CFU counts. Moreover, inoculation with strain A1501R did not result in significant changes in the total numbers of culturable bacteria, which remained at about 109 CFU per gram of dry soil, irrespective of the presence of the inoculant strain.
3.2. Molecular analysis of strain A1501R fate in bulk soil using a V6 probe
The sequence of the variable V6 region of 16S rDNA from strain A1501R was compared with se-quences of the NCBI database (Altschul et al., 1990). Out of over 4000 sequences, only two 16S rRNA
sequences (<0.05%) were homologous (Fig. 2). Both
sequences belonged to organisms classified as
Pseu-domonasspp. The next-nearest strains (Pseudomonas
alcaligenesandP. stutzeri) already had 3% sequence
divergence, and all other strains had 6% or more se-quence divergence. These included strains ofVibrio spp. and of different pseudomonads (P. stutzeri, P.
putida,P. aeruginosaandP. mendocina). In addition,
a FastA analysis with selected sequences (Fig. 2) showed high divergence with sequences from
Es-cherichia coli, P. testosteroni, Agrobacterium
tume-faciensandBacillus subtilis(Lane, 1991). Thus, the
Fig. 3. (A) Dot blot hybridization of PCR amplicons obtained with DNA of 86 strains isolated from A1501R-inoculated soil with the strain A1501R-specific V6 probe. Arrow indicates pos-itive clones (Pseudomonas spp. N2 and N3); (+) left (intense) dot=positive control (product generated with total genomic DNA of strain A1501R); right (faint) dot=negative control. (B) Dot blot hybridization of bulk soil DNA extracted over time from the microcosm study with the strain A1501R-specific V6 probe. For each rank, a threefold dilution scheme was used, from the top to the bottom. Rank 1–4: inoculated soil (respectively, from days 0, 15, 30 and 40); Rank 5: A1501R genomic DNA (upper well: 1000 ng); Rank 6–9: control soil (respectively, from days 0, 15, 30 and 40); Rank 10: negative control; Rank 11: A1501R genomic DNA (upper well: 500 ng); Rank 12: strain N2 genomic DNA.
a Biolog GN profile identical to that of strain A1501R. They were thus identified as the inoculant strain. Two remaining strains that showed weak signals (<10% of
positive control; Fig. 3A) were rifampicin-sensitive. These two strains showed identical substrate uti-lization profiles in Biolog GN plates, where they showed no close match to strain A1501R or to any
strain of the Biolog database (not shown). They were tentatively identified as “Pseudomonas” spp. The strains produced amplicons that comigrated in DGGE with the A1501R specific amplicon, suggesting their 986-1401 16S rDNA regions were highly similar (not shown).
The V6 probe was used in a dilution/dot blot ap-proach with DNA obtained from uninoculated and inoculated bulk soils from the microcosm study (Fig. 3B). The results revealed weak background signals in DNA obtained from uninoculated soils on days 0 and 40 (slots 6 and 9), whereas this background was not detected at other time points (slots 7 and 8). DNA of one cross-reacting strain (slot 12) also reacted with the probe. On the other hand, the inoculated soil samples consistently showed strong signals in several dilutions of the soil-derived DNA up to day-40 (slots 1–4). Only in the day-40 samples, the background signals equaled those from the inoculated soil. Quantification by scan-ning revealed initially strong signals in inoculated soils (day-0 and day-15) of about 10-fold background strength, after which signal intensity fell progressively down to background level after 40 days. Comparison with the positive control (strain A1501R genomic DNA, slots 5 and 11) suggested that initially an esti-mated 107copies of the target were present per gram of the inoculated soils, which declined to less than 106 over time. This roughly matched the data obtained by selective plating, and also supported the population density estimated via PCR–DGGE (Fig. 4; see below).
