PURIFICATION AND CHARACTERIZATION
OF
THEMOSTABLE CHITINASES FROM
BaciUus
licheni/ornris
MB-2
ARlS
TOHARISMAN
SCHOOL OF
GRADUATE PROGRAM
BOGOR AGRICULTURAL
UNIVERSITY
ABSTRACT
ARIS TOHARISMAN
.
Purification and Characterization of Themstable C h i t k s h m Bacillus Eicheniformis MB-2. Under the supervision of MAGGY T. SUHARTONO, ANTONIUS SUWANTO, and TRESNAWATI PURWADARSAChitinases are of great biotechnological interest and have received remarkable attention. A themophilic bacterium, Bacillus lichenifomis
MB-2,
from Tompaso hot spring, North Sulawesi Indonesia, secreted thermostable chitinases into culture media. Preliminary study showed that the enzymes occur in multiple formsand
were stable at a high temperature and a broad range of pH. The objectives of this research were to purify and characterize thermostable chitinasesfrom B. lichenrformis MB-2.
The extracellular chitinases were isolated by successive column
chromatographies on Phenyl Sepharose CL4B, DEAE Sephacel, and Superdex G-75. The purified enzymes had molecular weight of 67 (Chi-67) and 102 kDa
(Chi-102), estimated by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). Chi-67 and Chi-102
had
an endo- and exo- chitinolytic activities, respectively. The optimal temperature of Chi-67 was 70°C, whereas Chi-102 was 60"C.
The optimal pH for both enzymes was 6.0. Chi-67 wets stable below 60°C and over a broad pH range of 4-1 1 for 1 h. In contrast, Chi- 102 was not stable at temperature above 50 "C and only stable at its optimum pH for 1 h.Chi-47 was resistant to denaturation by urea, Tween-20 and Triton-X, but unstable toward organic solvents such as propanol, ethanol, DMSO (dimethyl sdfoxide), and PEG (polyethylene glycol), indicating that hydrophobic interaction of proteins plays an important role
in
maintaining enzyme activity. Ionic interactions are also important for Chi-67 fold as the activity was reduced by guanidine hydrochloride and NaCl (1 M). Chi- 1 02 was relatively more stable toward various organic solvents than Chi-67.Both enzymes hydrolyzed colloidal chitin, glycol chitin, or glycol chitosan, but were less active to regenerated chitin, fine powder of chitin, or methyl cellulose. The Michaelis constants &) for colloidal chitin, glycol chitin, 4- methylumbelliferyl N', W-diacetylchitobioside [MUF(GICNAC)~], 4- methyiumbelliferyl
N',
N', M-triacetylchitotrioside [MCTF(G~CNAC)~] for Chi-67were 3.08 mg ml-', 0.3 15 mg ml-', 0.02 pmol d-' and 0.02 pmol dl,
respectively. Meanwhile, the K, for colloidal chitin, glycol chitin and MUF(GlcNAc) for Chi-102 were 2.00 mg ml-', 1.32 rng ml-', and 0.03
m M
ml-',respectively. The first 13 N-tertninal amino
acids
of Chi-67 were determined to be SGKNYKIIGYYPS, which is identical to chitinase from B. licImenIfomrisPR-
DECLARATION
I hereby declare that this thesis is my own work and has not been accepted for the
award of any other degree or diplorna in any university or other institute of higher
leadng.
To the best of my knowledge and belief, this thesis contains no material previously published by any other person except where due acknowledgment has
been made.
Bogor, May 20U4
PURIFICATION AND CHARACTERIZATION
OF THERMOSTABLE CHITINASES FROM
BaciNus lichenifomis
MB-2
BY
ARIS TOHARISMAN
A DISSERTATION
Submitted
to Bogor Agricultural University
in partial fulfillment
of
the requirements for
the Doctorate Degree
in
Food Sciences
GRADUATE PROGRAM
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
Title
:Purification
and Characterization of
Thermostable
C
hitinases
from
BaciNus
lichen(ftorrnis
MB-2
Name
:Aris
Toharisman
Student
Number
:PO9600004
Study Program
:Food Sciences
Approved by:
1
.
Advisor BoardProf.
Dr.
Ir. Antonius Suwanto MemberDr.
Tresnawati Purwadaria MemtKr2. Head of Food Sciences Study Program of Graduate Program
BIOGRAPHY
Aris Toharisman was
born
on January 19, 1966 in Kuningan, West Java, thefourth of five children of M. Suwarman and Hj.
C,
Suwarman. The author finished his study at Senior High School (SMAN I Surnedang) in 1984 andstarted his undergraduate work at Bogor Agricultural University (IPB). The author received a Bachelor of Science degree
in
Agriculture from IPB and aMaster of Applied Science degree in Biotechnology from the University of New
South Wales Australia
in
1989 and 1997, respectively. Since 1989, the authorhas been working at the Indonesian Sugar Research Institute, involved in sugar-
industry waste treatment and its co-product utilization research. September 2000,
the author started his
PhD
program at Food Sciences Department ofPB,
with the scholarship from Indonesian Department of Agriculture.The
author had also got a scholarship for 5 months, March-July 2003, from Deutscher AkademischerAustausch Dienst (DAAD) to do Sandwich in Bioscience Programme at
Department of General Zoology and Endocrinology, the University of Ulm,
Germany. The author had done the project entitled Purification and
Foremost, I would like to express my sincere gratitude to my major supervisor:
Prof. Dr. Maggy T. Suhartono, for her thoughtful and invaluable guidance during
my study. It was a great pleasure to me to conduct research under her supervision. I am deeply indebted to my other supervisors, Prof. Dr. Antonius
Suwmto and Dr. Tresnawati Purwadaria, for their encouragement and
stimulaiing suggestions. Special thank is due to Prof. Margarethe Spindler-
Barth for having accepted me to do research at Department of General Zoology
and Endocrinology, UniversiGt Ulm, Germany. She gave me great help and
constant support during my work in her laboratow along the way.
I am grateful to all the staff and students of the Microbiology and
Biochemistry Laboratory, Research Center for Biotechnology, Bogor
Agricultural University: especially to ibu Ika Malikha, ibu Eni Sutnartini, ibu
Endang Yuli, ibu Rosita Lintang, pak Sumardi, ibu Yuyun, ibu Tati, mbak Nur Azizah, mbak Emma, Yanti Lim, Winda, Ace, Dim, teh Ida, Pudin, and Yanti
for their assistance and supports. I
thank
ibu Ekowati Chasanah for the cooperative spirit and fruitful discussion during my research.I am thankful to Prof. Dr. Margarethe Spindler-Bartb p u p especially to
Michael Wagemann, Natalja Mobius and Karin Dengler for dl their assistance on
laboratory works. Special
thank
goes to Dr. Yaya Rukayadi for sending mevaluable journals and other information and Dra Tami Indiyanti, MSc (Indonesian
I am grateful to both the Agricultural Department for the scholarship given to
me
and
tothe
Director of the Indonesian Sugar Research Institute for giving methe chance to study at the IPB. Also, I greatly appreciate hancial support fiom Bioproduct s Research Center, Y onsei University (Korea) and the scholarship
fiom Deutscher Akadernischer Austausch Dienst (DAAD).
