Effects of age, gender, and lifestyle factors on plasma
apolipoprotein A-IV concentrations
Zhiyong Sun
a, Ilona A. Larson
a, Jose M. Ordovas
a, James R. Barnard
b,
Ernst J. Schaefer
a,*
aLipid Metabolism Laboratory,Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts Uni6ersity,711 Washington Street, Boston,MA 02111, USA
bDepartment of Medicine,Laboratory of Kinesiology,Di6ision of Clinical Nutrition,Uni6ersity of California,Los Angeles,CA, USA
Received 17 February 1999; received in revised form 31 August 1999; accepted 15 September 1999
Abstract
Apolipoprotein (apo) A-IV is a protein component of triglyceride (TG)-rich lipoproteins and high density lipoproteins (HDL). Plasma apo A-IV levels were measured by immunoelectrophoresis and these values were related to other biological variables in 723 middle aged and elderly men and women (more than 90% of them were Caucasian) prior to participation in a lifestyle modification program. Apo A-IV may play an important function in regulating lipid absorption, reverse cholesterol transport, and food intake. The data are consistent with the following concepts: (1) apo A-IV levels are significantly and positively correlated with age (r=0.159,PB0.05) in all subjects, with plasma apo A-I levels in both men (r=0.194,PB0.001) and women (r=0.213,
PB0.001), and with apo E (r=0.111, PB0.05) and TG levels (r=0.120, PB0.05) in men; (2) apo A-IV levels are inversely correlated with body mass index (r=0.170, PB0.05) in women; (3) female subjects on hormone replacement therapy have significantly lower plasma apo A-IV levels (by 4.1%,PB0.05) than normal controls; (4) diabetic subjects have significantly higher apo A-IV levels (by 21%,PB0.01) than normal subjects; (5) there is no significant effect of smoking, alcohol intake, and apo A-IV-1/2 genotype on apo A-IV levels. The data indicate that plasma apo A-IV levels are significantly affected by age, diabetes, and hormone replacement therapy. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Lipoprotein; Apolipoprotein A-IV; Population study; Diabetes; Hormone replacement therapy
www.elsevier.com/locate/atherosclerosis
1. Introduction
Human apolipoprotein (apo) A-IV is an apolipo-protein synthesized in the intestine. Since its discovery in the late 1970s [1], many studies have been done to elucidate its physiologic and biochemical functions, ge-netic variations, and metabolism. The function of this apolipoprotein and its association with disorders such as coronary heart disease and Alzheimer’s disease are not completely understood. It has been suggested that apo A-IV may facilitate and/or mediate lipid absorp-tion, transport, and utilization. Apo A-IV synthesis and secretion increase after consuming a meal, especially one high in fat [2 – 4]. Weinberg et al. [5] reported that
plasma apo A-IV concentrations increased with in-creases in dietary fat content, in a dose-dependent manner. Animal studies have shown stimulation of apo A-IV synthesis in response to graded doses of dietary fat [6 – 10]. Apo A-IV is a critical protein component of chylomicrons, and when chylomicrons enter the circula-tion, they exchange apolipoproteins with high density lipoproteins (HDL) by picking up apo Cs and E from HDL and donating apo A-IV to HDL [11,12]. Further-more, apo C-II activates lipoprotein lipase (LPL) en-abling LPL-mediated TRL lipid hydrolysis, while apo E binds to specific receptors in the liver and other organs to facilitate TRL particle clearance. Apo A-IV may also participate in reverse cholesterol transport (RCT). It has been proposed that apo A-IV is involved in RCT by activating lecithin: cholesterol acyltransferase (LCAT) [13 – 15], enhancing cholesterol ester transfer protein (CETP) activity [16], and facilitating cholesterol * Corresponding author. Tel.:+1-617-556-3100; fax:+
1-617-556-3103.
E-mail address:[email protected] (E.J. Schaefer)
efflux from cells in different tissues into HDL [17 – 19]. Data also exists suggesting that apo A-IV may be involved in the regulation of food consumption [20]. Studies in rats have documented that apo A-IV infusion and injection can reduce food intake [21 – 23]. While the physiological mechanism of this effect is not clear, it has been suggested that apo A-IV may enter the central nervous system (CNS) to perform this function. Both serum and cerebrospinal fluid apo A-IV levels increase markedly as a result of lipid consumption [22 – 24]. Apo A-IV may inhibit gastric acid secretion in rats and reduce the severity of gastric ulceration by a mechanism involving the CNS [25,26]. Moreover, in a transgenic study by Duverger et al. [27], it was reported that apo A-IV had arteriosclerosis-protective potential in human apo A-IV gene transgenic mice. In another transgenic mouse study by Qin et al. [28], an antioxidant function of apo A-IV was noted.
