Other uses, including reproduction and distribution, or selling or
licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the
article (e.g. in Word or Tex form) to their personal website or
institutional repository. Authors requiring further information
regarding Elsevier’s archiving and manuscript policies are
encouraged to visit:
ContentslistsavailableatSciVerseScienceDirect
Animal
Reproduction
Science
journalhomepage:www.elsevier.com/locate/anireprosci
Association
study
and
expression
analysis
of
porcine
ESR1
as
a
candidate
gene
for
boar
fertility
and
sperm
quality
Asep
Gunawan
a,
Kanokwan
Kaewmala
a,
Muhammad
Jasim
Uddin
a,
Mehmet
Ulas
Cinar
a,
Dawit
Tesfaye
a,
Chirawath
Phatsara
a,b,
Ernst
Tholen
a,
Christian
Looft
a,
Karl
Schellander
a,∗aInstituteofAnimalScience,AnimalBreedingandHusbandryGroup,UniversityofBonn,53115Bonn,Germany bDepartmentofAnimalandAquaticSciences,FacultyofAgriculture,ChiangMaiUniversity,50200ChiangMai,Thailand
a r t i c l e i n f o
Articlehistory: Received13May2011
Receivedinrevisedform12August2011 Accepted24August2011
Available online 6 September 2011
Keywords: mRNA
Immunofluorescence Spermatogenesis Epididymis Spermatozoa
a b s t r a c t
MalefertilityisimpairedthroughthelackofESR1(EstrogenReceptor1)butlittleisknown abouttheESR1rolesinboarspermatogenesisandfertility.Therefore,thisresearchwas aimedatinvestigatingtheassociationwithspermqualityandboarfertilitytraitsinatotal of300boarsbothfrompurebredPietrainandPietrain×Hampshirecrosses.ASNPincoding regionofESR1g.672C>Tinexon1wasassociatedwithspermmotility(P<0.05)andplasma dropletrate(P<0.01)whilethepolymorphisminnon-codingregionofESR1g.35756T>Cin inton1wasassociatedwithnon-returnrate(P<0.05).Furthermore,toanalysethemRNA andproteinexpressionofESR1inboarreproductivetissues,atotalofsixboarsweredivided intotwogroups[GroupI(G-I)andGroupII(G-II)],whereG-Ihadrelativelybettersperm quality.ESR1expressionwashigherintissuescollectedfromG-Iboarsthanthoseof col-lectedfromG-IIboars,andthedifferenceinmRNAexpressionwassignificant(P<0.01) inheadofepididymis.TheESR1proteinexpressionresultsfromwesternblotcoincided withtheresultsofqRT-PCR.TheESR1proteinlocalizationobservedastrongstaininginthe cytoplasmofSertolicellinthetestis,intheepithelialcellsinheadandtailofepididymis, insmoothmuscleintailofepididymis,andinthepostacrosomalregionandtailofthe spermatozoa.TheseresultswillimprovetheunderstandingofthefunctionsoftheESR1in spermatogenesiswithinthereproductivetractandwillshedlightonESR1asacandidate intheselectionofboarwithgoodspermqualityandfertility.
© 2011 Elsevier B.V. All rights reserved.
1. Introduction
Despitebeingthe‘female’hormone,estrogenispresent inlowconcentrationsinmalebloodandinextraordinarily
highconcentrationinsemen(GanjamandAmann,1976).
∗ Correspondingauthor.Tel.:+49228732240;fax:+49228732284. E-mailaddresses:agun@itw.uni-bonn.de(A.Gunawan),
kkae@itw.uni-bonn.de(K.Kaewmala),judd@itw.uni-bonn.de
(M.J.Uddin),ucin@itw.uni-bonn.de(M.U.Cinar),dtes@itw.uni-bonn.de
(D.Tesfaye),chirawath.p@cmu.ac.th(C.Phatsara),etho@itw.uni-bonn.de
(E.Tholen),cloo@itw.uni-bonn.de(C.Looft),ksch@itw.uni-bonn.de
(K.Schellander).
The hormone estrogen is mediated through the nuclear
estrogen receptor which functions as ligand-dependant
transcription factor. The increasing interest in the role
ofestrogenin themalereproductivetractis mainlydue
to the demonstration that male fertility is impaired in
mice lacking estrogen receptor 1 (ESR1) (Eddy et al.,
1996). The evidence for ESR1 in different parts of the
male reproductive tract suggests a possible
physiologi-cal rolein spermatogenesisand sperm maturation. The
ESR1knockoutmicehaveprovidedevidencefora
signif-icantandcrucialroleofestrogensinmaintainingnormal spermatogenesis(Eddy et al., 1996). ESR1is involvedin
the reabsorption of luminal fluid during the transit of
0378-4320/$–seefrontmatter© 2011 Elsevier B.V. All rights reserved.
spermatozoa fromthetestistotheheadoftheepididymis whichisimportantfortheirsurvivalandmaturation
dur-ing epididymal storage (Couse and Korach, 1999). The
absenceofESR1leadstoreducedepididymalsperm
con-tent,reducedspermmotilityandfertilizingability(Eddy etal.,1996).Severalstudieshaveshownasignificant asso-ciationofESR1withsemenqualityorfertilityinhumans (Suzukietal.,2002;Guarduccietal.,2006;Lazarosetal., 2010; Safarinejad et al., 2010). Guarducci et al. (2006)
reported a significantassociation ofESR1polymorphism
with lower sperm count in men. Suzuki et al. (2002)
reportedtwosilentpolymorphismsinESR1being
associ-atedwithazoospermiaorsevereoligozoospermiainmen.
To our knowledge, onlyone studywasdevotedto
anal-ysetheassociationbetweenESR1polymorphismwithlitter sizeinsowsbutthisfailedtodetectanystatistically sig-nificantassociation(Munozetal.,2007).TheESR1mRNA
was reported tobe highly expressed in the epididymis,
testis,pituitary,uterus, kidneyand adrenalglandin rats (Kuiper et al., 1996). ESR1 was localized in head, body andtailofepididymisinthebonnetmonkey(Shayuetal., 2004),cat(Nieetal.,2002)andhorse(Hejmejetal.,2005; Parlevlietetal.,2006).Inpigs,ESR1islocatedon SSC1p24-25,whichwasevidencedastheQTLregionfortotalsperm
per ejaculate and close to the QTL for sperm motility
(Xingetal.,2008).Therefore,ESR1couldbeafunctionalas wellasapositionalcandidategeneformalereproduction
in pigs. But no study has yet been devotedto
unravel-ling itsassociationwith boarspermquality andfertility traits.
In spermatogenesis, beside the reproductive tissues
testis and epididymis, some otheraccessory glands and
tissuesarealsoinvolved.Spermatogenesisisthecomplex
processby whichimmaturegermcellsundergo division,
differentiation and meiosis togive rise to haploid
elon-gated spermatids (O’Donnell et al., 2001). When germ
celldevelopmentiscomplete,thematurespermatidsare
releasedfromtheSertolicellsintothetubulelumenand
proceedthroughtheexcurrentductsystem,knownasthe
rete testis,untiltheyenter the epididymisviathe
effer-ent ducts. During passage through the epididymis, the
spermatids undergo a series of biochemical changes to
becomethemotileandmaturespermatozoacapableof
fer-tilization (O’Donnellet al.,2001).Failurein anyof these
events leads to disturbances of male fertility. The role
ofESR1inboarspermatogenesiswithinthereproductive
tract is poorlyunderstood.For the betterunderstanding
of ESR1rolesin spermatogenesisin pigs,the expression
of ESR1 at different parts of reproductive tract,
includ-ing non-reproductive tissues, are important. Therefore,
the aims of this study were to investigate the
associa-tion ofESR1polymorphism withspermqualityandboar
fertility traits as well as to highlight the roles of ESR1
in boar spermatogenesis within the reproductive tract
by mRNA and protein expression and immunoreactive
ESR1localization.Thisworkmightbehelpful toget
fur-ther insight into the roles of ESR1 in spermatogenesis
in the boar. Theassociationof ESR1polymorphismwith
boar spermquality and fertility will supportthe
candi-dacy of this gene to be used in selection of breeding
boars.