Many thanks to my familes for their emotional and spiritual supports. I
must thank my wife. She has put a lot of enthusiasm and energy into eliminating
many short comings in various ways.
Many more persons participated in various ways to ensure my research succeeded and I am thankful to them all.
F W , I want to express my gratitude to
Allah
SWT whose blessings made this effort fruitful.LIST OF TABLES
...
Variation in chitin contents at d e r e n t organismsWorldwide market for chitin derived products in year 2002
...
...
Applications of chitin and its derivativesNomenclature of chitholytic and cellulolytic enzymes
...
...
Purification steps of some thermostable chithases
...
Properties of Some Bacterial C h i t h s
...
The pH stability of some chitinases
Activators
and
inhibitors of some kcterial chitinases...
...
Kinetic parameters of some chitinases...
Classification of extremophiles and example of applications of some their enzyme
Proposed structural thennostabiiizing mechanisms of protein
...
Chitinolyt ic index of the isolates fiom Tompaso grown at 5 S°C. .
Ammonium sulhte precipitation of chitinases produced...
by Bacillus licheniformis MB-2
Relative activity of crude-extract chitinases to wards
...
Various chit in substratesChit inase precipitation using 80% ammonium sulfate at
...
various pHYield
and
purity of chitimes treated with...
several methodsEffect of salts removal of precipitated chitinases
...
on enzyme yield and purityYield and purity ocf chitinases after binding
...
with 3 different substrates
....
Effect of heat treatment on pudlcation results of c h i t k s Protein recovery from HIC columns eluted with...
various conditions
...
Purification steps of endochitinase produced byB. lichenifomis MB-2
Purification steps of exochitinase produced by
...
B. lichenifomis MB-26-2 Relative activity of Chi-67 and Chi-102 towards
. . .
.
. .
.
. . ..
1 18 various chitin substrates6-3 Kinetic parameters of Chi-67 for natural and artificial chitins
. .
.
. .
123 6-4 Kinetic parameters of Chi- 102 for natural and arti6cimtl chitins. . .
123 7- 1 Chracterist ics of crude-extract, Chi-67 and Chi- 1 02 chitinases. . .
129 7-2 Characteristics of MB-2 pure chit inases and two other chitinases. 130 7-3 Comparison of N-terminal amino acid residues of chitinase. . .
.
.
.
136...
Time course of chitinases production by B, licheniformis MB-2 in medium containing 0.5% colloidal chitin (pH 7.0) at 5 5 O C
...
Chitinase yield of pellet
and
supematant precipitated at different ammonium sulfate concentrationActivities of endochitinase and exochitinase after
...
exchange buffer using ultrafiltration at 1 kDa(1, fdtrate; 2, effluent)
and
10 kDa (3, filtrate; 4, effluent)Percentage of chitbases bound by various chitin substrates
Elution profile of chitinase &om Phenyl Sepharose CL4B
...
c o b chromatography with gradients of ammonium sulfateat pH 7.0
Elution profle of chitinase from Phenyl Sepharose C L 4 B
...
column chromatography with gradients of ammonium sulfate at pH 8.0Elution profile of chit inase h m PhenyI Sepharose CL-4B
...
column chromatography with gradients of ammonium sulhte at pH 8.0 and decreasing pH gradient...
Elution profile of chit& from Phenyl Sepharose CL-4Bcolumn chromatography with gradients of ammonium sulfate at pH 8.0 and increasing pH gradient
Elution profile of chitinas. from Phenyl Sepharose
CL-4B
...
column chromatography with gradients of ammonium sulfate
at pH 8.0 and increasing urea gradient
...
Elution profle of chitinase fiom Phenyl Sepharose CL-4Bcolumn chromatography with gradients of ammonium sulfate at pH 8.0 and increasing propanol gradient
...
Elution profile of chit& from Phenyl Sepharose CL-4Bcolumn chromatography with gradients of ammonium sulfate at pH 8.0
and
increasing Tween-20 gradient...
Elution proiile of chitinase &om DEAE Sephacef column
chromatography with gradients NaCl at pH 8.0 (-) and increasing Tween-20 gradient
...
Elution profle of chitinase fiom Sephadex G-75 columnchromatography
The scheme of chitinase pu&cation steps
...
...
SDS-PAGE of peak fractions fiom different purification stepsSDS-PAGE and native PAGE of exochitinase fractions
...
fiom different p d c a t i o n stepsHeat stability of Chi-67
...
...
Heat stability of Chi- 1 02
pH stability ofchi-67
and
Chi-102 aRer 1 h and 4 h...
Effect of metal ions on Chi-67 andChi-
1 02 activities...
Synergism effect of Chi-67 and Chi- 1 02 for various...
chitin substratesChitinax activities on flourogenic substrates as a function of
...
incubation time...
Chitinase activities on flourogenic substrates as a functionof enzyme-
LIST OF APPENDICES
...
Sequences of 16s rRNA of MB-2 (1478 bases)
...
Phylogenetic Tree of Bacteria-Producing Chitinases...
Sequence Producing Alignments of Chi-67 N-terminal
...
Conserved Domain Search of Chi-67
...
Amino Acid Composition of Chi-67...
Predicted of Chi-67 Catalytic Domain Structure h mGenBank Database
...
Predicted of Chi-67 Substrate Binding Domain Structurefiom GenBank Database
1.
INTRODUCTION
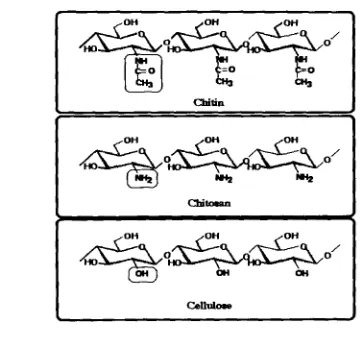
Chitin, an insoluble polysaccharide consisting of P-(14) linked N-ace@-D-
glucosamine (GicNAc) units, is the second most abundant polysacc&de
in
nature, after cellulose. It is widely distributed as structural component of
crustaceans, insects, and other arthropods, as we1 as component of the cell walls
of most fungi and some algae. About 10" tons of chitin is produced annually in the aquatic biosphere alone, however, only 0.1% of this material is cmently being
converted to valuable products.
Chitin and its derivatives exhibit interesting properties
and
constitute avaluable materials for biomedical, cosmetic, food and agricultural applications.
Currently they are used as immunoadjuvants, drug delivery systems, dietary
fibers, agrochemicals,
and
flocculants of wastewater sludge. In the f a d areaschitin and its derivatives are used for clarification of beverages, food wraps,
recovery of solid materials from f a d processing wastes
and
water, and irnmobiiization of enzymes. Chitin oligomers may promote the growth of Bifidus bacteria and suppress spoilage organisms, reduce toxinsand
detrimentalenzymes, prevent diarrhea and constipation, lower serum cholesterol, protect liver
function, and protect against cancer (Shahidi el al, 1 999).
Enzymatic degradation of chitin is performed by chitinases and appears to
occur in two steps. An endochitinase (EC 3.2.1.14) reduces the polymer to oligomers, which are subsequently degraded to monomers by exwhitinax-
chitobiase (P-N-acetylhexosaminidase, EC 3.2.1.52). Chitinases are found
in
aThe enzymes are also known to be synthesized by some of the protozoans,
coelenterates, nematodes, mollusks and +pods (Muzzarelli, 2002).