The effects of age, gender, and lifestyle factors (smoking, alcohol consumption, and use of medication for diabetes, cholesterol-lowering, thyroid disease, or hormone replacement therapy) on human plasma apo A-IV have not been well studied. Available data on relationships between plasma apo A-IV and various physiological parameters (age, gender, BMI, percent body fat, girth, blood glucose, and lipid levels) are reviewed in this manuscript. Elucidating such effects and relationships is helpful for understanding lipid metabolism and its links to coronary heart disease (CHD). Thus far, little is known about the relationship between apo A-IV and other apolipoproteins, how life style influences apo A-IV levels, and what impact health/medication status has on plasma apo A-IV lev-els. In order to further elucidate apo A-IV physiology, the influences of biological variables on human plasma
apo A-IV levels were assessed in 723 participants in the present study.
2. Subjects and methods
Seven hundred and twenty-three human subjects, who attended a residential lifestyle intervention pro-gram (The Pritikin Longevity Center, Santa Monica, CA) as previously reported [29], participated in this study. The participants consisted of 372 males and 351 females, respectively. More than 90% of them were Caucasians. Their physiological parameters of age, BMI (weight (kg)/height (m)2
), percent body fat, girth, and lipid profile are summarized in Table 1. Of all subjects, 10% were current smokers, and 74.6% con-sumed alcohol more than one drink/per week. With regard to the health status of these subjects, 10% were on medication for diabetes, 16.5% were on cholesterol-lowering medication, 14.8% were on thyroid medica-tion, and 35.9% of female subjects were on hormone replacement therapy.
Fasting blood samples were drawn from all subjects at the time of entry into the program (baseline) and were stored in 10 ml tubes containing either SST clot-activating gel (Bectin-Dickinson vacutainer system) for lipid and glucose measurements, or 0.1% EDTA for apolipoprotein measurements. Samples for lipid and glucose measurements were allowed to clot, and then were further centrifuged for 15 min at 2500 rpm to isolate serum. The total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), triglyceride (TG), and glucose levels were measured by standardized auto-mated enzymatic methods (Smith-Kline Beecham Labo-ratories). The low density lipoprotein cholesterol (LDL-C) was calculated by subtracting the HDL-C and TG/5 (an estimation of TG-rich lipoprotein cholesterol) from TC, as described by Friedewald et al. [30]. For samples with TG levels over 400 mg/dl, their TG-rich lipoprotein cholesterol levels were determined by ultra-centrifugation using the method reported by Havel et al. [31].
Plasma samples for apolipoprotein measurement were obtained by centrifugation of whole blood at 2500 rpm for 30 min. Plasma apo A-IV levels were measured by immunoelectrophoresis using a commercially avail-able kit (HYDRAGEL apo A-IV, Sebia, France). Plasma apo E levels were measured by an enzyme linked immunosorbent assay, also using a commercially available kit obtained from the Perimmune Corpora-tion, Rockville, MD. Plasma apo A-I and apo B100 were determined by immunoturbidimetric assays with a Spectrum CCX analyzer (Abbott Diagnosis) [32,33]. Within and between measurements, the coefficients of variation for all assays were B10%. For identification of apo A-IV-1/2 polymorphism, genomic DNA was Table 1
Biological characteristics of subjects (mg/dl, mean9S.D.)
Pvaluesa
bPercentage of body fat, estimated by skin-fold measurement
[51,52].
cIn inches.
Table 2
Effects of gender, smoking and alcohol on the apolipoprotein (apo) A-IV Levels
On-medication subjects
Normal subjects All subjects
Levels (mg/dl) Pvaluea n Levels (mg/dl) Pvalue n
n Levels (mg/dl) Pvalue
14.794.1 316 15.395.3
Total 407 723 15.094.6
Gender
14.994.1 123 16.696.1
249
Males 372 15.594.9
158
Females 14.594.0 nsb 193 14.494.5 B0.001 351 14.494.3 B0.01
Smoking
14.894.0 21
Yes 52 15.694.8 73 14.994.0
No 349 14.693.6 ns 293 15.295.3 ns 650 15.094.7 ns
Alcohol intake
15.194.05 231 15.194.90
318
Yes 549 14.894.45
89
No 14.794.09 ns 85 15.796.15 ns 174 15.495.18 ns
at-TestPvalue.
bNot statistically significant.