3.3. PCR–DGGE fingerprinting of bacterial communities in inoculated and control soil
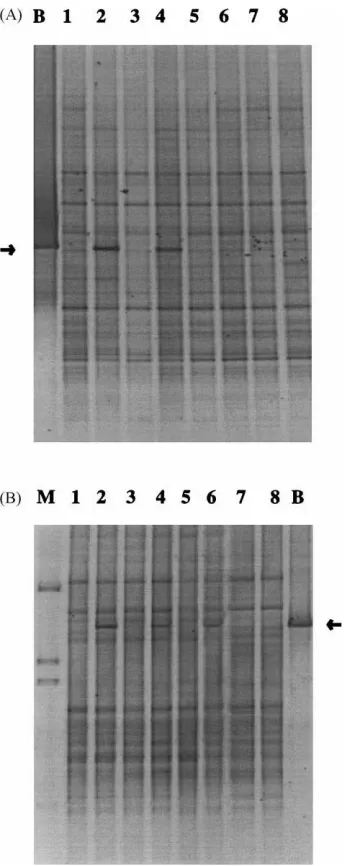
Fig. 4. PCR–DGGE fingerprinting of soil DNA obtained from uninoculated and inoculated soils: (A) bulk soils; (B) rice rhi-zosphere soils. B (in figure): 16S rDNA fragment amplified from strain A1501R using conserved primers; arrow indicates the A1501R-specific band. Lanes 1 and 2: day-0 samples; Lanes 3 and 4: day-15 samples; Lanes 5 and 6: day-30 samples; Lanes 7 and 8: day-40 samples. Odd numbers: amplified with DNA from uninoculated soils; even numbers: amplified with DNA from inoc-ulated soils. M: marker; products of, from top to bottom:Listeria innocuaALM105,Rhizobium leguminosarumbiovartrifoliiR62,
Arthrobactersp. A2,Burkholderia cepaciaP2 (faint band).
day-15 in bulk soil and up to day-30 in rhizosphere soil samples (Fig. 4A and B). This band migrated to the same position as the PCR product generated with strain A1501R. In later samples (e.g. day-30 and day-40), these bands in the profiles of inoculated soils became weaker and of similar intensity as comi-grating weak bands apparent in the profiles generated from uninoculated soils. These observations were confirmed by using the strain A1501R specific V6 probe, which identified the A1501R-specific bands, via strong signals, at the same time points indicated. The data suggested that the uninoculated soil samples might contain relatively low numbers of bacteria (es-timated, as numbers of target molecules, at≤105–106 cells per gram of soil) with similar 16S ribosomal target sequences.
Fig. 4 further shows that at least six to seven dom-inant bands and about 30 weak bands were present in almost all DGGE profiles. All dominant bands were very stable and similar between the profiles obtained for control and inoculated samples. The remaining weak bands were more variable during incubation, as compared to the major bands. Clustering of the bulk soil-derived profiles via the UPGMA (Dice coeffi-cient of similarity) revealed an internally great resem-blance (>90%) between all profiles, and no evidence for a trend towards an effect of inoculation (Fig. 5A). The rhizosphere-derived profiles clustered together at
about 80% similarity, and again no effect of inocu-lation was evident (Fig. 5B). There was an effect of incubation time (i.e. root growth), as at about 92% of similarity, three clusters could be formed, i.e. the day-40 samples, the day-30 samples and the day-0 plus day-15 samples (Fig. 5B). All of the differences occurred in the weak bands of the patterns.
3.4. Biolog GN community-level substrate utilization patterns
During 40 days, the substrate utilization patterns of the uninoculated as well as inoculated FSL soil microcosms were examined using Biolog GN mi-croplates. Among the 95 substrates, 10 could not be utilized at all by the microbial communities of the tested soil. These included erythritol, lactulose, xylitol, a-hydroxybutyric acid, a-keto-butyric acid, a-keto-valeric acid, sebacic acid, alaninamide, glycyl-l-glutamic acid and threonine. These characteristics were, thus, similar between the bacterial communi-ties of uninoculated and inoculated soil microcosms. On the other hand, utilization of four carbohydrate or polymer substrates, i.e. glycogen, arabinose, gentio-biose and mannose, occurred consistently, albeit with different rates, in all samples.