2. Materialsandmethods
2.1. Phenotypes
Samples and phenotypes from 200purebred Pietrain
(PI) and 100 Pietrain×Hampshire crossbred (PI×HA)
boars were used for association analysis in this study.
TheseanimalswereusedforAIincommercialpigherds
inNorth-WesternGermany.Detailsofthepopulationsand
phenotypesweredescribedpreviouslybyWimmersetal.
(2005), Lin et al (2006)and Kaewmala et al. (2011). In
brief, sperm samples from more than 39,000 ejaculates
wererepeatedlycollectedfromtheseboars.Whole
ejacu-lateswereobtainedfrompurebredPietrainandcrossbred
Pietrain×Hampshireboarsagebetweentwoandfiveyears
with an average age of 3.5 years. Sperm quality traits
included sperm concentration (SCON [×108ml]), semen
volumeperejaculate(VOL[ml]),spermmotility(MOT[%]),
plasmadroplets rate(PDR[%]) and abnormal
spermato-zoarate(ASR[%])andwereobtainedfromeachejaculate
withlightmicroscopyaccording totheguidelinesofthe
WorldHealthOrganization. Semenwas collected bythe
vinylglovehandmethodtwiceperweek.Foreachboar,
therepeatedmeasurementsofspermqualitytraitswere
available.Fertilitydata(non-returnratedata[NRR]at42 daysafterinsemination[%]andnumberofpigletbornalive [NBA]perlitter)ofeachboarwereavailableasthe devia-tionfromthepopulationmeanswithinsowbreed,parityof sow,farmandseasonclassesasdescribedearlierbyLinetal (2006)andKaewmalaetal.(2011).Thegeneraldescription ofspermqualitytraitsandboarfertilitytraitsareshownin
Table1.
2.2. Polymorphismstudy
Two single nucleotide polymorphisms detected by
Munoz et al. (2007) were used in this study: a cyto-sine(C) transversion toa thymine at g.672C>Tin exon
1 and a thymine (T) to a cytosine (C) transversion at
g.35756T>C in intron 1 of ESR1. For PCR amplification, the forward (5′-gttcaaatccctggttgcat-3′) and reverse (5′ -ctaggcgtctccccagattag-3′)primersweredesignedfromthe exon1 and the forward (5′-gacagcttccctgcagattc-3′)and reverse(5′-ttcatcatgcccacttcgta-3′)primersweredesigned
from the intron 1 of porcine ESR1 genomic sequence
(GenBankaccessionNo.ENSSSCG00000004083)usingthe
Primer3 tool (Rozen and Skaletsky, 2000). Polymerase
chainreactions(PCR) wereperformedina20lvolume
containing100ngofporcinegenomicDNA,1×PCRbuffer
(with1.5134mMMgCl2),0.25mMofeachdNTP,5pmolof
eachprimerand0.1UofTaqDNApolymerase(GeneCraft).
ThePCRreactionwasperformedunderthefollowing
con-ditions:initialdenaturationat95◦Cfor5minfollowedby 35cyclesof30s at95◦C,30s at52◦C, 30s at72◦Cand finalelongationof10minat72◦Cforthepolymorphism inexon1.ThePCRconditionswerethesameforthe
poly-morphisminintron1excepttheannealingtemperatureof
30sat56◦C.AftercheckingthePCRproductsin1.5%(w/v)
agarosegels,genotypingwasdonefollowing the
restric-tionfragmentlengthpolymorphisms(RFLPs)analysis.The
Table1
Means,standarddeviation(S.D.),samplesize,rangesoftraitsinPietrainandPietrain×Hampshire.
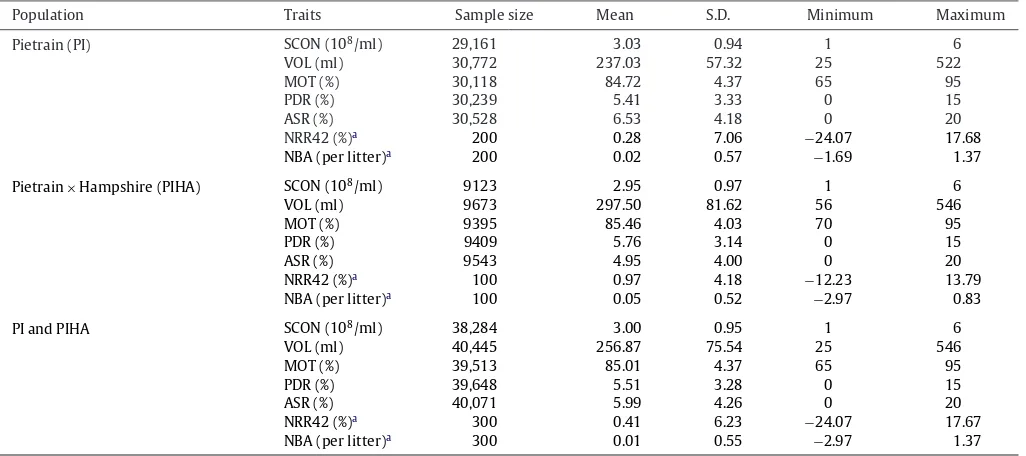
Population Traits Samplesize Mean S.D. Minimum Maximum
Pietrain(PI) SCON(108/ml) 29,161 3.03 0.94 1 6
VOL(ml) 30,772 237.03 57.32 25 522
MOT(%) 30,118 84.72 4.37 65 95
PDR(%) 30,239 5.41 3.33 0 15
ASR(%) 30,528 6.53 4.18 0 20
NRR42(%)a 200 0.28 7.06 −24.07 17.68
NBA(perlitter)a 200 0.02 0.57 −1.69 1.37
Pietrain×Hampshire(PIHA) SCON(108/ml) 9123 2.95 0.97 1 6
VOL(ml) 9673 297.50 81.62 56 546
MOT(%) 9395 85.46 4.03 70 95
PDR(%) 9409 5.76 3.14 0 15
ASR(%) 9543 4.95 4.00 0 20
NRR42(%)a 100 0.97 4.18 −12.23 13.79
NBA(perlitter)a 100 0.05 0.52 −2.97 0.83
PIandPIHA SCON(108/ml) 38,284 3.00 0.95 1 6
VOL(ml) 40,445 256.87 75.54 25 546
MOT(%) 39,513 85.01 4.37 65 95
PDR(%) 39,648 5.51 3.28 0 15
ASR(%) 40,071 5.99 4.26 0 20
NRR42(%)a 300 0.41 6.23 −24.07 17.67
NBA(perlitter)a 300 0.01 0.55 −2.97 1.37
a Fertility(NRR42,NBA)correctedwithfactors:parity,farm,seasonandbreed.
AluIforg.672C>Tandg.35756T>C,respectively(BioLabs).
The digestion was carried out in 10l of reaction
mix-tureofeachsampleandincubatedovernightat37◦Cfor g.35756T>Cand65◦Cforg.672C>T.DetectionofRFLPsof
300boarswascarriedoutbyelectrophoresisin3%(w/v)
agarosegels.
2.3. Statisticalanalysisforspermqualitytraits
TheassociationofESR1withboarspermqualitytraits
wasanalysedbyvarianceanalysis(PROCMIXED)usingthe
SASsoftwarepackage(ver.9.2;SASInstituteInc.,Cary,USA) asdescribedearlierbyKaewmalaetal.(2011).
yijklm=+breedi+seasonj+genotypek+agel
+ejaculationm+εijklm [Model 1]
whereyijklmarethespermqualitytraits(SCON,VOL,MOT, PDR,ASR);istheoverallpopulationmean;breediisthe fixed effect of the i-th breed (i=PI; PIHA, PIand PIHA); seasonjisthefixedeffectofthej-thseason(j=1through8; fourseasonsperyear,intotaleightseasonswithin2years
from January2000toDecember2001);genotypek isthe
fixedeffectofthek-thgenotype(k=1,2and3);agelisthe effectofboarage(covariable);ejaculationmisthe
perma-nentenvironmentaleffectofthem-thboar(random)and
εijklmistheresidualerror.