Table 3
The effects of disease/medication status on apolipoprotein (apo) A-IV levelsa
Diabetes(b)b CLM(c)b TM(d)b HRT(e)b Pvalues
Normal(a)
18.297.7 16.796.0
Males 14.894.7 17.195.8
(n=51)ba** (n=77) (n=24) – B0.001
(n=249)ab**
17.094.3 14.393.2 14.894.3
14.594.0 13.994.5
Females
(n=20)ba*,bc*,be** (n=42)cb* (n=82) (n=127)ea*,eb**
(n=158)ab* B0.01
17.897.4 15.995.3 15.494.7 14.794.1
All subjects
(n=71)ba**,bc**,bd** (n=119)bc** (n=106)bd** –
(n=407)ab** B0.001
a a,b,c,d,eThe different superscript letters represent that a statistically significant difference exists between the two means.
bSubject groups, Diabetes=on diabetic medication, CLM=on cholesterol lowering medication, TM=on thyroid medication, and HRT=on
hormone replacement therapy. *PB0.05;
**PB0.01; data analyzed by general linear models (GLM) procedure.
isolated from whole blood using the QIA amp Blood Kit (Qiagen). The 360 bp polymorphism within the apo A-IV gene was assessed as previously described by Tenkanen et al. [34].
SAS 6.12 and Systat 7.0 (SPSS) statistical programs were used to carry out hypothesis testing, correlation and regression analysis. A statistical P value less than 0.05 was considered as a significant boundary.
3. Results
The lipid and apolipoprotein concentrations as well as glucose levels of the study subjects are summarized in Table 1. With the exception of LDL-C, all of these variables differed significantly between males and fe-males. The apo A-IV plasma levels of different subject groups and the influence of gender, smoking, and alco-hol consumption on plasma apo A-IV concentration are shown in Table 2. No significant gender differences in apo A-IV levels were observed in normal subjects. However, for all subjects, the mean apo A-IV concen-tration of males (15.594.9 mg/dl) was significantly
higher than that of females (14.494.6 mg/dl) (PB 0.01). The gender difference in apo A-IV levels was also observed in the subjects on different types of medica-tions (16.696.1 mg/dl for males versus 14.494.5 mg/ dl for females, PB0.001). No significant effect of apo A-IV-1/2 polymorphism on apo A-IV levels was noted in either males or females. There was no significant effect of smoking and alcohol consumption on plasma apo A-IV concentrations.
medica-tion. Use of cholesterol-lowering medication or thyroid medication was not associated with any significant ef-fect on plasma apo A-IV.
To assess the relationships between apo A-IV plasma concentrations and other variables, multivariate regres-sion models were employed. After continuity tests for non-category variables, univariate correlation/ regres-sion screening was conducted. Then, the linear relation-ships between apo A-IV and selected variables were determined by multiple linear regression models. The results of these analyses are summarized in Table 4. After adjustment for age, gender, BMI, percent body fat, smoking, alcohol consumption, and health/ medica-tion status, the main variables which correlated signifi-cantly with apo A-IV concentrations were age and apo A-I levels. A correlation was also observed between apo A-IV and apo E in all subjects, in male subjects, and in subjects on medication both before and after adjust-ment. A weakly significant correlation between TG levels and apo A-IV levels was observed in male sub-jects only. A significant inverse correlation between plasma apo A-IV levels and BMI was noted in women, but it was not observed in men. After adjustment for other variables the correlation between apo A-IV levels and HDL-C in women was no longer significant. The correlation between apo A-IV and glucose also disap-peared after multiple adjustment.