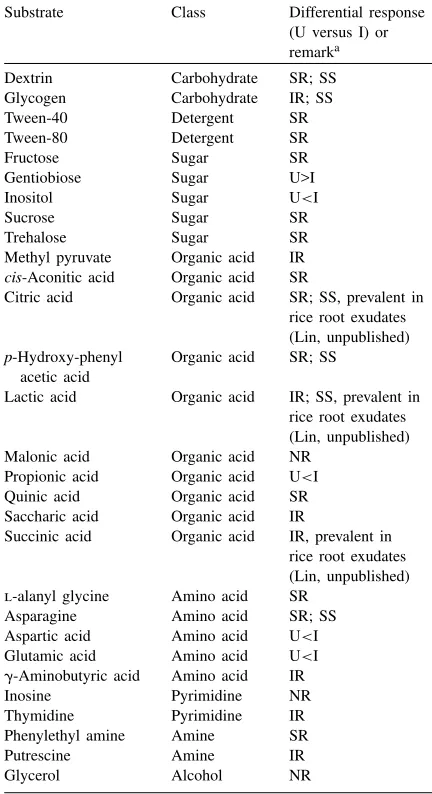
To assess the contribution of inoculation with A1501R to the substrate utilization patterns at the soil microbial community level, the responses to 29 dis-criminative substrates (Table 1) were analyzed; these substrates were selected on the basis of substrate cat-egories (Insam, 1997). Using this criterion, the effect of inoculation on community-level substrate utiliza-tion was not at all clear. In bulk soil, overall there were only up to five significant differences per time point between inoculated and control soils in the val-ues obtained. However, the differences were erratic and a clear trend could not be detected. Moreover, the patterns obtained with rhizosphere soil populations, sampled at day-23, were similar between inoculated and uninoculated soils.
On day-30, 24 of the 29 substrates were utilized similarly between control and inoculated bulk soil samples, whereas the utilization rates of the re-maining substrates were different (Table 1). Six se-lected substrates, i.e. dextrin, glycogen, citric acid, p-hydroxyphenylacetic acid, lactic acid or asparagine, were interesting since, in spite of the similar color
Table 1
Response of bacterial communities obtained on day-30 from uninoculated and inoculated soil microcosms to 29 selected sub-strates of the Biolog GN system
Substrate Class Differential response
Inositol Sugar U<I
Sucrose Sugar SR
Trehalose Sugar SR
Methyl pyruvate Organic acid IR
cis-Aconitic acid Organic acid SR
Citric acid Organic acid SR; SS, prevalent in rice root exudates (Lin, unpublished)
p-Hydroxy-phenyl acetic acid
Organic acid SR; SS
Lactic acid Organic acid IR; SS, prevalent in rice root exudates (Lin, unpublished) Malonic acid Organic acid NR
Propionic acid Organic acid U<I
Quinic acid Organic acid SR Saccharic acid Organic acid IR
Succinic acid Organic acid IR, prevalent in rice root exudates (Lin, unpublished)
l-alanyl glycine Amino acid SR
Asparagine Amino acid SR; SS Aspartic acid Amino acid U<I
Glutamic acid Amino acid U<I
g-Aminobutyric acid Amino acid IR
Inosine Pyrimidine NR
Thymidine Pyrimidine IR Phenylethyl amine Amine SR
Putrescine Amine IR
Glycerol Alcohol NR
aFor responses similar between uninoculated (U) and
inocu-lated (I) soils, the strength of the color development is shown, as follows: NR, no response (<5% of maximal color development);
IR, intermediate response (<50%); SR, strong response (50–
100%). SS, substrate selected for community analyses by PCR–DGGE.
3.5. PCR–DGGE community profiling applied to bacterial communities in selected Biolog wells
The DGGE profiles of soil microbial communities obtained on day-30 in the wells of the Biolog GN mi-croplates containing dextrin, glycogen, citric acid, p-hydroxy phenylacetic acid, lactic acid and asparagine were analyzed after incubation for 48 h (Fig. 6). These six substrates belong to different main groups of carbon substrates, i.e. carbohydrates, polymers, car-boxylic acid, amino acid and phenolic components. All butp-hydroxyphenylacetic acid were utilized by strain A1501R.