2.4. Statisticalanalysisforfertilitytraits
TheassociationanalysisbetweenESR1andthefertility traitswascarriedoutusingthefollowinggeneralizedlinear model(PROCGLM)inSAS(Kaewmalaetal.,2011).
yijkl=+breedi+genotypej+yeark+εijkl [Model 2]
whereyijk istheboarfertilitytraits(NRRandNBA);is the overallpopulationmean;breediisthefixed effectof
thei-thbreed(i=PI,PIHA,PIandPIHA);genotypej isthe fixedeffectofthej-thgenotype(j=1,2and3);yearkisthe fixedeffectofthek-thboaryearofbirth(k=1through3: boarbornbefore1996,in1996–97andin1998–99),εijkis theresidualerror.
Achi-square(2)testwasconductedtotestthe
popula-tionsforHardy–Weinbergequilibrium.Leastsquaremean
valuesfortheESR1genotypeswerecomparedbyt-testand P-valueswereadjustedbytheTukey–Kramercorrection.
2.5. SelectionofanimalsformRNAandprotein expression
The reproductive (testis, head, body and tail of
epi-didymis, vas deferens, bulbourethral gland, vesicular
glandsandprostategland),non-reproductive(brain, mus-cleandliver)tissuesfrom6breedingboarswithdivergent
phenotypes were collected from the AI station (SuisAG,
Sempach,Switzerland)for mRNAand proteinstudy. For
differential expression study between reproductive and
non-reproductivetissuesbysemi-quantitativePCRstudy,
mRNAfromall6boarswaspooledtogetheraccordingto
thetissues.Ontheotherhand,thedifferentialmRNAand proteinexpressionstudyindifferentreproductivetissues
fromtwodivergentgroupsofanimalswasperformedby
Table2
Means,standarddeviation(S.D.),numberofboarsandrangesoftraits selectedformRNAandproteinexpressionstudy.
Traits Selected animals (n=6)
G-I (n=3)
G-II (n=3)
Mean S.D. Mean S.D. Mean S.D.
SCON(106/ml) 262.32 87.97 335.94 50.78 188.70 22.54
[image:4.595.41.554.94.322.2] [image:4.595.307.551.686.768.2]semi-quantitativePCR,qRT-PCRandwesternblot, respec-tively. Forthesepurposes,the6boarsweredividedinto two groups:groupI(G-I) andgroupII(G-II),eachgroup containing3boarsonthebasisofSCON,SVOLandSMOT (Table 2). TheSCON (average spermconcentration) was highly negatively (r=−0.8) correlated with SVOL
(aver-agesemenvolume),whereasSCONwashighlypositively
(r=0.7)correlated with SMOT (average spermmotility).
Moreover,SVOLwashighlynegatively(r=−0.8)correlated
with SMOT.Forthe animalsof G-Ithe SCONwashigher
thanthe mean(262.32×106ml),SMOTwashigherthan
the mean (76.59%) and SVOL waslower thanthe mean
(215.24ml/ejaculation)of respectiveparameters.Forthe animalsofG-IItheseparameterswereviceversa.The sig-nificantdifferencebetweenthetwogroupswascalculated usingproct-testinSAS.ThereweredifferencesforSCON (P<0.05) and for SVOL (P<0.01) between G-I and G-II,
whereasforthe SMOTthedifferencewasnotsignificant
(P=0.12).
2.6. Semi-quantitativePCR
TotalRNAwasisolatedusingTRIreagent(Sigma)from
different reproductive and non-reproductive tissues of
breedingboars mentioned above(Section2.5). RNA was
purified using RNeasy Mini Kit (Qiagen) according to
the manufacturer’s instructions. Total RNA was treated
using on-column RNase-Free DNase set (Promega) and
quantifiedsphectrophotometrically(NanoDrop,ND8000).
Furthermore,RNAintegritywascheckedby2%agarosegel
electrophoresis.First-strandcDNAweresynthesizedfrom
individualRNAusingSuperscriptIIenzyme(Invitrogen).
cDNA amplification was performed by using specific
forward and reverse primers (forward: 5′
-agggagagg-agtttgtgtg-3′ and reverse: 5′-tctccagcagcaggtcatag-3′)
derivedfromporcineESR1sequence(GenBankaccession
AF035775). Amplificationwas performedwith an initial
heatingat95◦Cfor5minfollowedby35cyclesof95◦Cfor 45s,annealingtemperatureat60◦Cfor1minand72◦Cfor
1min,onthePCRThermalCycler(BioRad).PCRproducts
wereelectrophoresedona1.5%agarosegelandvisualized
upon staining with ethidium bromide. Amplification of
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
servedashousekeepinggene.
2.7. QuantitativeReal-TimePCR(qRT-PCR)
ForqRT-PCR,totalRNAwasisolatedusingTRIreagent
(Sigma) from different reproductive tissues of the two
divergent groupsof animals (G-Iand G-II)as previously
described (Section 2.5). Total RNA isolation and cDNA
synthesis wasdescribedinthe previoussection (Section
2.6).Thesameprimerpairusedinsemi-quantitativePCR
was also used in qRT-PCR. Nine-fold serial dilution of
plasmid DNA was prepared and used as a template for
the generation of the standard curve. In each run, the
96-wellmicrotiterplatecontainedeachcDNAsample,
plas-midstandardsforthestandardcurvesandano-template
control.To ensurerepeatabilityofthe experiments,each
sample was run in three replications. qRT-PCR was set
upusing2lfirst-strandcDNAtemplate,7.6ldeionized
H2O, 0.2M of upstream and downstream primers and
10l 1× Power SYBR Green I master mix with ROX as
reference dye (BioRad). The thermal cycling conditions
were3minat94◦Cfollowedby40cyclesof20sat94◦C
and 1min at 60◦C. Experiments were performed using
theABIprism®7000(AppliedBiosystems)qRT-PCRsystem.
An amplification-based threshold and adaptive baseline
were selected as algorithms. The housekeeping gene
GAPDH(forward:5′-acccagaagactgtggatgg-3′andreverse: 5′-acgcctgcttcaccaccttc-3′)derivedfromporcinesequence
(GenBankaccessionNo.AF017079)wasusedforthedata
normalization.Finalresultswerereportedastherelative
abundancelevelafternormalizingwithmRNAexpression
levelofthehousekeepinggene.DifferencesinESR1mRNA
expressionwereanalysedwiththesimplet-testinSAS soft-ware(SASInstituteInc.,ver.9.2).Valuesof P<0.05were consideredtoindicatestatisticallysignificantdifferences.
2.8. Westernblotting
Theproteinwasextractedfromdifferentreproductive
tissues(testis,head,bodyandtailofepididymis)fromthe twodivergentgroupsofbreedingboarsasusedinqRT-PCR. However,forwesternblotstudy,proteinsfromthethree
G-Iboarswerepooledtogetherandproteinsfromthethree
G-IIboarswerepooledtogetheraccordingtothetissues.
Theproteinextractedfromtissueswasseparatedby
SDS-PAGE(gradient 4–18%). Subsequentlythe proteins were
transferred onto a nitrocellulosemembrane (Amersham
Biosciences).Afterblockinginblockingbuffer(20mMTris
pH7.5,150mMNaCl,0.05%Tween-20and1%
Polyvinylpy-rolidone)atroomtemperaturefor1h,themembranewas
incubatedwiththeanti-ESR1antibodypurifiedfromrabbit polyclonalantibody(Cat.nr.543;SantaCruz)inthe block-ingmedium(diluted1:500)overnightat4◦C.Non-specific
bindingof antibodywas washedoffwith sixchangesof
0.1% PBST (10min totime). The horseradish peroxidase
conjugatedgoatanti-rabbitIgGsecondaryantibody(Cat.nr.