4. Discussion
Plasma lipoprotein levels are clearly related to the risk of cardiovascular disease. Apo A-IV is one of the critical apolipoproteins within TRL and HDL, and has been implicated in multiple steps of lipid metabolism as mentioned above. The present study has examined the relationships between plasma apo A-IV levels and age, gender, BMI, percent body fat, smoking, alcohol con-sumption, apo A-I, apo E, TG, TC, LDL-C, HDL-C, glucose and health/medication status. The mean plasma
apo A-IV levels in the study population for men, women, and all subjects are 15.594.9, 14.494.3, and 15.094.6 mg/dl, respectively. These values are similar to the values reported by Menzel et al. (16.598.5 mg/dl,n=180), Ehnholm et al. (14.991.6, mg/dl), and Li et al. (17.296.5 mg/dl, n=285). In all subjects on medication, there was a gender effect with male subjects having significantly higher apo A-IV concentrations than female subjects. However, the gender difference was not seen in subjects off medication. An explanation for this result is that diabetic subjects had increased apo A-IV levels and the majority of these subjects were male. Conversely, subjects on hormone replacement therapy had decreased apo A-IV levels, all of whom were female (Table 3). Therefore, diabetes and hor-mone replacement therapy were largely responsible for causing the observed gender differences in apo A-IV levels in the entire study population. After excluding the diabetic and on hormone replacement therapy sub-jects, the gender difference was no longer observed between any two groups. Ehnholm et al.[35] reported that males had significantly higher apo A-IV concentra-tions by 16% than did females. The subjects in Ehn-holm’s study were university students in Europe with an age range of 18 – 26 years old and a large proportion of the female subjects were using oral contraceptives. The female subjects who used contraceptives had sig-nificantly lower apo A-IV levels by 17% than the values of other female subjects. These data suggest that the gender differences observed in the study of older sub-jects and Ehnholm’s study of university students may have been partly due to hormonal therapy.
The impact of health/medication status on apo A-IV levels is summarized in Table 3. Apo A-IV levels of the different subject groups were compared using GLM analysis with adjustment for age. It was observed that diabetic subjects had significantly higher apo A-IV lev-els than did normal subjects for the male, female, and total groups. Verges et al. [36,37] and Attia et al. [38] have also reported that diabetic patients have increased
Table 4
The Correlation/regressions between apolipoprotein (apo) A-IV and selected variablesa
Normal subjects
All subjects Males Females On-medication subjects
(n=407) (n=351)
(n=723) (n=372) (n=316)
0.159**b 0.158*
Age 0.176** 0.181** 0.128*
0.20** 0.194**
Apo A-I 0.213** 0.236** 0.198**c
0.103** 0.111**
Apo E 0.035 0.044 0.186**
−0.053
Triglyceride 0.064 0.120** 0.039 0.089
0.001
HDL-C 0.009 0.128* 0.089 0.094
Glucose 0.176* 0.162* 0.164* 0.053 0.237*
BMI 0.065 0.051 −0.170** 0.066 0.060
aThe numbers in table are univariate correlation coefficients.
bBoth * and ** representPB0.05, and ** also representsPstillB0.05 after adjustment by age, gender, BMI, percentage of body fat, smoking,
alcohol intake, and health/medication status.
Table 5
Apolipoprotein (apo) A-IV levels in diabetes and controls (mean9
S.D., mg/dl)
Reference Diabetes Controls Pvalues
Verges1997[37]
Females 11.993.5 B0.001
(n=40) (n=50)
Attia1997[38]
27.091.0
Total subjects 13.093.0 B0.001 (n=22)
In correlation analysis, weakly independent signifi-cant correlations were found between apo A-IV and the following variables: apo A-I, apo E, age, TG levels, and BMI (Table 4). It was also found that glucose levels and HDL-C were univariately correlated with apo A-IV in certain subject groups. However, these univariate correlations disappeared after multiple adjustments, in contrast to the former variables. The significant inde-pendent correlation between apo A-I and apo A-IV may be explained by the following: (1) apo A-IV can associate with apo A-I containing particles (even though the content and range of the association remain discrepant [3,39 – 41]); (2) the gene expression of apo A-I and apo A-IV may be coordinately regulated since these genes are located in the same gene cluster on chromosome 11 [42 – 45]; and (3) apo A-IV and apo A-I may share common receptors on cell membrane for their clearance [18], and therefore their fractional catabolic rates may be linked.