First, during incubation, the numbers of total bac-teria in all wells increased from about 4×104 to over 108CFU per milliliter (Table 2). The density of strain A1501R was initially about 3×102 CFU per milliliter (0.75% of the total bacterial CFU). In the lactic acid-containing well it increased to over 108 CFU per milliliter, whereas it remained lower (around
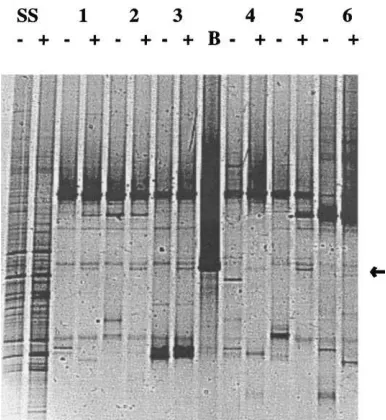
Fig. 6. DGGE patterns obtained directly from uninoculated (−) and inoculated (+) soil, and from six wells of the Biolog GN system inoculated with the microbial community from these soils. SS: DGGE fingerprints of the bacterial communities in uninoculated and inoculated soils (30 days); 1–6: profiles of selected microbial communities in Biolog wells. 1, Dextrin; 2, glycogen; 3, citric acid; 4,p-hydroxy-phenylacetic acid; 5, lactic acid; 6, asparagine and B, profile of strain A1501R.
105CFU per milliliter) in wells containing four other substrates (dextrin, glycogen, citric acid, p-hydroxy phenylacetic acid). In the asparagine-containing well, strain A1501R was not found. Hence, A1501R was a dominant strain in the lactic acid-utilizing bacterial community, whereas it was a minor player in those degrading the other substrates.
Fig. 6 provides, next to the soil-derived PCR–DGGE fingerprints, the fingerprints showing the effect of soil inoculation on microbial communities with po-tential to oxidize the selected substrates. The DGGE profiles of the bacterial communities selected with the six substrates were quite different from those obtained directly with soil DNA in that a strongly re-duced number of bands was generally observed. For three substrates, i.e. dextrin, glycogen and citric acid, the profiles were similar between uninoculated and inoculated soils, with the exception of a consistent extra band from the inoculated soils which comi-grated with the band generated with strain A1501.
For p-hydroxyphenylacetic acid, this comigrating
band from inoculated soil was also observed in oth-erwise divergent profiles. Moreover, the lactic acid well representing the uninoculated soil showed only two strong bands, whereas those from the inoculated soils showed, next to other bands, one stronger band which comigrated with the A1501R-derived band. Therefore, the presence of strain A1501R affected the composition of the bacterial communities capa-ble of utilizing these five substrates, in particular lactic acid, since it made part of these functional communities.
Table 2
Bacterial populations in six selected wells of Biolog GN plates inoculated with bacterial suspensions obtained from soil from microcosms 30 days after the introduction of inoculant strain A1501Ra
Bacterial numbers in wells of Biolog GN microplates (CFU per milliliter) containing the following substrates
Dextrin Glycogen Citric acid P-hydroxy-phenylacetic acid Lactic acid Asparagine
Total counts 4.4×108 9.9×108 4.7×108 8.7×108 6.6×108 5.5×108
A1501R counts 2.0×105 3.0×105 2.1×105 1.6×107 7.8×108 <102
Percentage of A1501R in total 0.05 0.03 0.05 1.8 100 [0]
aBacterial density established before inoculation: total: 4×105 CFU per milliliter; A1501R: 3×102CFU per milliliter.
4. Discussion
As intensively farmed systems that remain flooded for most of the cropping season, rice paddy soils have unique ecological features. Reichardt et al. (1997) as-sumed that the microbial biomass in soils used for rice cropping plays a significant role as a passive nutrient pool as well as due to the presence of microbial cata-lysts that govern nutrient availability. In addition, these soils often become largely anoxic and methanogenic (Grosskopf et al., 1998), and gradients of oxygen can occur around the rice roots (Ueckert et al., 1990). Fur-thermore, in continuously cropped rice fields, nitro-gen depletion can occur (Reichardt et al., 1997), and nitrogen-fixing organisms might thus serve directly as major sources of nitrogen (Inubushi and Watanabe, 1986). In paddy soils, conditions for nitrogen fixa-tion by associative fixers indeed seem adequate due to frequently occurring low oxygen tensions and the availability of root-derived carbonaceous and other compounds (Lin and You, 1989; Ueckert et al., 1990). In China, associative nitrogen fixers, including the in-oculants based on strain A1501, have been experimen-tally applied to rice since 1989 (You and Zhou, 1991; You et al., 1995), without knowledge on the putative fate and potentially adverse effects of the inoculants used.