Sc2004;SantaCruz)wasusedasthe secondaryantibody
(diluted 1:5000). The membrane was incubated for 1h
atroomtemperaturewith secondaryantibody,followed
by washing with six changes of 0.1% PBST (10min to
time).ThechemiluminescewasdetectedbyusingtheECL
plus westernblottingdetection system (Amersham
Bio-sciences)andwasvisualizedbyusingKodakBioMaxXAR
film(Kodak).GAPDHwasusedasaloadingcontrolandfor
normalization.Themembranewasstrippedbyincubation
in2%SDS,100mMTris–HCl,0.1%-mercaptoethanolfor
30minat60◦Candre-probedwithGAPDHantibody(Cat.nr. Sc20357;SantaCruz).
2.9. Proteinlocalizationbyimmunofluorescence
Due to the limitations of fresh samples from G-I
and G-II boars, we collected different fresh
reproduc-tivetissueswerecollectedfrom ahealthybreedingboar
afterslaughteringforproteinlocalizationby
immunofluo-rosence.Immunofluorescencestainingwasperformedon
Table3
Genotype,allelefrequenciesandthechi-squaretestoftheporcineESR1gene.
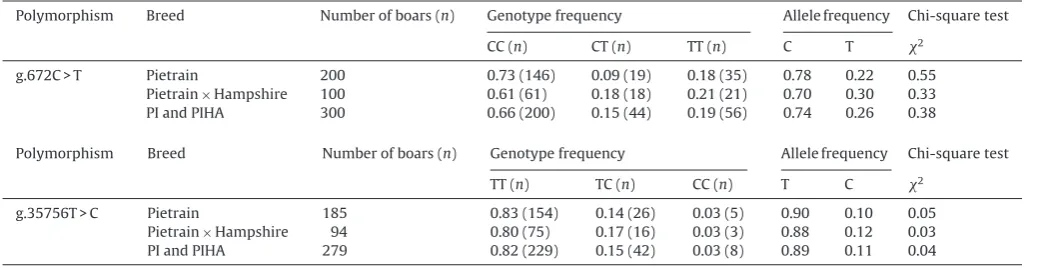
Polymorphism Breed Numberofboars(n) Genotypefrequency Allelefrequency Chi-squaretest
CC(n) CT(n) TT(n) C T 2
g.672C>T Pietrain 200 0.73(146) 0.09(19) 0.18(35) 0.78 0.22 0.55 Pietrain× Hampshire 100 0.61(61) 0.18(18) 0.21(21) 0.70 0.30 0.33 PIandPIHA 300 0.66(200) 0.15(44) 0.19(56) 0.74 0.26 0.38
Polymorphism Breed Numberofboars(n) Genotypefrequency Allelefrequency Chi-squaretest
TT(n) TC(n) CC(n) T C 2
g.35756T>C Pietrain 185 0.83(154) 0.14(26) 0.03(5) 0.90 0.10 0.05 Pietrain× Hampshire 94 0.80(75) 0.17(16) 0.03(3) 0.88 0.12 0.03 PIandPIHA 279 0.82(229) 0.15(42) 0.03(8) 0.89 0.11 0.04
temperaturewith5%bovineserumalbumininPBS(50nM sodiumphosphate,pH7.4;0.9%NaCl).Sectionswere incu-bated overnight at 4◦C with the ESR1 rabbitpolyclonal primaryantibody(Cat.nr.543;SantaCruz)dilutedat1:50 inPBSTfollowedbysixtimes(10minpertime)washing withPBS. Then,thesectionswereincubated 1hatroom temperaturewiththebiotinylateddonkeyanti-rabbit IgG-B as a secondary antibody (Cat.nr. Sc2090; Santa Cruz) (dilution 1:200) which was conjugatedwith fluorescein isothiocynate (FITC). Later on sections werewashed six times (10min per time) with PBS. Finally, the samples werecounterstainedwithvectashieldmountingmedium (VectorLaboratories)containing40,6-diamidino-2-phenyl indole(DAPI)andcoveredwithacoverglassslip.The stain-ingwasobservedby confocallaserscanningmicroscope (Carl Zeiss). In case of negative controls, PBS was used insteadoftheprimaryantibody.
3. Results
3.1. Polymorphismstudy
Twosinglenucleotidepolymorphisms(SNPs)were con-firmedinexon1andintron1ofESR1inthePIandPI×HA populations.Animalsofthesepopulationsweregenotyped atg.672C>Tinexon1andatg.35756T>Cwhichwerethe SNP segregatingwithin the populations.The SNPs were confirmed by PCR-RFLP. The DNA restriction fragments obtainedforg.672C>TofESR1-BstNIpolymorphismwere: 190,117,50,32and17bpfortheCCgenotype;190,117,50, 32,17and12bpfortheCTgenotype,and190,117,50and 12bpfortheTTgenotype.TheDNArestrictionfragments obtainedforg.35756T>CofESR1-AluIpolymorphismwere: 93,48,40and5bpfortheTTgenotype;133,93,48,40and 5bpfortheTCgenotype,and133,93,and5bpfortheCC genotype.
The calculated genotype and allele frequencies of porcine ESR1 gene are shown in Table 3. In this study,
threegenotypeCC,CTandTTwerefoundforbothSNPsat
g.672C>Tand g.35756T>C inour populations.
Homozy-goteCCwasmorefrequentandhomozygoteTTwasrare
atSNPg.672C>TwhereasincaseofSNPg.35756T>C,the
homozygoteTTwasthemorefrequent,andhomozygoteCC
wasrareinourpopulations.Thechi-squaretestrevealed
thatthelocusofESR1wasinHardy–Weinbergequilibrium
inbothPIandPI×HApopulations(Table3).
3.2. Associationanalysis
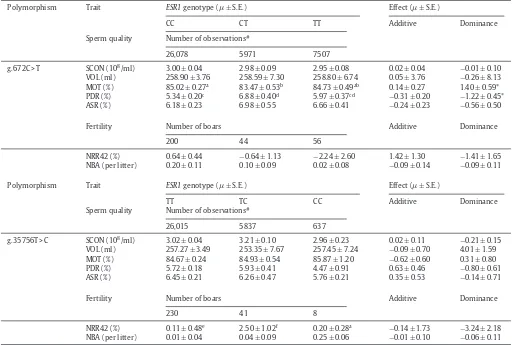
Association analysis of g.672C>T withsperm quality andfertilitytraitsrevealedsignificant(P<0.01)association
withMOTandPDR.SemenfromanimalswithgenotypeCT
hadsignificantlylowerspermmotilityandhigherplasma
dropletratethananimalswith CCand TT(Table4). The
results also indicated that there were dominant effects
(P<0.05)onMOTandPDRinourpopulation.Inthecase ofg.35756T>C,theassociationanalysisshowedsignificant (P<0.05)associationwithNRR.AnimalswiththeTC geno-typehada significantly(P<0.05)higherNRRthanthose withgenotypeTTandCC(Table4).
3.3. mRNAexpressionbysemi-quantitativePCR
ESR1gene expressionwas higherin headof the
epi-didymis than in body and tail, moderate in testis, vas
deferens,bulbourethralglandsandlowerexpressionwas
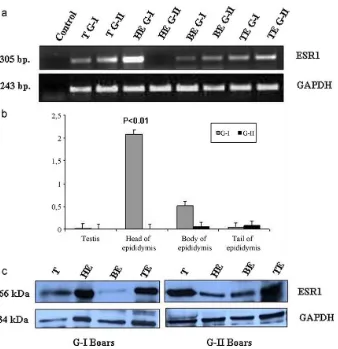
foundinnon-reproductivetissue(brainandliver)except skeletalmuscle.Thesemi-quantitativereverse transcrip-tionPCRresultofGAPDHshowednoremarkabledifferences amongtissues(Fig.1).
3.4. mRNAandproteinexpressionstudyintissuesfrom G-IandG-IIboars
TheESR1mRNAwasexpressedintestis,bodyandtail
of epididymis from both the G-I and G-IIboars but the
higherexpressionwasshowninheadofepididymisofG-I
thanthatofG-IIboarsinsemi-quantitativePCR(Fig.2a).
ThesemRNAexpressionresultsofsemi-quantitativePCR
appeared to be consistent with the results of the
qRT-PCR. The ESR1 mRNA expression was higherin headof
epididymisofG-Iboars(P<0.01),whereastheexpression
[image:6.595.39.558.94.228.2] [image:6.595.311.546.659.744.2]Table4
AssociationbetweenESR1genotypeswithspermqualityandfertilitytraits.