Chitinases are of great biotechnological interest and have received remarkable
attention. First, these enzymes may be used to convert chitin-containing biomass into biologically reusable forms such as oligosaccharides, chitobiox
and
N-
acetylglucosamine. Secondly, chitinases may be exploited for the control offungal
and
insect pathogens of plants. Thirdly, chitinase inhibitors potentiallyinhibit growth of chitin-containing pathogens and plague insects that
need
chitinases for normal development. Chitinase inhibitors have generated a lot of
interest given their potential as insecticides, fungicides, and antimalarials. Efforts are going on throughout the world to enhance the production and purity of
chitinases. Some characteristics of chitinases have been reported but only a few
thermostable chitinolytic enzymes are known.
Exploration of extremozymes with their unusual stability towards heat, organic
solvents, extreme pHs, detergents, and resistance to common protein denaturing
agents has attracted quite a number of researchers as such features confer a
number of advantages with r e s p t to industrial applications. Usage of
themostable enzymes in biotechnological processes will allow faster reaction
times and reduction of contaminations, reduce energy costs
in
large scalefermentations, less viscosity and kter solubility of chemicals intended to be
~ r d u d at high temperatures, and reduce pathogen contamination and efficient
product recovery p m s s e s such as distillation. Investigation of thermostable
enzymes is fundamental for the elucidation of the structural basis for their
it may be possible to engineer proteins designed for the conditions required in various industrial processes. Additionally, such enzymes are also suited for
studying structure-function relationships and mechanisms for promoting and
maintaining tertiary structures at extreme conditions.
Thermophilic microorganisms that grow optimally at temperature of a b u t 50
to over 90 "C are potential sources of thermostable chitinases. The natural
environment for thermophilic microorganisms are multifaceted throughout
Indonesia, including crater, terrestrial hot spring, and deep-sea thermal vents.
Many microorganisms producing various thermostable enzymes such as
protease (Mubarik er al, 2000; Wahyuntari et al, 2000), keratinase
(Lintang
et al, 2002), chitin deacetylase (Toharisman et al, 2001, 2002a), chitinase (Natsir et al, 2002; Suhartono et al, 20031, and chitosanase (Azizah et al, 2002; Haliza et al, 2002) have been isolated in our laboratory. Furthermore, we screened more than50 therrnophiles from Tompaso hot spring (North Sulawesi), able to hydrolyse
chitin. Among them, isolate MB-2 identified as Bacillus licheniformis MB-2,
secreted a variety of thermostable enzymes including chitinases into culture
media. Preliminary study with zymograrn analysis showed that partial purified
chitinases occur in multiple forms and were stable at a high temperature and a
broad range of pH. However, these chitinases have not yet been purified and fkrther characterized.
The objectives of the research are to purify and characterize thermostable
chitinases from B. licheniformis MB-2. Pure enzymes are required for research and analysis in biochemical and clinical chemistry, whereas information of
as understanding of structural-function of protein (enzyme). Thus, there is a
rapidly increasing demand for enzymes of high purity with comprehensive
characteristics. Characterization of the purified enzymes includes analysis of
substrates specificity, optimum pH and temperature, heat stability, the effect of
cations, stability of enzymes towards specific inhibitors and denaturant
substances, analysis of molecular weight, enzyme kinetics, and N-terminal
amino acid sequencing.
This research should greatly contribute for developing marine industry in
Indonesia, as the country presently has 170 shrimp processing units with capacity
of
500 000 tons per year. It is predicted that Indonesia produces chitin of about1 00 000 tons annually. Enzymatic conversion of chitinous materials into valuable
and marketable products may drive the development of marine industries in
2.
LITERATURE
REVIEW
2.1. Chitin
2.1.1. Structure and Distribution
Chitin is a polymer formed primarily of repeating units of P-1-4-linked 2
wtamido-2deoxy -b-D-glucopyranose residues (GlcNAL). Its structure is similar
to the structure of cellulose, except that acety1arnino groups have replaced the
hydroxyl groups in position C-2 (Fig 2-1). Chitin polymer tends to form
microfibrils of about 3 nrn in diameter that are stabilized by hydrogen b n d s formed between the amine and carbonyl groups (Gooday, 1994).
Chitin
[image:22.630.122.482.376.716.2]I
X-ray diffraction analysis suggested that micrwrystalline structure chitin
occurs in a-,
p-
and y- arrangements. in the a form, all chains exhibit an anti-parallel orientation; in the
0 form, the chains are
arranged in a parallel manner; and in the y form sets of two parallel strands dtemate with single anti-parallelstrands. The anti-parallel arrangement allows tight packaging into chitin
microfibrib, consisting of 20 single chitin chains that are stabilized by
a
highnumber of hydrogen bonds formed within and between the molecules. In contrast,
in the
p-
and y-chains, packing tightness and numbers of inter-chain hydrogen bonds are reduced, resulting in an increased number of hydrogen bonds withwater. The high degree of hydration and reduced packaging tightness result in
more flexible and soft chitinous structures (Goosen, 1997).
The most abundant form of chitin is a-chitin. It is found in the hydrozoa,
nematodes, rotifers, mollusks and arthropods. 0-chitin, a less stable and more
degradable form of chitin, is occurred in mollusks, squid pen, diatoms, cuttlefish
bone, insect exoskeletons and cocoons, and is major component of cell walls. The
y form is found in stomach lining of squid (Gooday, 1990).
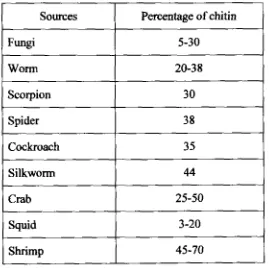
Chitin has a very wide distribution among organisms (Table 2-1) and slightly
different structure and associated proteins and minerals. Variations in the
amounts of chitin may depend on physiological parameters
in
naturalenvironments. Chitin is always found cross linked to other structural
components such as protein and glucan. In insects and other invertebrates, chitin
is always aswiated with specific proteins, whereas in fungal walls it is found to
covalently bonded to glucans, either directly or via peptide bridges (Gooday,
Table 2.1. Variation in chitin contents at different organisms (Skj ak-Braek and Sandford, 1 989)
Chitin varies in crystallinity, degree of covalent bonding to other components
such as glucms, and degree of deaeetylation. Degree of chitin acetylation is between 0 to 1 OOO/o, but the natural chitin is approximately 16% deacetylated.
Deacetylation of above 80% chitin units is generally termed as chitosan
(Goosen, 1 997).
Chitosan is composed primarily of GlcNAc and GlcN (2-amino-2-deoxy-P-~-
glucopyranose) residues, Unlike most polysaccharides, chitosan has three types
of reactiond functional groups, an amino group as well as both primary and
secondary hydroxy l groups at the C-2, C-3, and C-6 positions, respectively. Chemical modifications of these groups provide numerous useful materials in
Sources
Fungi
Worm
Scorpion
Spider
Cockroach
Silkworm
Crab
Squid
Shrimp
Pacentage of chitin
5-30
20-38
30
38
3 5
44
25-50
3-20
[image:24.624.193.462.137.405.2]different field of applications. The molecular weight of chitosan is about 0.1-0.5 x
10' Dalton, whereas native chitin is about 1 - 2 x1
o6
Dalton (Goosen, 1997).In 2002 about
id
tons of chitin were recovered industriallyh m
marineindustry, approximately 65% of which was converted into glucosamine, 25% into
chitosan and 10% into chitooligosacchrtrides. The world market of chitin and its
derivatives was estimated to reach $ 1 3 7 million (Table 2-2).