The results obtained in this study indicated that the introduced population ofA. faecalisA1501R reached a maximum of about 108CFU per gram of dry soil dur-ing the first two days in flooded bulk soils or 15 days in corresponding rhizosphere soils, and then showed a decay to a roughly stable level of about 106 CFU per gram of dry soil. The decay was also visible in the hybridization study of soil DNA with the specific V6 probe. In addition, the A1501R specific band in
the DGGE fingerprints obtained with total microbial community DNA from inoculated bulk or rhizosphere soils faded from initially strong bands to weak ones in the course of the experiment. Assuming, on the ba-sis of previous direct microscopic counts, a total size of the bacterial community in the order of 109 cells per gram of soil and considering the fact that down to 0.1% of the total population might still produce visible bands when analyzed via PCR–DGGE (Muyzer et al., 1996; Heuer and Smalla, 1997a,b), this agreed well with the data from the direct plating and soil DNA hy-bridization analyses. The most likely explanation for this contention is that all three detection methods pri-marily assessed the fate of culturable cells of strain A1501R, which declined slowly. Similar slow declines of inoculant bacteria in FSL soil have been found be-fore for fluorescent pseudomonads (van Overbeek et al., 1997). Also, Compeau et al. (1988) described this behavior for a range of rifampicin-resistant mutants of this bacterial group. Although the silt loam soil used is known to confer some protection to inoculant strains, progressive predation by protozoa is the likely cause of the declines observed (van Veen et al., 1997).
and the inculant’s response, as this will advance our understanding of the rhizospheric nitrogen fixation activity.
The PCR–DGGE fingerprinting was intended to un-ravel potential bacterial community shifts as a result of the inoculant release (Muyzer et al., 1996; Duineveld et al., 1998). However, the DGGE profiles obtained revealed a picture of great stability in the dominating bands, which presumably represent numerically dom-inant species (Heuer and Smalla, 1997a,b). This sta-bility was seen both in time, and when rhizospheres or bulk soils of inoculated versus uninoculated soils were compared. Therefore, we interpret the data as indica-tive of the stable presence, irrespecindica-tive of the presence or absence of the A1501R inoculant strain, of these major contributors to the patterns, as assessed on the basis of the resolving power of the PCR–DGGE sys-tem. Similar observations have been recently made by Duineveld et al. (1998) for natural bacterial popula-tions in the Chrysanthemum rhizosphere. The greater variability of patterns at the level of weaker bands, in particular in the rhizosphere-derived patterns, might indicate that the underlying minority populations were more variable. Introduction of strain A1501R thus did not impact the dominant members of the microbial communities in flooded FSL soil. Microbial commu-nities in active ecosystems may possess the capacity to maintain structural stability and consequently to blur effects of introduction of bacterial inoculant strains. Reichardt et al. (1997) also reported that a comparison between bulk and rhizosphere soil revealed no sig-nificant differences in microbial community structure in rice fields, as evidenced by comparing groups and ratios of phospholipid fatty acids (PLFA). However, in the current study an effect of time was noted in the rhizosphere samples, and this was indicative of popu-lation changes, at the weak band level, presumably as a result of root growth (Figs. 4B and 5B). Therefore, it is likely that, with the exception of the intended effect, an ecological factor such as (a change in) root exudation pattern has a stronger bearing on microbial community structure/activity than the addition of a single inoculant strain.