Polymorphism Trait ESR1genotype(± S.E.) Effect(± S.E.)
CC CT TT Additive Dominance
Spermquality Numberofobservations#
26,078 5971 7507
g.672C>T SCON(108/ml) 3.00± 0.04 2.98± 0.09 2.95± 0.08 0.02± 0.04 −0.01± 0.10
VOL(ml) 258.90 ± 3.76 258.59± 7.30 258.80± 6.74 0.05± 3.76 −0.26± 8.13 MOT(%) 85.02± 0.27a 83.47± 0.53b 84.73 ± 0.49ab 0.14± 0.27 1.40± 0.59*
PDR(%) 5.34± 0.20c 6.88± 0.40d 5.97± 0.37cd −0.31± 0.20 −1.22± 0.45*
ASR(%) 6.18± 0.23 6.98± 0.55 6.66± 0.41 −0.24± 0.23 −0.56± 0.50
Fertility Numberofboars Additive Dominance
200 44 56
NRR42(%) 0.64± 0.44 −0.64± 1.13 −2.24 ± 2.60 1.42± 1.30 −1.41± 1.65 NBA(perlitter) 0.20± 0.11 0.10 ± 0.09 0.02± 0.08 −0.09± 0.14 −0.09± 0.11
Polymorphism Trait ESR1genotype(± S.E.) Effect(± S.E.)
TT TC CC Additive Dominance
Spermquality Numberofobservations#
26,015 5837 637
g.35756T>C SCON(108/ml) 3.02± 0.04 3.21± 0.10 2.96± 0.23 0.02± 0.11 −0.21± 0.15
VOL(ml) 257.27 ± 3.49 253.35± 7.67 257.45± 7.24 −0.09± 0.70 4.01± 1.59 MOT(%) 84.67± 0.24 84.93± 0.54 85.87 ± 1.20 −0.62± 0.60 0.31± 0.80 PDR(%) 5.72± 0.18 5.93± 0.41 4.47± 0.91 0.63± 0.46 −0.80± 0.61 ASR(%) 6.45± 0.21 6.26± 0.47 5.76± 0.21 0.35± 0.53 −0.14± 0.71
Fertility Numberofboars Additive Dominance
230 41 8
NRR42(%) 0.11± 0.48e 2.50 ± 1.02f 0.20± 0.28a −0.14± 1.73 −3.24± 2.18
NBA(perlitter) 0.01± 0.04 0.04 ± 0.09 0.25± 0.06 −0.01± 0.10 −0.06± 0.11
#Repeatedmeasurements.
a,bP<0.01;c,dP<0.001;e,fP<0.05.*P<0.05.
differences werenon-significant in the testis, body and tailofepididymisinbetweenG-IandG-IIboars(Fig.2b).
ESR1proteinwith66kDamolecularweightwasdetected
intestis,head,bodyandtailoftheepididymisinbothof G-IandG-IIboars(Fig.2c).Thewesternblotresultshowed thattheESR1proteinexpressionwashigherinheadofthe epididymisinG-Iboars.Thisproteinexpressionseemedto beconsistentwiththeresultsoftranscriptionlevels.
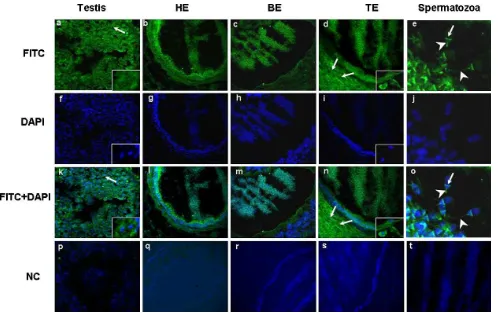
3.5. LocalizationofESR1proteininboarreproductive tissuesbyimmunofluorescence
Sections of testis, head, body and tail of epididymis
werestainedthroughthesameopticalpanelfortheESR1
proteinexpression(Fig.3).ImmunoreactiveESR1protein
was observed as strong staining in cytoplasmof Sertoli
cells but poorly in germ cells and Leydig cells in testis (Fig.3a).Spermatozoawithinthelumenoftheepididymis
headwerefoundtobepositivelyimmunostainedwithESR1
(Fig.3b).Similarly,spermatozoawithinthelumenofthe
epididymisbodyandtailwerepositivetoimmunoreactive
ESR1protein(Fig.3candd).Theimmunofluorescenceof ESR1 proteinwas specificallylocalizedin the post
acro-somalregion(arrow)and tailofthesperm(arrowhead)
whenspermatozoawithintheepididymiswereexamined
(Fig.3e).Moreprecisely,theepithelialcellsintheheadand
tail ofthe epididymis expressedhigherimmunoreactive
ESR1protein(Fig.3bandd),whereasthe epithelialcells
inthe epididymisbodypoorlyexpressedthetarget
pro-tein(Fig.3c).Inthecaseoftheepididymisheadandbody, thesterociliacellswereremarkablyhigherimmunostained whileintheepididymistailtheproteinwasstrongly local-izedinthesmoothmuscle(Fig.3b–d).
4. Discussion
4.1. AssociationofSNPwithboarspermqualityand fertilitytraits
ThisstudyrevealedassociationofESR1withsperm qual-ityandfertility traitsinboars (Table4).Theexonic SNP g.672C>Twas found tobe significantly associated with spermqualitytrait:spermmotilityandplasmadropletrate. AssociationstudyofESR1israreinboars,butLazarosetal. (2010)reported twoSNPsofESR1at397(T>C)and351 (A>G)hadapositiveeffectonspermqualityinhumans.
Moreover,Suzukietal.(2002)reportedapolymorphism
ofESR1inexon4associatedwithidiopathicazoospermia
in humans.Theexistence ofESR1at the postacrosomal
[image:7.595.44.561.94.439.2]Fig.2.mRNAandproteinexpressionofESR1inreproductivetissues(testis,head,bodyandtailoftheepididymis).(a)ESR1mRNAexpressionindifferent reproductivetissuesfromG-IandG-IIboarsbysemi-quantitativePCR.(b)ESR1mRNAexpressionindifferentreproductivetissuesfromG-IandG-IIboars byqRT-PCR.(c)ProteinexpressionindifferentreproductivetissuesfromG-IandG-IIboarsbywesternblotting.(T:Testis,HE:Headofepididymis,BE: Bodyofepididymis,TE:Tailofepididymis.)
estrogensinmalegametematurationandmotility.Ithas
beenreportedthatestrogenproducedlocallyisinvolvedin theregulationofspermmotility(Lazarosetal.,2010).ESR1
polymorphism may influence theselocally acting
estro-gen levelswith effect onsperm motility (Carreauet al.,
2002). Abnormal sperm production and reduced
fertil-ity have been reported in transgenic male mice lacking
ESR1 (Eddy et al., 1996). Infertility from lacking ESR1is reportedmainlyduetothedisruptionoffluidreabsorption
in efferentductuleswhichincreasedthebackpressureof
theaccumulatingluminalfluidsthatleadstoaprogressive degenerationofthetesticulartissueandseverelyimpaired
spermatogenesis (Eddy et al., 1996; Hess et al., 1997;
CouseandKorach,1999).TheidentifiedSNPisconfirmed
in exon whichmay playimportant rolein transcription
process.Polymorphisminthecodingregioncouldhavea
directeffectthroughchangingthenucleotidesequenceand structureofgene,possiblyleadingtochangesinmRNA syn-thesis,splicing,maturation,transportation,translationor degradation(IidaandAkashi,2000).
TheintronicSNPg.35756T>Cwassignificantly associ-atedwiththefertilitytraitnon-returnrate(NRR)(Table4).