Table 2-2. Worldwide market for chitin derived products in year 2002 (Sandford, 2003)
2.1.2. Application
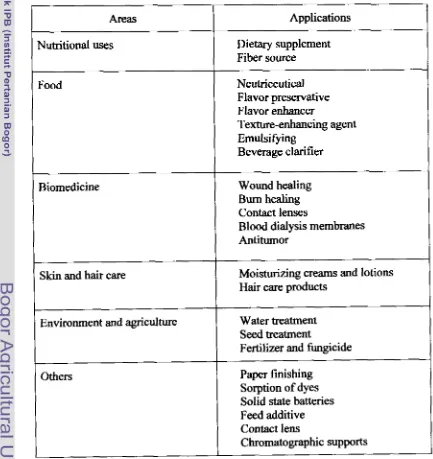
During the past 20 years, a substantial amount of work has been published on
chitin and its derivates and their potential use in various applications such as in food, agriculture, medicine, biotechnology, pharmacology, and waste treatment
(Table 2-3).
In
food processing, chitin and its derivates may beused
for clarification ofwine, beer and juices, deacidification of fruit juices, formation of biodegradable
films, production of flavor compounds, preservation of foods from microbial Derivative Chitom Glucosamine Oligosaccharides Quantity (Tom)
6
700
4 000
500
Price ($/ton)
10 000 10 000
60 000
I
Value ($ million)
67 40 30
deterioration, recovery of waste material from
focd
processing discards, andreducing
warmed over flavor in precooked meats (Shahidi et al, 1999). They areused as food supplement
and
may reduce plasma cholesterol and triglycerides dueto their ability to bind dietary lipids (Koide, 1998).
Chitin
has
been reported to regulate some interactions between plantpathogenic fungi and their plant host. The fungi-resistant properties of chitin
derivates have resulted in their application as fertilizer, soil stabilizer, and seed protector (Kohle et al, 1984; Kauss et al, 1989; Shirnosaka et el, 1993 and 1995;
Leger et a/, 1996).
h the biomedical area, chitin and its derivates have been observed to accelerate
wound healing properties and the attainment of an attractive skin surface. It has
been suggested that the mechanism by chitin and its derivates act at wound site
involves activation of neutrophiles and macrophages. These compounds stimulate
the migration of plymorphonuclear and mononuclear cells and accelerate the reformation of connective tissue and angiogenesis (Bianco et al, 2000). Because of its high oxygen permeability, the chitin derivate was used as a material for contact and intramular lenses.
It
has also been found to expedite blocd clotting and can form complexes with other natural polymers, such as collagenand
keratin,
to form materials with unique biomedical properties (Sand ford, 1 989). The fact that chitin and its derivates are biodegradable and biocompatiblemakes them particularly appropriate for
use
in drug delivery systems (Thacharodiand
Rao, 1995; Genta et al, 1997; Zecchi et al, 1997). This property is extremely valuable in cancer chemotherapy since the agents are often highly toxic andBecause of their binding and ionic properties, chitin derivates can be used as a
flocculating agent to remove heavy metals and other contaminants h m
wastewater. Current applications
in
this area include treatment of sewage [image:27.620.86.519.291.750.2]effluents, paper mill wastes, metal-finishing residues,
and
radioactive wastes (Guibal er al, 1997).Table 2-3. Applications of chitin and its derivatives
1
Areas1
Applications1
Nutritional uses Dietary supplement
Fiber source
Texture-enhancing agent Emulsifying
Beverage clarifier
Food Neutriceutical
Flavor preservative Flavor enhancer Biomedicine
1
Wound healing Burn healing Contact lensesB l d dialysis membranes Antitumor
Skin and hair care
Others
Moisturizing creams and lotions Hair care products
Environment and agriculture
Paper finishing Sorption of dyes Solid state batteries Feed additive Contact lens
Chromatographic supports Water treatment
Seed treatment
Chitin oligomers or chitin oligosaccharides [chitooligosaccharides),
customarily used for saccharides having the degree of polymerization of 2-10,
are biologically active compounds. The products have activities as elicitors,
anti bacterial agents, immuno-enhancers
and
1 y sozyme inducers ( Ai ba, 1 994; Shahidi et a], 1999; Wang el al, 2002a md 2002b). Aiba (1994) reported that (GlcNAc), (n=2-5) are useful in agriculture and medicine, meanwhile (GlcNAc),activate macrophages and the immune system. Another chitooligosaccharide,
(GICNAC)~, is claimed to be a potent anti-metastatic agent against
mouse
bearing Lewis lung carcinoma (Roseman etat,
1999). Monomer of chitin, GlcNAc, is avaluable pharmacological agent in the treatment of a wide variety of ailments
including gastritis, fwd allergies, inflammatory bowel disease (IBD) and
diverticulitis. It does not have m y established negative side effects (Haynes et
al, 1999).
2.13. Biodegradation of Chitin
Chitin degradation towards chitosoligosaccharides, GlcNAc, GlcN and other
derivatives can be obtained by various treatments. The most used method is
chemical treatment using strong acid at high temperatures for extended of time.
Unfortunately, it is not easily controlled and environmentally unsafe. The product
has a broad range of molecular weight and a heterogeneous extent of deacetylation, so it is not suitable in food, cosmetics and pharmaceuticals
industries, which need high purity grade
and
uniform prducts.In
addition, themethod i s disadvantageous due to the occurrence of side reactions, energy-
1997; Yoon et al, 1998; Kolodziejska et al, 2000). Enzymatically process, on the other hand, could be employed under mild conditions, would not yield side
product, and results
in
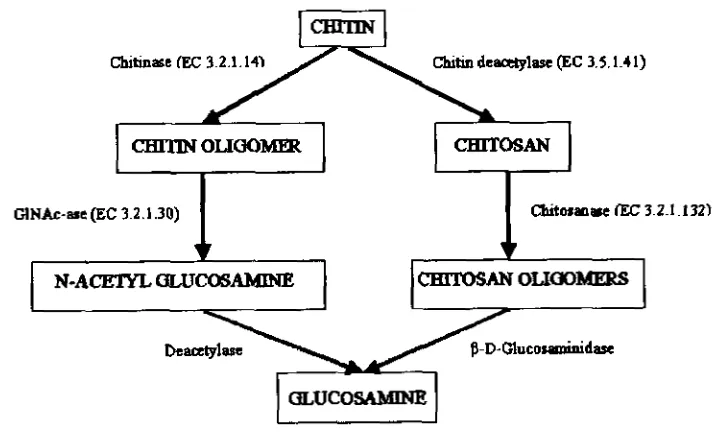
specific products with good quality.There are two possible pathways of chitin biodegradation (Fig 2-2, Gooday,
1990). First, it involves the initial hydrolysis of 1,4 P-glycosidic bond of chitin,
[image:29.622.151.509.356.575.2]Second, it is the deacetylation of chitin to chitosan. The fonner is accomplished by chitholytic enzymes and the latter by chitin deacetylase
and
chitosanase.Figure 2-2. Enzymatic pathways of chitin degradation (Gooday, 1 990)
Cbitinase [eC 3.2.1.141 Chitin d e q l a w (EC 3.5.1.41)
~ O ~ C # ) M W CHlTOS AN
Chitinolytic enzymes catalyze the hydrolysis of chitin by cleaving the bond
between the C1 and C4 of two consecutive N-acetylglucosamines, chitin deacetylase (EC 3 -5.1.4 1 ) modify chitin into chitosan molecule through
GIN&-MC @C 3.2.1.30) Cbitolana~ (EC 3.2.1 -132)
f 7
N - A m mWAMmE cmTOSAN 0LICK)MERS
deacetylation mechanism, while chitosanase
(EC
3 -2.1.1 3 2 or 3 -2.1 -99) hydrolyzechitosan to chitosan oligomers (Somashekar and Joseph, 19%; Cohen-Kupiec and
Chet, 1998).