Biolog GN community-level substrate utilization analysis, by virtue of its resolving power on the ba-sis of the utilization of 95 sole carbon sources by the community, is a potentially adequate method to as-sess functional community shifts. Using the method,
metabolic fingerprints are generated which may serve as indicators for community structure (Garland, 1997). However, as the response to the substrates offered in the Biolog wells is only due to the activity of bacteria directly selected in the wells, the response measured does not assess in situ activity but should rather be re-ferred to as “potential activity”. In the current study, changes of the Biolog patterns were observed over time between control and inoculated soils; however, these differences were ephemeral and no trend was detectable. The introduction of strain A1501R thus did not produce a persistent impact on the potential functional diversity measured by Biolog. On the other hand, PCR–DGGE analysis of communities thriving in selected substrates (such as lactic acid) showed that there could be a drastic effect of the presence of strain A1501R in the community under conditions of selec-tion, as the populations selected in the Biolog wells were quite different between inoculated and uninocu-lated soils. In fact, in lactic acid wells, strain 1501R dominated the population obtained from the inocu-lated soil, whereas it was a contributor to the response on several other substrates (Fig. 6). Moreover, only a few bacterial types out of the total diversity in soil could thrive in the Biolog wells, which confirmed the above inference about the strong selection for quick adaptors to the copious substrates offered by Biolog. It has been shown that these responders often belong to (different subclasses of) the proteobacteria (Smalla et al., 1998). Even in the presence of such indigenous competitors, the inoculant strain was very capable of thriving on lactic acid, which underpins its competi-tiveness in the rice rhizosphere.
This study further demonstrated that the V6 region-based probe amplified with total genomic DNA of
A. faecalis strain 1501R showed a high degree of
as a unique cluster targeted by PCR/DGGE and the V6 based probe. The usefulness of the probe was, thus, twofold. On the one hand, it facilitated the de-tection of strain A1501R when present at sufficiently high density in soil, and, on the other hand, it al-lowed the detection of indigenous strains of this par-ticular cluster. The results of the dot blot experiment (Fig. 3A and B) illustrated this point. The level of cross-reacting strains in the FSL soil limited the use of the probe for detection of strain A1501R targets to lev-els of about 106cells per gram of soil or higher. Below this threshold, natural bacteria with similar sequences would be primarily detected. It would be interesting to understand the natural role of this bacterial group in rice paddy soils, and compare it to that of strain A1501.
As evidenced via the analysis of the community structure of the bacterial responders to a limited number of Biolog substrates (viz., lactic acid), the association of strain A1501R with rice seedlings may affect the potential functional diversity of the bacte-rial community in rice soils to a greater extent than their structural diversity. This is all the more striking given the potential abundance of lactic acid in the rice rhizosphere. Unfortunately, in this paper, data that directly link the presence of the inoculant with effects from the plant, i.e. the release of lactic acid, were not produced. Moreover, effects of the inoculant strain on the plant (such as growth promotion) were also not assessed. However, it may be assumed that the putative plant-beneficial effects of strain A1501R re-semble those reported in previous work in China (e.g. You et al., 1995). The current research on inoculant fate might provide the basis for an environmentally sound strategy for the application of genetically mod-ified derivatives of strain A1501 in (commercial) rice cropping in China.
Acknowledgements
We thank Anneke Keijzer-Wolters and Ludwina Lankwarden for excellent technical assistance, and Dr. L.S. van Overbeek for critically reading the manu-script. This work was supported by a grant from the Chinese and Dutch governments (Ministries of Agriculture) and by a grant from the National High Biotechnology Development (863) plan in China.
References
Akkermans, A.D.L., van Elsas, J.D., de Bruijn, F.J. (Eds.), 1995. Molecular Microbial Ecology Manual. Kluwer Academic Publishers, Dordrecht, the Netherlands.
Altschul, S.F., Gish, W., Miller, W., Myers, E.W., Lipman, D.J., 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403–410.
Compeau, G., Al-Achi, J.B., Platsouka, E., Levy, S.B., 1988. Survi-val of rifampin-resistant mutants ofPseudomonas fluorescens
and Pseudomonas putida in soil systems. Appl. Environ. Microbiol. 10, 2432–2438.
De Leij, F.A.A.M., Sutton, E.J., Whipps, J.M., Fenlon, J.S., Lynch, J.M., 1995. Impact of field release of genetically modified
Pseudomonas fluorescenson indigenous microbial populations of wheat. Appl. Environ. Microbiol. 61, 3443–3453. Duineveld, B.M., Rosado, A.S., van Elsas, J.D., van Veen, J.A.,
1998. Analysis of the dynamics of bacterial communities in the rhizosphere of Chrysanthemum via denaturing gradient gel electrophoresis (DGGE) and substrate utilization patterns. Appl. Environ. Microbiol. 64, 4950–4957.