Reproductive efficiency of boars is usuallymeasured by
non-returnrate, whichisdefined aspercentage of sows
notappointedforasecondinseminationwithinaperiod
ofdaysafterthe firstinsemination.Male fertilitycanbe regardedasaresultofbothfertilizingabilityofthesperm cellsaswellasthe numberoffertilizedoocytesandthe
viability of the embryo. But, under the field conditions
onlytheoutcomeoftheinseminationscanberecorded,i.e. whetherornotthefemaleshavereturnedforrepeat insem-ination(Stalhammaretal.,1994).However,littleisknown
aboutthepolymorphismandassociationofporcineESR1
withfertilitytraitlikeNRRin pigs.AnESR1genevariant atc.1227C>Tinexon5showedapositiveeffectonlitter sizeinsows(Munozetal.,2007).It isimportanttonote that,thedetectedSNPatESR1g.35756T>Cwasinintron 1whichissubjectedtolessfunctionalconstraintandmay changetheprimarystructureoftheESR1.Though‘silent’, itcouldaffectESR1functionbyalteringthemRNAstability (Caponetal.,2004).Moreover,thereisincreasingnumber ofreports describingthe roleofintronin regulatingthe expressionlevelofageneortissuespecificexpression pat-tern(JiangandLe,2000;Paganietal.,2004).Traitsrelated tofertilityofboarsareoflowheritability(h2=0.01–0.06)
and are stronglyaffected by environmental and genetic
effectsofthe boaritself,the sow andthe offspring(See, 2000). Therefore, effects of single loci are expected to
be low and require a higher number of animals to be
[image:8.595.129.467.68.417.2]Fig.3. LocalizationofESR1proteinindifferentpartsofboarreproductivetissues.(a)ImmunofluorescencedetectionofESR1incytoplasmofSertolicells andgermcells.SertolicellswerestainedwithESR1(arrows)andthenucleiwerecounterstainedwithDAPI(arrow).(bandc)ESR1proteinlocalization inepithelialcellsintheheadandbodyoftheepididymisandinthespermatozoawithinthelumenoftheepididymis.(d)LocalizationofESR1proteinin epithelialcells,inspermatozoawithinthelumenoftheepididymisandremarkableexpressionwasinsmoothmuscleinthetailoftheepididymis.Smooth muscleinthetailoftheepididymiswasstainedwithESR1(arrows).(e)TheESR1localizedinacrosomalregion(arrow)andtailofspermatozoa(arrowhead) withinepididymis.(f–j)ThecellnucleiwerecounterstainedwithDAPI.(k–o)Mergedimages.(p–t)Negativecontrol.(T:Testis,HE:Headofepididymis, BE:Bodyofepididymis,TE:Tailofepididymis.)
moderate to medium heritability (h2=0.19–0.37) (See,
2000).However,twoSNPsof ESR1areidentifiedin both
exonandintronwhichmighthaveanimportanteffectson
spermqualityandfertilitytraitsinpig.
4.2. ESR1mRNAandproteinexpressioninboar reproductivetissues
Thephysiologicalroleofestrogens inmale
reproduc-tion is now extensively revisited (Carreau et al., 2002) butexpressionstudyisrareinboars.Inthisstudy,mRNA
expressionanalysisbysemi-quantitativePCRandqRT-PCR
demonstratedthattheporcineESR1geneexpressionwas
higher in reproductive tissuesthan in non-reproductive
tissues(Fig.1).Importantly,ESR1expressedhigherinthe
headofepididymiscollectedfromG-Iboarsthanthatof
G-II boars. The highest expression of ESR1 in the head
of the epididymis was in agreement with the previous
reportsdescribingthatthehighestconcentrationof estro-genreceptorwasfoundintheepidydimisheadinmiceand macaques(Schleicheretal.,1984).Itiswelldocumented thatmaleestrogenisinhighpresenceintheefferentducts
in the headof epididymis (Hess and Carnes, 2003). The
presenceofESR1intheheadoftheepididymishasimmense importancesinceestrogenplaysacrucialfunctioninfluid
reabsorptioninthisregioninthemale(Hessetal.,1997). Thisfluidreabsorptionisimportantforspermmaturation andformaintainingthenumberofspermintransit(Janulis etal.,1996).Anydisruptionoftheestrogenreceptorgenein malemiceresultedwithdecreasedfertilityandtesticular
weightandverylowspermcounts(LubahnandGolding,
1993). Moreover, ESR1 abundance is detected in
differ-entpartsof malereproductivetract andspermatozoain
mice(Zhouetal.,2002),humans(Aquilaetal.,2002)and rodents(Pelletier et al., 2000)which coincides withour findings.Theevidenceofestrogenindifferentpartsofthe malereproductivetractsuggestsapossiblephysiological
roleof estrogen in spermatogenesisand sperm
matura-tion.Furthermore,thelowerESR1transcriptabundancein theheadoftheepididymisofG-IIboarsinthisstudymay
indicate alack ofestrogen actionwhich maycontribute
tothe poor spermqualityand fertility. It isknown that
spermanditsmembraneproteinsundergoseveral
modifi-cationsduringmaturationwithintheepididymis.Estrogen
antagonist treatment in mice showed that lack of ESR1
functionintheepididymisheadcausesfailureoffluid
reab-sorption, impairedsperm production and loss of sperm
motility(Shayuetal.,2004).InfertilityinlackingESR1is
reportedmainlydue tothedisruption offluid
[image:9.595.54.552.70.383.2]degenerationofthetesticulartissueandseverelyimpaired spermatogenesis(Eddyetal.,1996;Hessetal.,1997;Couse andKorach,1999).Moreover,swellinganddamageof sem-iniferoustubulesandthedilutionoftheessentialproteins secretedfromepididymisalsocontributedtothe infertil-ity(Eddyetal.,1996;Hessetal.,1997;CouseandKorach, 1999).
Inthisstudy,theproteinexpressionanalysisby
west-ernblottingshowedthatESR1antibodyrecognizedaband
at66kDainalltissues.Thebandissimilartothe
molecu-larweightofhuman(66kDa)andmouse(66kDa)ESR1and
veryclosetothatofthestallion(65kDa).Although,the lev-elsofproteinindifferenttissuesfromG-IandG-IIwerenot
asdistinguishableasmRNA,theESR1proteinexpression
wasremarkablyhigherintheheadoftheepididymisofG-I boarsthanthatofG-IIboars(Fig.2b).Thevasdeferensinthe headoftheepididymisarethemajorsitesforESR1 expres-sionwhichregulatesthetesticularfluidreabsorptionand
increasesthe spermcellconcentrationasthey enterthe
epididymis(Hessetal.,1997).Moreover,theepididymisis involvedindifferentproteinabsorptionaswellasin pro-teinsecretion(Syntinetal.,1999).Theheadandbodyofthe epididymisarereportedtosecrethigheramountsof differ-entproteinsinboarsincomparisontootherfarmanimals (Syntinetal.,1999).Theseproteinsecretionsareregulated
by estrogen and the proteinsare involved in the sperm
membraneremodelling,intheinitiationofspermmotility, andespeciallyinsperm-egginteraction(Pearletal.,2007). Ithasbeencalculatedthat50–90%ofthetotalprotein leav-ingthetestisisabsorbedbytheefferentductsinthehead oftheepididymis(Clulowetal.,1994).
4.3. ProteinlocalizationofESR1
ESR1wasfoundtobeexpressedstronglyin the
cyto-plasmofSertolicells,expressedongermcellsandLeydig cells in testis (Fig. 3a). These results are in agreement with previous results localizingESR1in Sertoliand Ley-digcells(HessandCarnes,2003)andinLeydigandgerm cellsinrat(Pelletieretal.,2000).Pelletieretal.(2000) sug-gestedthatintheLeydigcellstheESR1mightbeinvolved
in the secretion and maturation of germ cells. Estrogen
arereportedtobeinvolvedinmaintainingtheSertolicell function (O’Donnellet al., 2001)as well asin
establish-ingSertoli–germcelladhesion(MacCalmanandBlaschuk,
1994).However,thecellulardistributionpatternsofESR1 proteinbetweenspeciescouldbedifferent.