23.
Chitinase2.2.1. Biologica1 Role
Different organisms p d u c e a wide variety of chitinases that exhibit different
substrate specificities and other properties useful for various functions. In
bacteria, chitinases play roles in nutrition (Bati ZU et al, 2000; Tsujibo et al, 2000; Folders et al, 2001) and parasitism (Grenier et al, 1993; Wenuganen, 1996;
Khaeruni, 1998; Kobayashi et al, 2002; Lutz et al, 2003; Malik et
al,
2003a and2003b), whereas in fungi, protozoa and invertebrates, they role in rnorphogenesis
(Bakkers ef al, 1997). Plant chitinases function in a self-defense mechanism against pathogenic fungi (Hou et al, 1998; Minic et 01, 1998; Vander er 01, 1998; Velazhahan et a!, 2000). Baculovirus, which are used for biological control of
insect pests, also produce chitinase for pathogenesis (You et al, 2003). Chitinase
activity
in
human serumhas
recently been described. The possible role suggested is a defense against fungal pathogens (Escottand
Adams, 1995; Langer el 01,2002).
Chitinase from marine organisms play a crucial role in the recycling of
chitinous materials for maintenance of the ecosystem in the marine environment.
Many bacteria and fungi containing chitinolytic enzymes convert chitin
into
Chitinase plays an important role in insect growth
and
development. It involves in molting and cuticle turnover by hydrolyzing the structural polymerchitin, a principal component of the insect exoskeleton and gut lining. Chitinase
activity has been identified in the molting fluids
and
midguts of several insectsincluding Bornbyx mori, Manducu sexta, Ae&s aegypti,
and
the wasp Chelonus (Royer et al, 2002). Chitinase is also important in the life cycle of arthropods (Spindler et al, 1 997).Plants do not have an immune system and instead use various defense mechanisms to protect their vegetative and reproductive organs against pathogen
infection. One response to pathogenic attack involves expression of pathogenesis-
related proteins such as chitinases (Andersen et al, 1997). The enzymes are capable of releasing chitin oligomers that elicit a series of defense reaction from
the fungus invading the plant (Nishizawa et a/, 1999; Staehelin et a), 2000). Purified barley and bean chitinases inhibited the growth of fungal hyphae (Leah at
al, 1991). It has also been demonstrated that transgenic plants that over express chitinases have increased resistance to fungal attack (Wiendi, 2002). In root
nodules, chitinases may protect the symbiotically infected zone from external
pathogens (Minic et al, 1998).
2.2.2. Application
Chitinases have
many
industrial and agricultural applications such aspreparation of chitooligosacc~des, biocontrol of plant pathogenic fungi,
production of single-cell protein, fungal protoplast technology and chitochemical
2002). Chitinases can be exploited to produce chitmligosaccharides
from
chitin.A chitinase from Vibrio alginolyticus
was used
to prepare chitopentaose and chitotriose fiom colloidal chitin ( M m el al, 1992). Strepdomyces chitinase wasutilized for the enzymatic hydrolysis of colloidal chitin. The chitobiose produced
was subjected to chemical modifications to produce novel disaccharides
derivatives of 2-acetamido-2-deoxy-~al1oppose moieties that are potential
intermediates for the synthesis of an enzyme inhibitor (Ohtakara et al, 1 W). Fungal formulations containing chitinases are perceived as safe alternatives to
chemical pesticides for insect treatment. Entomophatogenic fungi e.g. Beauveria bassima, Metharizizrm anisoplue and Verticillium Iecanii (Leger ef a/, 1996; Freimoser et al, 2003) are parasites of various pests including potato beetle, sugarcane frog hopper and aphids. A numlxr of soil bacteria produce chitinolytic enzymes that can also be used to inhibit fungal infection (Lodto et a/, 1996).
The enzymatic conversion of waste chitin to yeast single-cell protein (SCP) has
been investigated. Revah-Moiseev and Carroad (1981) used the S. marcescens
chitinase system to hydrolase the chitin and Pichia kuclriavezevii to yield the SCP. Vyas and Deshpande (1 99 1) showed that the M. verrucaria chitinase complex and
S. cerevisiae can also be used for SCP production h m chitinous waste. The total protein and nucleic acid contents of produced SCP were 61% and 3.1%,
respectively.
Since chitin is the major structural component in the cell walls of most fungi,
chitinolflc enzymes play a significant role in protoplast isolation. H d y n et a1
isolation and found that high chitinase levels permit effective mycelia
degradation.
The lectins, due to their specific monosaccharide-binding properties, can
be
used to locate sugar residues in thin section of plant and fungi. Similarly,
hydrolytic enzymes like chitinase can be also employed to Iucate fungal pathogens
that possessd chitinous cell wall. The chitinase-gold complex can be
used
forthis purpose (Peters and Latka, 1997).
2.23. Classification
Based
on amino acid sequences homology of glycosyl hydrolases, chitinaseswere grouped into two evolutionarily unrelated groups, designed as families 18
and 19 (Henrissat and Davies, 1997). Family 1 8 chitinases include most of the
chitinases from bacteria, fmg, insects, plants (class I11 and
V
chitinases), and animals. Family 19 chitinases include classes I,ll
andIV
of the plant chitinases(Meins et ai, 1992; Gijzen et al, 2001) and a chitinase from Streptomyces griseus (Ohno et a/, 1996). Class I
and
IV of plant chitimes consist of the sequence with a highly conserved main structure and an N-terminal Cys-rich domain. ClassIV
chitinases are smaller
than
class I chitinases due to deletionsin
both the cysteine-rich domain and the catalytic domain. Class I1 chitinases are structurally homologous to class I and
JV
but lack the Cys-rich domain. Class III chitinasesshow little sequence similarity to the enzymes
in
classes I,II,
orlV
and
are morethey possess a different substrate specificity (Roberts and Selitrenikoff, 1 988; Collinge et al, 1993).
Family 18 chitinase is further classified into 3 groups: A, B,
and
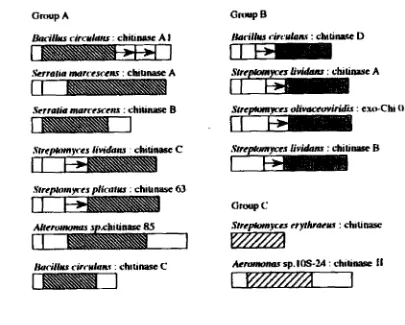
C, based onthe amino acid sequence of individual catalytic domains (Fig 2-3). Chitinases
in
group A have an insertion domain between the seventh and eight P-strands of the
(P/(~)~-barrel basic structure, whereas chitinases in group B and C do not have
such an insertion domain (Uchiyama et a], 2001 ; Suzuki et al, 2002).