Fujii, T., Huang, Y., Higashitani, A., Nishimura, Y., Iyama, S., Hirota, Y., Yoneyama, T., Dixon, R.A., 1991. Effect of inocula-tion withKlebsiella oxytocaandEnterobacter cloacaeon dinit-rogen fixation by rice–bacteria associations. In: You, C.B. (Ed.), The Associative Nitrogen Fixation in the Rice Rhizosphere. Agricultural Publishing House, Beijing, pp. 363–371. Plant Soil 103 (1987) 221–226.
Garland, J.L., 1997. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 24, 289–300.
Garland, J.L., Mills, A.L., 1991. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level-sole-carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–2359.
Grosskopf, R., Janssen, P.H., Liesack, W., 1998. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl. Environ. Microbiol. 64, 960–969.
Heuer, H., Smalla, K., 1997a. Application of denaturing gradient gel electrophoresis and temperature gradient gel electrophoresis for studying soil microbial communities. In: van Elsas, J.D., Trevors, J.T., Wellington, E.M.H. (Eds.), Modern Soil Microbiology. Marcel Dekker, New York, pp. 353–370. Heuer, H., Smalla, K., 1997b. Evaluation of community-level
cata-bolic profiling using BIOLOG microplates to study community changes in potato phyllosphere. J. Microbiol. Meth. 30, 49– 61.
Heuer, H., Hartung, K., Wieland, G., Kramer, I., Smalla, K., 1999. Polynucleotides that target a hypervariable region of 16S rRNA genes to identify bacterial isolates corresponding to bands of community fingerprints. Appl. Environ. Microbiol. 65, 1045– 1049.
Insam, H., 1997. A new set of substrates proposed for community characterization in environmental samples. In: Insam, H., Rangger, A. (Eds.), Microbial Communities-functional Versus Structural Approaches. Springer, Berlin, pp. 289–260. Inubushi, K., Watanabe, I., 1986. Dynamics of available nitrogen
in paddy soils. II. Mineralized N of chloroform-fumigated soil as a nutrient source for rice. Soil Sci. Plant Nutr. 32, 561– 577.
Knight, B.P., McGrath, S.P., Chaudri, A.M., 1997. Biomass carbon measurements and substrate utilization patterns of microbial populations from soils amended with cadmium, copper, or zinc. Appl. Environ. Microbiol. 63, 39–43.
Lane, D.J., 1991. 16S/23S rRNA sequencing. In: Stackebrandt, E., Goodfellow, M. (Eds.), Nucleic Acid Techniques in Bacterial Systematics. Wiley, New York, pp. 115–175.
Lin, M., 1997. Field release of genetically modified associative diazotrophs in China. China-EC Newslett. 11, 14–15. Lin, M., You, C., 1989. Root exudates of rice (Oryza sativaL.)
and their interaction withAlcaligenes faecalis. Sci. Agric. Sin. 22, 6–12.
Lin, M., Ping, S.Z., You, S.B., 1992. Effect of inoculation with
Alcaligenes faecalison excretion of protons by rice roots and microecology of the rice rhizosphere. Acta Phytophysiol. Sin. 18, 233–238.
Muyzer, G., Hottentrager, S., Teske, A., Wawer, C., 1996. Denaturing gradient gel electrophoresis of PCR-amplified 16S rDNA — a new molecular approach to analyse the genetic diversity of mixed microbial communities. In: Akkermans, A.D.L., van Elsas, J.D., de Bruijn, F.J. (Eds.), Molecular Microbial Ecology Manual 3.4.4. Kluwer Academic Publishers, Dordrecht, the Netherlands, pp. 1–23.
Myers, R.M., Maniatis, T., Lerman, L.S., 1987. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Meth. Enzymol. 155, 501–527.
Reichardt, W., Mascarina, G., Padre, B., Doll, J., 1997. Microbial communities of continuously cropped, irrigated rice fields. Appl. Environ. Microbiol. 63, 233–238.
Sambrook, J., Fritsch, E.F., Maniatis, T., 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Smalla, K., Cresswell, N., Mendonca-Hagler, L.C., Wolters, A., van Elsas, J.D., 1993. Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification. J. Appl. Bacteriol. 74, 78–85.