We localizedESR1 in the head, body and tail of the
epididymis. Whenspermare releasedfrom their ‘nurse’
(Sertoli) cells in the testis, theyare transported in fluid secretedbythe Sertolicellstoacollectingarea,therete
testis. Fromthere, the dilute suspensionof spermenter
thethin-walledefferentductsintheepididymisheadand theepitheliaofefferentductsexpressenormousestrogen receptors(Fisheretal.,1997).Thespermpassthroughthe
epididymis wheretheymatureand progresstoward the
vas deferens. Moreover, the ESR1 in the epididymis are
reportedtomodulatesecretionofproteinssuchasoscilin thatpromotesthematurityandviabilityofthe spermato-zoa(MowaandIwanaga,2001).Themostintensesignals ofESR1arereportedinepithelialcellsintheheadandtail
oftheepididymisinratsandmonkeysandsuggestedtobe responsibleforsemenconcentration(Fisher etal.,1997; Hessetal., 1997).Our studyidentified astrongsignalof ESR1inthesmoothmusclelayerinthetailoftheepididymis
whichisinaccordancewithaprevious
immunohistochem-icalstudyinhumanandrabbitwhichstatedthatestrogen isessentialfortransportingthespermatozoaby influenc-ingepididymalsmoothmusclecontractilityaswellasfor ejaculationwiththehelpofoxytocin(Filippietal.,2002). ItisimportanttonotethattheexpressionofESR1in
dif-ferentpartsof theepididymiscouldvaryamong species
sinceithasbeenlocalizedinallpartsofepididymisinthe rat(Pelletieretal.,2000),mouse(Zhouetal.,2002),bonnet monkey(Shayuetal.,2004),horse(Parlevlietetal.,2006), cat(Nieetal.,2002)anddog(Nieetal.,2002).
We found that the ESR1 is remarkably localized in
spermwithinthelumenoftheepididymis.ESR1was
espe-ciallyimmunolocalizedinthe postacrosomalregionand
tail of the sperm in this study (Fig. 3e). This result is
ingoodagreementwithpreviousstudieswhichlocalized
immunoreactiveESR1inthepostacrosomalregionofthe
spermhead(Solakidietal.,2005)andinthetailpieceofthe sperm(Durkee etal.,1998)inhumans.Thepost
acroso-malregionofspermisinvolvedinthesperm–eggplasma
membranefusion(Liuetal.,2008).ThelocalizationofESR1
in the post acrosomal region implies its involvementin
thefertilizationprocess(Ramalho-Santosetal.,2002).Itis importanttonotethatthereareotherproteinsidentified
inthepostacrosomalregionofspermsuchasequatorin
andoscilinwhichareimportantforsuccessfulfertilization (Montagetal.,1998;Solakidietal.,2005).Ourlocalization ofESR1inthetailofporcinespermcoincidedwithprevious reportsinhumans(Durkeeetal.,1998;Aquilaetal.,2002). Moreover,Aquilaetal.(2002)reportedthattheESR1might beinvolvedinspermsurvivalandmotility.Impaired motil-ityofspermhasbeenreportedinmicelackingfunctional ESR1insperm(Eddyetal.,1996)andinESR1knockoutmice (HessandCarnes,2003).Lowfertilizationrateshavebeen reportedincaseofimmotilespermatozoa(Nijsetal.,1996).
5. Conclusion
Associations of ESR1 gene polymorphisms with boar
spermqualityand fertilitytraitshavebeen describedfor
thefirst time,providingevidencethatESR1mightbean
importantcandidategeneforboarspermqualityand fertil-ity.However,thisstudyhastobevalidatedinotheranimal populationsinordertoevaluateitspotentialinselective breeding.Finally,theESR1mightplayarolein spermatoge-nesisvalidatedthroughassociationstudyandbyprofiling
ofmRNAandproteinexpressioninnon-reproductiveand
reproductivetissuesincludingspermatozoa.
Acknowledgments
This research was financially supported by FBF
(Förderverein Biotechnologieforschung e. V., Bonn,
Germany). We thank Dr.A. Niggemeyer, Dr.S. Brüning,
Dr. M. Friedrichs and Dr. A. Riesenbeck (GFS Artificial
InseminationStation:Die GenossenschaftzurFörderung
Hofer, Dr.H. Lutherand Dr.K. Caspari(SuisAG Artificial
Insemination Station, Sempach, Switzerland) for their
support during the sample collection and the
arrange-ments ofthereproduction records.We arealsothankful
to Prof. Dr. C. Knorr (Institute of Veterinary Medicine,
Georg-August-University, Goettingen, Germany) for his
co-operation.
References
Aquila,S.,Sisci,D.,Gentile,M.,Middea,E.,Siciliano,L.,Ando,S.,2002. Human-ejaculated spermatozoacontainactiveP450aromatase. J. Clin.Endocrinol.Metab.87,85–90.
Capon,F.,Allen,M.H.,Ameen,M.,Burden,A.D.,Tillman,D.,Barker,J.N., Trembath, R.C.,2004. Asynonymous SNPofthe corneodesmosin geneleadstoincreasedmRNAstabilityanddemonstrates associa-tionwithpsoriasisacrossdiverseethnicgroups.Hum.Mol.Genet.13, 2361–2368.
Carreau,S.,Bourguiba,S.,Lambard,S.,Galeraud-Denis,I.,Genissel,C., Lev-allet,J.,2002.Reproductivesystem:aromataseandestrogens.Mol. Cel.Endocrinol.193,137–143.
Clulow,J.,Jones,R.,Hansen,L.,1994.Micropunctureandcannulation stud-iesoffluidcompositionandtransportintheductuliefferentestestis oftherat:comparisonswiththehomologousmetanephricproximal tubule.Exp.Physiol.79,915–928.
Couse,J.,Korach,K.,1999.Estrogenreceptornullmice:whathavewe learnedandwherewilltheyleadus?Endocr.Rev.20,358–417. Durkee,T.,Mueller,M.,Zinaman,M.,1998.Identificationofoestrogen
receptorproteinandmessengerribonucleicacidinhuman spermato-zoa.Am.J.Obstet.Gynecol.178,1288–1295.
Eddy,E.,Washburn,T.,Bunch,D.,Goulding,E.,Gladen,B.,Lubahn,D., Korach,K.,1996.Targeteddisruptionoftheestrogenreceptorgene inmalemicecausesalterationofspermatogenesis andinfertility. Endocrinology137,4796–4805.
Filippi,S.,Vannelli,G.B.,Granchi,S.,Luconi,M.,Crescioli,C.,Mancina, R.,Natali,A.,Brocchi,S.,Vignozzi,L.,Bencini,E.,Noci,I.,Ledda,F., Forti,G.,Maggi,M.,2002.Identification,localizationandfunctional activityofoxytocinreceptorsinepididymis.Mol.Cel.Endocrinol.193, 89–100.
Fisher,J.,Millar,M.,Majdic,G.,Saunders,P.,Fraser,H.,Sharpe,R.,1997. Immunolocalisationofoestrogenreceptor1within thetestisand excurrentductsoftheratandmarmosetmonkeyfromperinatallife toadulthood.J.Endocrinol.153,485–495.
Ganjam, V., Amann, R., 1976. Steroid content of fluids and sperm entering and leaving the bovine epididymis, in epididymal tis-sue, and in accessory sex gland secretions. Endocrinology 99, 1618–1630.
Guarducci,E.,Nuti,Becherini,L.,Rotondi,M.,Balercia,G.,Forti,G.,Krausz, C.,2006.Estrogenreceptor2promoterpolymorphism:stronger estro-genactioniscoupledwith lowerspermcount.Hum. Reprod.21, 994–1001.
Hejmej,A.,Gorazd,M.,Kosiniak-Kamysz,K.,Wisniewska,B.,Sadowska, J.,Bilinska,B.,2005.Expressionaromataseandandrogenreceptorsin reproductivetissueofthestallionandasinglecryptochidvisualized bymeansofimmunohistochemistry.Domest.Anim.Endricinol.29, 534–547.
Hess,R.,Carnes,K.,2003.Theroleofoestrogenintestisandmale repro-ductivetract:areviewandspeciescomparison.Anim.Reprod.1,5–30. Hess,R.,Gist,D.,Bunick,D.,1997.Estrogenreceptor(alphaandbeta) expressionintheexcurrentductsoftheadultmaleratreproductive tract.J.Androl.18,602–611.