~ t r e a # r r r w e ~ dimmiridis : ex* Chi 0 1
SrrcIUmn w s dintru : chi u nasc 63
Aemmmm sp. IOS-2.4 : chili- f 1
[image:34.626.123.528.339.664.2]Based on the mode of action, chitinases were classified into endochitinases
and exochitinases. Endochitham (EC 3.2.1.14) cleave chitin randomly at
internal site generating soluble low molecular mass muftimers of GlcNAc such as
chitotetraose, chitotriose, and the dirner of di-acetylchito biose. Exochitinase can
be divided into two subcategories: chitobiosidases (EC 3.2.1.29) which catalyze the progressive release of di-acetylchito biose starting at the non-reducing end of
the chitin microfibril; and 1 -4-~~N-acetylglucosamidase (EC 3.2.1 -30) which
cleave the oligomeric product of endochitinase and chitobiosidases generating
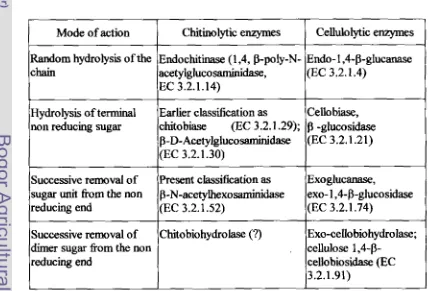
[image:35.624.89.517.441.732.2]monomers of GlcNAc (Cohen-Kupiec and Chet, 1998). This class5cation is almost parallel to the cellulolytic complex (Table 2-4).
Table 2-4. Nomenclature of chitinokytic and celluloZytic enzymes (Patil et al, 2000)
Cellulolytic enzymes
Endo- 1 ,CP-glucanase (EC 3.2.1.4)
Cello biase, -gluwsidase (EC 3.2.1.21)
Exoglucanase,
exo- l,4-b-glucosidase
(EC 3.2.1.74)
Exo-cello biohydrolase; cellulose 1,4-P-
cellobiosidase (EC 3.2.1.91)
Mode of action
Random hydrolysis of the chain
Hydrolysis of terminal non reducing sugar
Successive removal of sugar unit from the non reducing end
Successive removal of dimer sugar fiom the non reducing end
Chitinolytic enzymes
Endochitinase (1,4, P-ply-N- a&y1gluco saminidase,
EC 3.2.1.14)
Earlier classification as
chitobiase (EC 3.2.1 -29); P-D-Acetylgluco~dase (EC 3.2.1.30)
Present classikation as p-N-acetylhexoddase (EC 3.2.1.52)
The classification of endo and exochitinase depends mainly on the substrate.
For example, the Streptomyces chitinase complex degrades pure cry stdine
P-
chitin of diatom spines only from the non-reducing ends to yield diacetylchitobiose, whereas colloidal chitin is degraded to a mixture of oligomers
and diacetylchitobiose. Some 1 -4-P-N-acetylgluwsamidases can also act weakly
as exochitinases cleaving monomer units
f k m
the non-reducing ends of chitinchain (Gooday, 1 994).
23.4. Structure and Catalytic Mechanism
Crystal structure of chitinases has been successively reported. The crystal
structure of 26 kDa chitinase from barley seeds (Hordem vulgare L.), which is classifid into family 19, was first solved. After
that,
the structures of a 60 kDac h i w e A from S. murcescens and a 29 kDa chitinase from rubber tree (Hevea
brasiliensis) were reported. Both are family 18 enzymes.
Comparison between the structures of family 18 and 19
chitinases
revealed aclear different in their structures. The family 18 chitinase has a typical (dB)s
barrel structure composed of eight a-helices and an eight stranded &sheet (Fig 2-
4). In addition to the main barrel domain, it has an N-termid p-strand-rich
domain
and
a small (a+P) domain. The crystal structures of other family 18chitinases exhibit similar barrel structure. Apparently, in family 18 chitinases, the sequence homology results in the similarity in three-dimensional structure
(Fukamizo, 2000; van Aalten et al, 2001; Watanabe el al, 2003). On the other
hand, barley chitiaase (family 1 9) is composed of two loks, each of which is rich
(GICNAC)~, the substrate binding cleft is estimated to lie between the two lobes. The hypothetical binding cleft is composed of two a-helices and three-stranded
p-
sheet (Fukamizo, 2000).
Fig 2-5. A ribbon drawing of barley chitinase (family 19 chitinase) revealing a mixture of secondary structure, including 10 a-helical segments and one three-stranded
P
sheet. The structure shows a globular protein with high a-helical content and an elongated cleft nmning the length of the protein, presumably for substrate binding and catalysis (Robertus and Monzingo, 1 999)Family 18 bacterial chitinases contain the consensus sequences SXGG and
DXXDXDXE. The sequences SXGG and DXXDXDXE are substrate-binding
and active sites, respectively. Several runs of conserved amino acids for family
18 h m Coccidioides immitis (Yang et al, 1997), Trichderma harzianurn (Garcia et al, 1 994), Aphanocladium album (Blaiseau and Lafay, 1 992), and Serratia murcescens (Brurberg et al, 1994) are shown in Fig 2-6. A section of signature sequence for the family 19 is shown
in
Fig 2-7, This represents the [image:38.616.211.436.91.351.2]tuberosum (Gaynor, 1 988), Arabidopsis thulium (Sarnac ef 02, 1 9901, and pea,
Pisum sativum (Chang et al, 1995).