Smalla, K., Wachtendorf, U., Heuer, H., Liu, W.-T., Forney, L., 1998. Analysis of BIOLOG GN substrate utilization patterns by microbial communities. Appl. Environ. Microbiol. 64, 1220– 1225.
Ueckert, J., Hurek, T., Fendrik, I., Niemann, E.-G., 1990. Radial gas diffusion from roots of rice (Oryza sativaL.) and Kallar grass (Leptochloa fusca L. Kunth) and effects of inoculation withAzospirillum brasilenseCd. Plant Soil 122, 59–65. Ueda, T., Suga, Y., Yashiro, N., Matsuguchi, T., 1995. Remarkable
N2-fixing bacterial diversity detected in rice roots by molecular
evolutionary analysis. J. Bacteriol. 177, 1414–1417.
van Elsas, J.D., Smalla, K., 1995. Extraction of microbial community DNA from soils. In: Akkermans, A.D.L., van Elsas, J.D., de Bruijn, F.J. (Eds.), Molecular Microbial Ecology Manual 1.3.3. Kluwer Academic Publishers, Dordrecht, the Netherlands, pp. 1–11.
van Elsas, J.D., Wolters, A., 1995. Polymerase chain reaction (PCR) analysis of soil microbial DNA. In: Akkermans, A.D.L., van Elsas, J.D., de Bruijn, F.J. (Eds.), Molecular Microbial Ecology Manual 2.7.2. Kluwer Academic Publishers, Dordrecht, the Netherlands, pp. 1–10.
van Elsas, J.D., Hekman, W., van Overbeek, L.S., Smit, E., 1991. Problems and perspectives of the application of genetically engineered microorganisms to soil. Trends Soil Sci. 1, 373– 392.
van Elsas, J.D., Mäntynen, V., Wolters, A.C., 1997. Soil DNA extraction and assessment of the fate of Mycobacterium chlorophenolicum strain PCP-1 in different soils by 16S ribosomal RNA gene sequence based most-probable-number PCR and immunofluorescence. Biol. Fert. Soils 24, 188–195. van Elsas, J.D., Duarte, G.F., Rosado, A.S., Smalla, K.,
1998. Microbiological and molecular biological methods for monitoring microbial inoculants and their effects in the soil environment. J. Microbiol. Meth. 32, 133–154.
van Overbeek, L.S., van Veen, J.A., van Elsas, J.D., 1997. Induced reporter gene activity, enhanced stress resistance, and competitive ability of a genetically modified Pseudomonas fluorescensstrain released into a field plot planted with wheat. Appl. Environ. Microbiol. 5, 1965–1973.
van Veen, J.A., van Overbeek, L.S., van Elsas, J.D., 1997. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 61, 121–135.
Winding, A.K., 1994. Fingerprinting bacterial soil communities using Biolog microtiter plates. In: Ritz, K., Dighton, Giller, K.E. (Eds.), Beyond the Biomass: Compositional and Functional Analysis of Soil Microbial Communities. Wiley, Chichester, UK, pp. 85–94.
Wunsche, L., Bruggemann, L., Babel, W., 1995. Determination of substrate utilization patterns of soil microbial communities: an approach to assess population changes after hydrocarbon pollution. FEMS Microbiol. Ecol. 17, 295–306.
You, C.B., Zhou, F.Y., 1989. Non-nodular nitrogen fixation in wetland rice. Can. J. Microbiol. 35, 403–408.
You, C.B., Zhou, F.Y., 1991. Non-nodular endorhizospheric nitrogen fixation in wetland rice. In: You, C.B. (Ed.), The Associative Nitrogen Fixation in the Rice Rhizosphere. Agricultural Publishing House, Beijing, pp. 305–314. You, C.B., Song, W., Wang, H.X., Li, J.P., Lin, M., Hai, W.L.,
1991. Association of Alcaligenes faecalis with wetland rice. Plant Soil 137, 81–85.
You, C.B., Lin, M., Fang, X.J., 1995. Field release of genetically engineered associative diazotrophs and its risk assessment. J. Agric. Biotechnol. 1, 34–41.