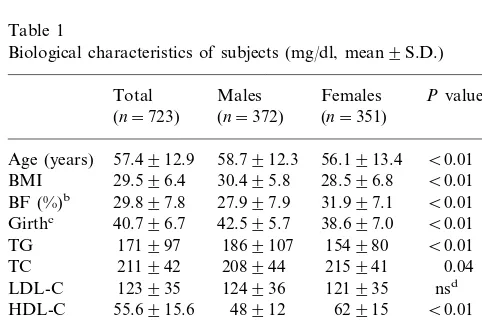
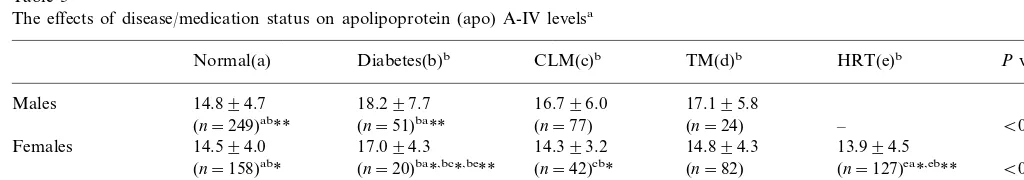
In the present study, a weak but significant correla-tion was noted between age and apo A-IV levels, the cause of which is not clear. It may involve changes in body composition with aging, which could affect apolipoprotein turnover rates, including that of apoA-IV. Schaefer et al. [46] documented that plasma apo B levels increased following aging in their population study. Millar et al. [47] reported that the fractional catabolism of human LDL apo B100 decreased with aging, resulting in increased plasma apo B100 levels in older subjects. Thus, the correlation between age and apo A-IV may be due to the normal aging process. Age was also significantly correlated with apo A-I levels both in univariate and multivariate regression models (PB0.001 in both models) and apo A-I was also significantly correlated with apo A-IV. These data indi-cate co-correlations among these three variables. The disappearance of the age-apo A-IV correlation after adjustment in male subjects and subjects on medication suggests that health/medication status can affect apo A-IV levels. This adjusted effect was further modified by the finding that diabetic subjects in both groups had correlation trend-lines significantly opposite that of the normal group, as shown in Figs. 1 and 2. These oppos-ing effects serve to neutralize the correlation relation-ships between age and apo A-IV levels. In all subjects and in females, this opposing effect was not strong enough to eliminate the correlation. Only 9.8% of all subjects and 5.7% of females were diabetic.
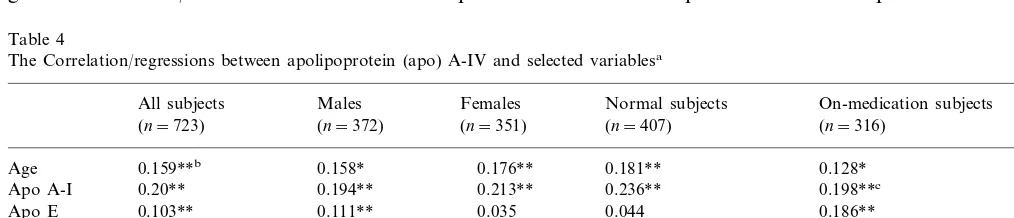
The correlation between apo E and apo A-IV levels was detected in all subjects, male subjects, and in subjects on medication. The existence of diabetes also played a major role in the correlation. If diabetic subjects are excluded from the groups mentioned above, the correlation between apo E and apo A-IV levels is no longer significant. Fig. 3 shows the correla-tion plot of all subject group sorted by diabetes. A apo A-IV levels (Table 5). The etiology of such
significant correlation was only present in diabetic sub-jects (r=0.256, P=0.03). Therefore, the presence of diabetics contributes greatly to the significant correla-tion between apo E and A-IV levels.
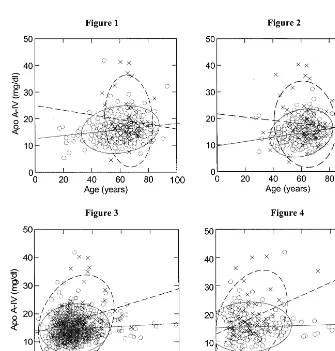
The relationship between TG and apo A-IV is con-troversial. Lagrost et al. [48] reported that there was a significant linear correlation (r=0.61, PB0.001) be-tween the concentrations of plasma apo A-IV and TG. Verges [41] reported that hypertriglyceridemia resulted in elevated plasma apo A-IV levels. However, Ghiselli et al. [49] and Utermann et al. [50] found that plasma apo A-IV levels were identical in normolipidemic and hypertriglyceridemic subjects. In the current study, it was found that the correlation between the two
vari-ables existed only in the male subjects. The reason for this result is, at least partially, that diabetic subjects had increased apo A-IV and TG levels and most of them were found in the male subject group (51/71). Fig. 4 shows the correlation plot between apo A-IV and TG levels for the two types of subjects. The matched trend lines have reversed directions, indicating that the corre-lation is present only in diabetic subjects (r=0.338,
PB0.001), but it does affect entire male subject group resulting in a significant correlation between TG and apo A-IV concentrations. The correlation between TG and apo A-IV was also reported by Verges et al. [36] in their study of diabetics. With regard to the relationship of glucose and apo A-IV levels, a univariate correlation
Fig. 1. Correlation plots. The crosses and dashed lines represent diabetic subjects, the rings and solid lines represent non-diabetic subjects. The circles cover 90% of subjects of represented groups. In all figures, diabetic subjects have significantly different line slopes compared to non-diabetic subjects, for male subjects.