In addition to the difference in
3D
structure, chitinases of the two familiesshow the difference in catalytic mechanism (Fig 2-8 and Fig 2-91, Family 118
chitinase hydrolyze the glycosidic
bond
with retention of the anornericconfiguration (Sasaki et al, 2002), whereas family 19 chitinases with inversion (Ohno et al, 1996). Substrate assisted catalysis is the most widely accepted model for the catalytic mechanism of family 18 (Brameld et al, 1998), whereas a general acid and base mechanism has been suggested to be the catalytic mechanism of family 19 (Ohno er al, 1996). Family 1 8 c h i t h s hydrolyse GlcNAc-GlcNAc and GlcNAc-GlcN, meanwhile family 19 chitinases hydrolyse GlcNAc-GlcNAc
and GlcN-GlcNAc linkage (Ohno et al, 1996; Mitsutorni eb
ai,
1997). Family 18 chitinases are sensitive to allosarnidin, but a family 19 chitinase from higherplants has been shown to
k
insensitive (Koga et aI, 1999).130 170
Chi-ci LSIGGWTYSPNF FDGIDIDWEYPED
Chi-th LSIGGWTWSTNF FDGIDlDWEYPAD Chi-aa LSlGGWTWSTNF FDGDIDWEYPAD Chi-sm PSIGGWTLSDPF FDGVDIDWEFPGG
60
70
80 90 100Chi-hv KREVAAFLAQTSHE'ZTGGWATAPDGAFAW GYCFKQERGASSDYCTPSAQWPCAPGK
Chi-st KREIAAFLA QTSHEmCCWASAPDGPYAWGYCFLR ERGNPGDYCPPSQQWPCAPGK
Chi-at KReVMFGQTSHETLY;GWATAPDCPYSWGYCFKQEQNPD YCEPSATWPCASGE
Chi-ps KREIAAFLG QTSHEITGGWPTAPDGPYAWGYCFLR EQNP
-
SWCQASSEFPCASGKFig 2-7. Conserved amino acid on the active site of family 19 chitinases. The sequence numbers correspand to barley chitinase, Hordeum vulgare
(Chi-hv);
Chi-st,
Solanurn iuberosum;Chi-at,
Arabidopsis thuliana; Chi-ps, Pasum safivumThe catalytic mechanism of W y - 1 8 chitinase was first reported by Watanabe
et al (1990) for chitinase A1 h m B. circulam. The Glu204 residue was considered to be a proton donor
in
its catalysis since site-directed mutagenesis of the residue completely e h t e d its activity. The glutamic residue was alsofound to & conserved in family 18. In S, marcescem chitinase A and B, the catalytic carboxylate corresponding to Glu204 of B. circulans chitinase AT is Glu3 1 5 and
Gh
144, respectively (Vaaje-Kolstad et al, 2004).In the consensus region of the catalytic domain of family- 1 8 chitinases, there
are several consewed carboxylic amino acid residues. For example, Asp200 and
Asp202 in chitinase A1 from 3. circulam, Asp3 1 1 and Asp313
in
S. marcescens (Watanabe et al, 1992 and 1993). The location of these residues does not correspond to that of the second carboxyhtein
lysozyrne (Asp52) or in family 19 barley chitinase (Glu89). Thus, the second carbxylate cannot be identikd in anyh d y 18 chitiwise. Therefore, the family 18 c h i t b s should have a different mechanism of catalysis. Recent studies on the family 18 chitimes indicate that
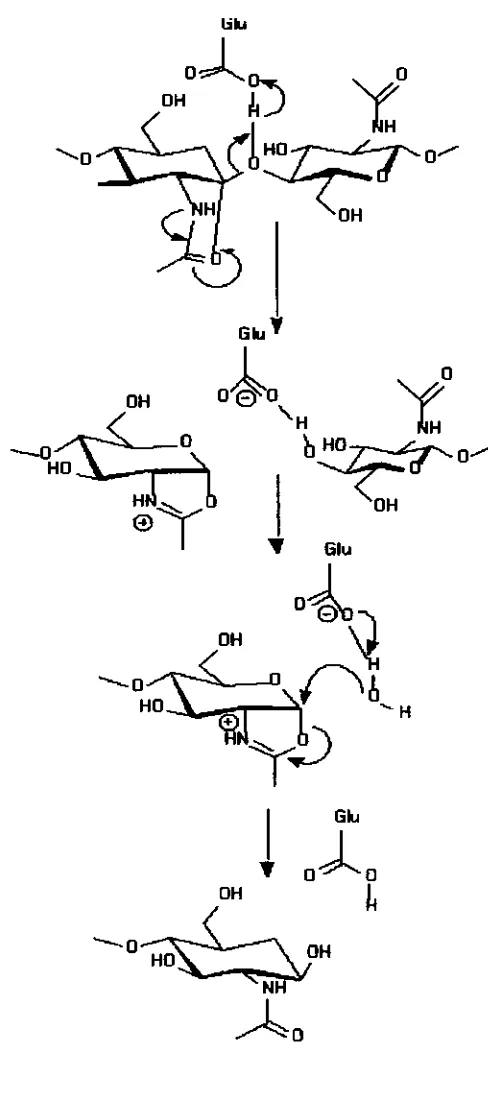
the catalytic reaction of the enzymes takes place through a substrate-assisted
As shown in Fig 2-8, a putative oxocarboniurn ion intermediate is stabilized by
an anchimeric assistance of the sugar N-acetyl group after donation of a proton
from the catalytic carboxylate to the leaving group. Such a stabilization might
occur either through a charge interaction between the C 1 carbon and the carbonyl
oxygen. The mechanism does not require the second carboxylate and can rationalize the anomer retaining reaction of the enzyme without the second
carboxylate (Fukarnizo, 2000).
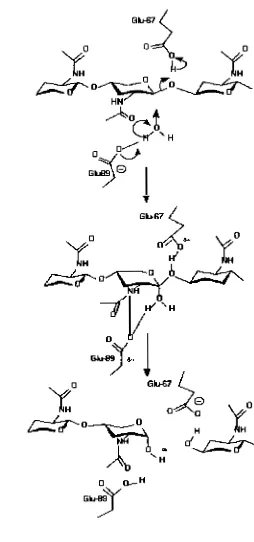
The catalytic mechanism of family 19 chitinase is shown in Fig 2-9. At first,
the general acid, Glu67, protonates the
P-
1,4-glycosidic oxygen atom forming anoxacarbonium ion intermediate. The water molecule, activated by the general
base (Glu89), attacks the C1 atom of the intermediate state for the a-side to
complete the reaction.
The
separated location of the two catalytic residues mightbe permit the water molecule to be located in between the anomeric C1 atom and
the carboxyl oxygen of the general base (Glu89). This location of the water
molecule would result in the anomeric inversion of the reaction products. From
the molecular dynamic simulations, however, Glu89 was found not only to
activate the nucleophilicity of the water molecule but also to act as a stabilizer of
the carbonium ion intermediate (Brameld and Goddard, 1 998). In addition, the
simulation study indicated that the (G~CNAC)~ substrate binds to barley chitinase
with all sugar residues in a chair conformation, no sugar residue distortion was
2.2.5. Chitin Binding Domain
Chitinases generally consist of multiple functional domains, such as C-terminal
domains which binds especially to insoluble chitin (chitin binding domains, ChBD), fibronectin type III-like donins (FnIIIDs), and a N-terminal catalytic
domain. The fimtion of each dolllain except for the ChBD has not been yet elucidated (Morimoto at al, 1997; Gao et al, 2003). The ChDB has been
demonstrated to be important in the degradation of insoluble chitin (Wu et al,
2001 ; Jee el d, 2002; Royer et al, 2002; Orikoshi
er
al, 2003).B, circulans
WL-
1 2 produces ChiAl , Chic 1and
ChiD 1 as the initial products of the three chitinase genes (Alam et a!, 1996). ChiAlhas
the highest hydrolyzmg activity against insoluble chitin. This chitinase comprises the catalytic domain (CatD), two FnlIIDs, and the C-terminal ChBD ( Watanabe et 01, 1 992; 2003), The deletion of FnIIIDs did not affect chitin binding but decreased hydrolysis ofinsoluble substrate like colloidal chitin. However, hydrolysis of soluble substrate
either carboxymethy 1 or chitin-oligomer was not significantly affected. In
addition to its involvement in binding to insoluble substrate, the type I11 dornain
could possibly
IE
involved in the exo-hydrolytic mechanism (Watanabe et al, 1992; Tsujib at al, 1998b).Suzuki
et a1 (1999) identified the chitinase C1 and C2 fiom S. marcescens 21 70. Chitinase C 1 lacks a signal sequence and consists of a catalytic domain belonging to glycoside hydrolase family 18, FnlIID and a C-terminal. ChitinaseC2 corresponds to the catalytic domain of C1 and is probably generated by proteolytic removal of the FnIIID and ChBD. The loss of the C-terminal portion
but not towards colloidal chitin and glycol chitin, illustrating the importance of
the ChBD for the efficient hydrolysis of crystalline chitin.