Iida,K.,Akashi,H.,2000.Atestoftranslationalselectionat‘silent’sitesin thehumangenome:basecompositioncomparisonsinalternatively splicedgenes.Gene261,93–102.
Janulis, L.,Hess,R.,Bunick, D.,Nitta,H.,Janssen, S.,Asawa, Y.,Bahr, J.,1996.Mouse epididimalspermcontaintactiveP450aromatase which decrease as sperm traversethe epididymis. J. Androl.17, 111–116.
Jiang,W.,Le,B.,2000.StructureandexpressionofthehumanMEP1Agene encodingthealphasubunitofmetalloendopeptidasemeprinA.Arch. Biochem.Biophys.379,183–187.
Kaewmala,K.,Uddin,M.J.,Cinar,M.U.,Grosse-Brinkhaus,C.,Jonas,E., Tesfaye,D.,Phatsara,C.,Tholen,E.,Looft,C.,Schellander,K.,2011. Association study and expression analysis of CD9 as candidate geneforboarspermqualityandfertilitytraits.Anim.Reprod.Sci., doi:10.1016/j.anireprosci.2011.02.017.
Kuiper,G.,Enmark,E.,Puelto-Huiko,M.,Nilsson,S.,Gustafsson,J.,1996. Cloningofanovelestrogenreceptorexpressedinratprostateand ovary.Proc.Natl.Acad.Sci.U.S.A.93,2925–2930.
Lazaros,L.A.,Xita,N.V.,Kaponis,A.I.,Zikopoulos,K.A.,Plachouras,N.I., Georgiou,I.A.,2010.Estrogenreceptoralphaandbetapolymorphisms areassociatedwithsemenquality.J.Androl.31,291–298.
Lin,C.L.,Tholen,E.,Jennen,D.,Ponsuksili,S.,Schellander,K.,Wimmers,K., 2006.Candidategenemarkersforspermqualityandfertilityofboar. Anim.Reprod.Sci.92,349–363.
Liu,G.Q.,Zhu,J.J.,Wang,Z.Y.,Jiang,X.P.,Dafalla,M.M.,2008.Analysisof spermstorageabilityusingdurationoffertilityinhens.Br.Poult.Sci. 49,770–775.
Lubahn,D.B.,Moyer,J.S.,Golding,T.S.,1993.Alterationofreproductive functionbutnotprenatalsexualdevelopmentafterinsertional dis-ruptionofthemouseestrogenreceptorgene.Proc.Natl.Acad.Sci. U.S.A.90,11162–11166.
MacCalman,C.D.,Blaschuk,O.,1994.GonadalsteroidsregulateN-cadherin mRNAlevelsinthemousetestis.Endocr.Rev.2,157–163.
Montag,M.,Parrington,J.,Swann,K.,Lai,F.,VanderVen,H.,1998. Pres-enceandlocalizationofoscillininhumanspermatozoainrelationto integrityofthespermmembrane.FEBSLett.423,357–361. Mowa,C.N.,Iwanaga,T.,2001.Expressionofestrogenreceptor-alphaand
-betamRNAsinthemalereproductivesystemoftheratasrevealed byinsituhybridization.J.Mol.Endocrinol.26,165–174.
Munoz,G.,Ovio,C.,Estell,J.,Silio,L.,Fernadez,A.,Rodriguez,C.,2007. Association with litter size of new polymorphism on ESR1 and ESR2 genesina Chinese–Europeanpig line. Genet.Sel. Evol. 39, 195–206.
Nie,R.,Zhou,Q.,Jassin,E.,Saunders,P.,Hess,R.,2002.Differential expres-sionofoestrogenreceptorsalphaandbetainthereproductivetract ofadultmaledogsandcats.Biol.Reprod.66,1161–1168.
Nijs,M.,Vanderzwalmen,P.,Vandamme,G.,1996.Fertilizingabilityof immotilspermatozoaafterintracyctoplasmicsperminjection.Hum. Reprod.11,2180–2185.
O’Donnell,L.,Robertson,K.M.,Jones,M.E.,Simpson,E.R.,2001.Estrogen andspermatogenesis.Endocr.Rev.22,289–318.
Pagani,R.,Song,M.,McEnery,M.,Qin,N.,Tsien,R.W.,Toro,L.,Stefani, E.,Uchitel, O.D.,2004.Differentialexpressionofalpha1andbeta subunitsofvoltagedependentCa2+channelattheneuromuscular
junctionofnormalandP/QCa2+channelknockoutmouse.
Neuro-science123,75–85.
Parlevliet,J.,Pearl,C.,Hess,M.,Famula,T.,Roser,J.,2006. Immunolocaliza-tionofestrogenandandrogenreceptorsandsteroidconcentrations inthestallionepididymis.Theriogenoloy66,755–765.
Pearl,C.,Berger,T.,Roser,J.,2007.Estrogenandandrogenreceptor expres-sioninrelationtosteroidsconcentrationsinadultboarepididymis. Domest.Anim.Endocrinol.33,451–459.
Pelletier,G.,Labrie,C.,Labrie,F.,2000.Localizationofoestrogen recep-toralpha,oestrogenreceptorbetaandandrogenreceptorsintherat reproductiveorgans.J.Endocrinol.165,359–370.
Ramalho-Santos,J.,Schatten,G.,Moreno,R.,2002.Controlmembrane fusionduringspermiogenesisandtheacrosomereaction.Biol.Reprod. 67,1043–1051.
Rozen,S.,Skaletsky,H.,2000.Primer3ontheWWWforgeneralusersand forbiologistprogrammers.MethodsMol.Biol.132,365–386. Safarinejad,M.R.,Shafiei,N.,Safarinejad,S.,2010.Associationof
polymor-phismsintheestrogenreceptorsalpha,andbeta(ESR1,ESR2)with theoccurrenceofmaleinfertilityandsemenparameters.J.Steroid Biochem.Mol.Biol.122,193–203.
Schleicher,G.,Drew,U.,Stumpt,W.,Sar,M.,1984.Differentialdistribution ofdihydrosteroneandestrodialbindingsiteintheepididymisofthe mouse:anautoradiographicstudy.Histochemistry,1139–1147. See,M.,2000.SelectionforAIstudtraits.In:ProceedingsofNationalSwine
ImprovementFederation,Nashville,Tennessee,USA,7–8December, p.25.
Shayu, D.,Chennakesava, C., Soundarajan, R., Rao, J., 2004. Effect of ICI 182780onestrogenreceptor expression,fluidabsorptionand spermmotilityintheepididymisofthebonnetmonkey.Reprod.Biol. Endocrinol.3,3–10.
Solakidi,S.,Psarra,A.,Nikolaropouluos,S.,Sekeris,C.,2005.Estrogen receptoralphaandbetaandandrogenreceptor(AR)inhumansperm: localizationofEstrogenbetaandARinmitochondriaofthemidpiece. Hum.Reprod.20,3481–3487.
Stalhammar,E.,Janson,L.,Philipsson,J.,1994.Geneticstudiesonfertility inAIbulls.II.Environmentalandgeneticeffectsonnon-returnrates ofyoungbulls.Anim.Reprod.Sci.34,193–207.
Syntin, P.,Dacheux, F.,Druart,X., Gatti, J., Okamura,N.,Dacheux, J., 1999. Characterization and identification of proteins secreted in thevariousregionsoftheadultboarepididymis.Biol.Reprod.55, 956–974.
Wimmers,K.,Lin,C.,Tholen,E.,Jennen,D.,Schellander,K.,Ponsuksili,S., 2005.Polymorphismsincandidategenesasmarkersforspermquality andboarfertility.Anim.Genet.Sel.Evol.36,152–155.
Xing,Y.,Ren,J.,Ren,D.,Guo,Y.,Wu,Y.,Yang,G.,Mao,H.,Brenig,B.,Huang, L.,2008.Awholegenomescanningforquantitativetraitlociontraits relatedtospermqualityandejaculationinpigs.Anim.Reprod.Sci. 114,210–2118.