Fig. 2. Correlation plots. The crosses and dashed lines represent diabetic subjects, the rings and solid lines represent non-diabetic subjects. The circles cover 90% of subjects of represented groups. In all figures, diabetic subjects have significantly different line slopes compared to non-diabetic subjects, for on-medication subjects.
Fig. 3. Correlation plots. The crosses and dashed lines represent diabetic subjects, the rings and solid lines represent non-diabetic subjects. The circles cover 90% of subjects of represented groups. In all figures, diabetic subjects have significantly different line slopes compared to non-diabetic subjects, for all subjects.
was found in all groups except the normal subject group. However, after the exclusion of diabetic subjects and/or multiple adjustment, all significant correlation disappeared. These findings further demonstrate the influence of diabetes on plasma apo A-IV levels.
In summary, in the present population study we found that plasma apo A-IV levels were positively correlated with age and plasma apo A-I levels. More-over, it was noted that diabetic subjects had signifi-cantly higher apo A-IV levels than controls, and that their plasma TG levels were correlated with apo A-IV levels. These data indicate that diabetes can have a significant effect on apo A-IV metabolism. Female hor-mone replacement therapy has a lowering effect on plasma apo A-IV levels. No influence of apo A-IV-1/2 polymorphism, cholesterol lowering medication, smok-ing, and alcohol consumption on plasma apo A-IV was observed. Plasma glucose levels, HDL-C levels, and apo B concentrations were also not independently corre-lated with plasma apo A-IV levels. The present study indicates that age, apo A-I, hormone replacement ther-apy, and diabetes are significant factors affecting apo A-IV metabolism.
References
[1] Weisgraber KH, Bersot TP, Mahley RW. Isolation and charac-terization of an apoprotein from the d less than 1.006 lipo-proteins of human and canine lymph homologous with the rat A- IV apoprotein. Biochem Biophys Res Commun 1978;85:287 – 92.
[2] Green PH, Glickman RM, Riley JW, Quinet E. Human apolipo-protein A-IV. Intestinal origin and distribution in plasma. J Clin Invest 1980;65:911 – 9.
[3] Bisgaier CL, Sachdev OP, Megna L, Glickman RM. Distribu-tion of apolipoprotein A-IV in human plasma. J Lipid Res 1985;26:11 – 25.
[4] Miyata Y, Koga S, Ibayashi H. Alterations in plasma levels of apolipoprotein A-IV in various clinical entities. Gastroenterol Jpn 1986;21:479 – 85.
[5] Weinberg RB, Dantzker C, Patton CS. Sensitivity of serum apolipoprotein A-IV levels to changes in dietary fat content [see comments]. Gastroenterology 1990;98:17 – 24.
[6] Delamatre JG, Roheim PS. The response of apolipoprotein A-IV to cholesterol feeding in rats. Biochim Biophys Acta 1983;751:210 – 7.
[7] Apfelbaum TF, Davidson NO, Glickman RM. Apolipoprotein A-IV synthesis in rat intestine: regulation by dietary triglyceride. Am J Physiol 1987;252:G662 – 6.
[8] Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 1990;31:1613 – 25. [9] Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV
in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol 1992;262:G1002 – 6.
[10] Kalogeris TJ, Fukagawa K, Tso P. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J Lipid Res 1994;35:1141 – 51.
[11] Goldberg IJ, Scheraldi CA, Yacoub LK, Saxena U, Bisgaier CL. Lipoprotein ApoC-II activation of lipoprotein lipase. Modula-tion by apolipoprotein A-IV. J Biol Chem 1990;265:4266 – 72.
[12] Weinberg RB, Spector MS. Human apolipoprotein A-IV: dis-placement from the surface of triglyceride-rich particles by HDL2-associated C-apoproteins. J Lipid Res 1985;26:26 – 37. [13] Chen CH, Albers JJ. Activation of lecithin: cholesterol
acyltrans-ferase by apolipoproteins E-2, E-3, and A-IV isolated from human plasma. Biochim Biophys Acta 1985;836:279 – 85. [14] Emmanuel F, Steinmetz A, Rosseneu M, Brasseur R, Gosselet
N, Attenot F, Cuine S, Seguret S, Latta M, Fruchart JC, et al. Identification of specific amphipathic alpha-helical sequence of human apolipoprotein A-IV involved in lecithin:cholesterol acyl-transferase activation. J Biol Chem 1994;269:29883 – 90. [15] Steinmetz A, Utermann G. Activation of lecithin: cholesterol
acyltransferase by human apolipoprotein A-IV. J Biol Chem 1985;260:2258 – 64.