Unlike cellulose binding domain (CBD), the tertiary structure of ChsD remains unclear. Chitin differs chemically from cellulose only in that each C2 hydroxyl (-OH) group in cellulose is replaced by an acetamide (-NHCOCH3) group in chitin, hence the mechanism by which ChBD binds to chitin was expected to be similar to the mechanism by which CBD
bind
to ceILulose(Ikegami el al, 2000).
The most accepted model for the binding of
CBD
to cellulose is that aromaticrings arranged in the flat face of a CBD
are
stacked on every other pyranose ringof plysaccharides through hydrophobic interactions. The involvement of
arormdic residues
in
the interactions has been observed using NMR, site-directedmutagenesis, and chemical modscation. In CBD, the st WWst motif is widely
c o r n e d . This motif is also found in Aeromonas caviae extracellular chitinase A, Alteromonas sp. strain
0-7
chitinax 85, Janthinobacterium lividurn chitinase 85 (Ikegami et al, 2000).2.2.6. Dual Active Sites
Microorganisms posses two catalytic domains on a single polypeptide have
been rare reported. Tanaka er a1 (2001 j found that the chitinase fiom the hyperthermophilic archaeon T bdahraensis KOD1 has an interesting multidomain structure containing dual catalytic domains and triple chit in-binding
domains. From the analysis of hydrolysates of N-acetyl-chitooligosaccharides
(ChiA
A
5 and ChiAA4). ChiAA5 showed exochitinolytic activity that mainlyhydrolyzed the second glycosidic bond and slightly hydrolyzed the third one
h m
the nonreducing end of chitin chains. On the other hand, ChiAA4 showed
endochitinolytic activity that randomly hydrolyzed glycosidic bonds other than the terminal
b n d
at the non reducing end.Howard ef a1 (2004) recently reported that chititme B of Microbulbifer
degradam 2-40 is a modular protein that
is
predicted to contain two catalyticdomains. Each of domains was shown to be catalytically active against chitin.
The presence of both domains
in
a single reaction increased the amount of reducing sugars released from native chitin to 140% above the theoretical combined rate, indicating that the domains function cooperatively to degradechitin.
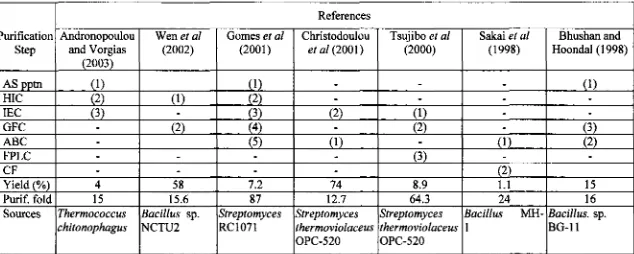
227. Purification
Chithases have been purified using the conventional protein purification
techniques like ammonium sulfate hctionation, gel filtration, ion-exchange
chromatography, af5nity chromatography, isoelectric focusing, and hydrophobic
chromatography. Purification process of c h i t b e s is usually conducted in series
of steps. However, Ilyina et a1 (1995) proposed one step purdication system
using an affinity chromatography sorbent based on cross-linked chitin. The sorbent retained selectivity one of the four extracellular chitinases in the culture
supernatant produced by Streptomyces kurssanovii.
M c a t i o n of thennostable c h i t i s were performed with similar procedures
themostable chitinases
h m
various bacterial sources are shown in Table 2-5.The first issue in handling
an
extracellular enzyme is to recover the enzyme ofinterest from the fermentation medium. In classical process, the culture filtrate of
fermentation is fmt treated
with
ammonium sulfate to obtain crude proteinprecipitate. However, Wen et a1 (2002) reported that the step was not applicable
in their works. They proposed two steps purification system using Phenyl
sepharose and Sephadex G-75 purifying an extracellular chitinase from the culture
supernatant of Bacillus sp. NCTU2. Similarly, Sakai ef a1 (1998) purified three
thermostable chitinases from Buci~~ecs. MH-1 using two steps of purifications. Chitinases were first adsorbed on colloidal chitin
and
then separated onchromatofocusing. Three isoforms of endochitinases ((L, M, and S) were
purified to homogeneity with the yield and purification fold of 0.67-2.2% and 18-
24, respectively.
The two-steps chromatographic p r d u r e s for large-scale purScation of the
recombinant thermostable chitinase cloned from S. thermoviolaceus were performed using metal-&nity chromatography, followed by ion-exchange
chromatography. The pure protein
was
highly active and hermostable, with a 12.7 purification fold (Christdoulou et al, 2001). A thermostable actinomycete chitinase was purified by concentration, precipitation, hydrophobic interaction, gelfiltration and adsorption p r d u r e s (Gomes et al, 2001). Thermostable chitinase from Bacillus sp. BG 11 was purified by chitin-binding affinity and gel
chromatography. This scheme resulted in a homogeneous enzyme preparation
Table 2-5. Purification steps of some thermostable chi tinasec'
"The number in the bracket indicates the order steps of purification; AS pptn, ammonium sulfate precipitation; HIC, hydrophobic interaction chromatography; IEC, ion exchange chromatography;
GFC,
gel filtration chromatography; ABC, affinity binding chromatography; FPLC, fast performance liquid chromatography; CF, chromatofocusingPurification step AS pptn HIC IEC
GFC
ABC FPLC CF Yield (%) Purif. fold SourcesReferences Andronopoulou
and Vorgias (2003)
(1
1
(2) (3)
-
-
-
4 15 Therrnococcus chitonophagusWen et a1
(2002) (1)
-
(2)-
-
58 15.6 Bacillus sp. NCTU2B
hus han and Hoondal ( 1 998)(1)
-
(3) (2) 15 16 Bacillus. sp. BG-11 Gomes et al(2001)
(I)
(2)
(3)
(4)
( 5 )
-
7.2 87
SfPeptomyces
RC1071
Sakai st a1
(1 998)
-
-
-
-
(1) - (2) 1.1 24Bacillus MH- 1
Christodoulou et a1 (2001)
-
-
(2)-
(1)-
-
74 12.7 Streptomyces ~hermoviolaceus OPC-520Tsujibo el
d
[image:48.829.92.726.169.423.2]2.2.8. Characteristics
There is considerable heterogeneity in the reported biochemical properties of
bacterial chitinases. The enzymes display a wide range of molecular mass,
temperature
and
pH optima, pI and thermostability (Table 2-6). Several chitinasesfrom higher plants such as carrot and from insects such as the tobacco hornworm
and silkworm
are
glycoproteins. The enzymes h m microorganisms likeThermococcus kodakaraensis, Aeromonas and Rhizopus were reported to be glycoproteins (Koga, 1996; Hollis et al, 1997; T& er al, 2001; Merzendorfer and Zimoch, 2003).
Some chitinases can do the trmsglycosylation reaction, which is the reverse of
hydrolysis. Such chitinases are found in microorgani