[16] Main LA, Ohnishi T, Yokoyama S. Activation of human plasma cholesteryl ester transfer protein by human apolipoprotein A-IV. Biochim Biophys Acta 1996;1300:17 – 24.
[17] Weinberg RB, Patton CS. Binding of human apolipoprotein A-IV to human hepatocellular plasma membranes. Biochim Bio-phys Acta 1990;1044:255 – 61.
[18] Steinmetz A, Barbaras R, Ghalim N, Clavey V, Fruchart JC, Ailhaud G. Human apolipoprotein A-IV binds to apolipoprotein A-I/A-II receptor sites and promotes cholesterol efflux from adipose cells. J Biol Chem 1990;265:7859 – 63.
[19] Eckardstein AV, Huang Y, Wu S, Sarmadi AS, Schwarz S, Steinmetz A, Assmann G. Lipoproteins containing apolipo-protein A-IV but not apolipoapolipo-protein A-I take up and esterify cell-derived cholesterol in plasma. Arterioscler Thromb Vasc Biol 1995;15:1755 – 63.
[20] Tso P, Liu M, Kalogeris TJ. The role of apolipoprotein A-IV in food intake regulation. J Nutr 1999;129:1503 – 6.
[21] Sparks CE, Tennenberg SD, Marsh JB. Catabolism of the apolipoproteins of HDL in control and nephrotic rats. Biochim Biophys Acta 1981;665:8 – 12.
[22] Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest 1993;91:1830 – 3.
[23] Fujimoto K, Machidori H, Iwakiri R, Yamamoto K, Fujisaki J, Sakata T, Tso P. Effect of intravenous administration of apolipoprotein A-IV on patterns of feeding, drinking and ambu-latory activity of rats. Brain Res 1993;608:233 – 7.
[24] Tso P, Chen Q, Fujimoto K, Fukagawa K, Sakata T. Apolipo-protein A-IV: a circulating satiety signal produced by the small intestine. Obes Res 1995;3(Suppl. 5):689S – 95S.
[25] Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Mechanism of action of intracisternal apolipoprotein A-IV in inhibiting gastric acid secretion in rats. Gastroenterology 1995;109:1583 – 8.
[26] Okumura T, Taylor IL, Fukagawa K, Tso P, Pappas TN. Apolipoprotein A-IV acts centrally in the brain to reduce the severity of gastric ulceration in the rat. Brain Res 1995;673:153 – 6.
[27] Duverger N, Tremp G, Caillaud JM, Emmanuel F, Castro G, Fruchart JC, Steinmetz A, Denefle P. Protection against athero-genesis in mice mediated by human apolipoprotein A-IV. Science 1996;273:966 – 8.
[28] Qin X, Swertfeger DK, Zheng S, Hui DY, Tso P. Apolipo-protein AIV: a potent endogenous inhibitor of lipid oxidation. Am J Physiol 1998;274:H1836 – 40.
[29] Barnard JR. Effects of life-style modification on serum lipids. Arch Intern Med 1991;151:1389 – 94.
[30] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499 – 502.
[32] Contois HJ, McNamara JR, Lammi-Keefe JC, Wilson FPW, Massov T, Schaefer EJ. Reference intervals for plasma apolipo-protein A-I determined with a standardized commercial im-munoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem 1996;42:507 – 14.
[33] Contois HJ, McNnamara RJ, Lammi-Keefe JC, Wilson FPW, Massov T, Schaefer JE. Reference intervals for plasma apolipo-protein B determined with a standardized commercial immuno-turbidimetric assay: results from the Framingham Offspring Study. Clin Chem 1996;42:515 – 23.
[34] Tenkanen H. Genotyping of apolipoprotein A-IV by digestion of amplified DNA with restriction endonuclease Fnu4HI: use of a tailored primer to abolish additional recognition sites during the gene amplification. J Lipid Res 1991;32:545 – 9.
[35] Ehnholm C, Tenkanen H, de Knijff P, Havekes L, Rosseneu M, Menzel HJ, Tiret L. Genetic polymorphism of apolipoprotein A-IV in five different regions of Europe. Relations to plasma lipoproteins and to history of myocardial infarction: the EARS study. European Atherosclerosis Research Study. Atherosclerosis 1994;107:229 – 38.
[36] Verges BL, Vaillant G, Goux A, Lagrost L, Brun JM, Gambert P. Apolipoprotein A-IV levels and phenotype distribution in NIDDM. Diabetes Care 1994;17:810 – 7.
[37] Verges BL, Lagrost L, Vaillant G, Petit JM, Cohen M, Gambert P, Brun JM. Macrovascular disease is associated with increased plasma apolipoprotein A-IV levels in NIDDM. Diabetes 1997;46:125 – 32.
[38] Attia N AT, Lahrichi M, Balafrej A, Kabbaj O, Girad-Globa A. Response of apolipoprotein A-IV and lipoproteins to glycaemic control in young people with insulin-dependent diabetes mellitus. Diabet Med 1997;14:242 – 7.
[39] Bisgaier CL, Sachdev OP, Lee ES, Williams KJ, Blum CB, Glickman RM. Effect of lecithin:cholesterol acyltransferase on distribution of apolipoprotein A-IV among lipoproteins of hu-man plasma. J Lipid Res 1987;28:693 – 703.
[40] Lagrost L, Gambert P, Boquillon M, Lallemant C. Evidence for high density lipoproteins as the major apolipoprotein A-IV-con-taining fraction in normal human serum. J Lipid Res 1989;30:1525 – 34.
[41] Verges B, Rader D, Schaefer J, Zech L, Kindt M, Fairwell T, Gambert P, Brewer HB. In vivo metabolism of apolipoprotein A-IV in severe hypertriglyceridemia: a combined radiotracer and stable isotope kinetic study. J Lipid Res 1994;35:2280 – 91.
[42] Vergnes L, Taniguchi T, Omori K, Zakin MM, Ochoa A. The apolipoprotein A-I/C-III/A-IV gene cluster: ApoC-III and ApoA-IV expression is regulated by two common enhancers. Biochim Biophys Acta 1997;1348:299 – 310.
[43] Metzger S, Levy Y, Arnon R, Chajek-Shaul T. Co-regulation of apo A-I, apo C-III and apo A-IV gene expression in human intestinal biopsies. Eur J Clin Invest 1996;26:71 – 5.
[44] Cohen JC, Wang Z, Grundy SM, Stoesz MR, Guerra R. Varia-tion at the hepatic lipase and apolipoprotein AI/CIII/AIV loci is a major cause of genetically determined variation in plasma HDL cholesterol levels. J Clin Invest 1994;94:2377 – 84. [45] Boguski MS, Birkenmeier EH, Elshourbagy NA, Taylor JM,
Gordon JI. Evolution of the apolipoproteins. Structure of the rat apo-A-IV gene and its relationship to the human genes for apo-A-I, C-III, and E. J Biol Chem 1986;261:6398 – 407. [46] Schaefer EJ, Lamon-Fava S, Cohn S, Ordovas JM, Castelli WP,
Wilson PW. Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study. J Lipid Res 1994;35:779 – 92.
[47] Millar JS, Lichtenstein AH, Cruchel M, Dolnikowski GG, Hachey DL, Cohn JS, Schaefer EJ. Impact of age on the metabolism of VLDL, IDL, and LDL apolipoprotein B100 in men. J Lipid Res 1995;36:1155 – 67.
[48] Lagrost L, Gambert P, Meunier S, Morgado P, Desgres J, d’Athis P, Lallemant C. Correlation between apolipoprotein A-IV and triglyceride concentrations in human sera. J Lipid Res 1989;30:701 – 10.
[49] Ghiselli G, Krishnan S, Beigel Y, Gotto AM Jr. Plasma metabolism of apolipoprotein A-IV in humans. J Lipid Res 1986;27:813 – 27.
[50] Utermann G, Beisiegel U. Apolipoprotein A-IV: a protein occur-ring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. Eur J Biochem 1979;99:333 – 43.
[51] Borkan GA, Hults DE, Cardarelli J, Burrows BA. Comparison of ultrasound and skinfold measurements in assessment of sub-cutaneous and total fatness. Am Phys Anthropol 1982;58:307 – 13.
[52] Martin AD, Ross WD, Drinkwater DT, Clarys JP. Prediction of body fat by skinfold caliper: assumptions and cadaver evidence. Int J Obes 1985;9:31 – 9.