www.elsevier.com / locate / bres
Research report
Polycyclic neuromodulation of the feeding rhythm of the pond snail
Lymnaea stagnalis by the intrinsic octopaminergic interneuron, OC
´
*
Christopher J.H. Elliott , Agnes Vehovszky
Department of Biology, University of York, PO Box 373, York YO10 5YW, UK
Accepted 12 September 2000
Abstract
We have examined the role of the octopamine-containing buccal OC interneuron in the fictive feeding rhythm generated by depolarizing a modulatory interneuron, SO, in the isolated central nervous system (CNS) of Lymnaea stagnalis. Before stimulating the SO, the initial fictive feeding rate was 2.060.37 bites / min (mean6S.E.). When the SO was stimulated, the fictive feeding rate more than doubled, increasing by 5.462.6 bites / min. Prestimulation of OC facilitates the ability of the modulatory neuron SO to drive fictive feeding 4 s later. Following OC stimulation, the increase in SO-driven feeding rate was 10.861.6 bites / min, significantly more than when only the SO was stimulated (P,0.02, paired t-test on five preparations). OC activity is not required during the SO stimulation for this enhancement. The maximum of the SO driven rhythm occurs between 6 and 12 s after the end of the OC stimulation at 20 bites / min. This is the maximum feeding rate of intact Lymnaea in sucrose. Facilitation is mimicked by bath applied octopamine at 5mM. Facilitation is specific to OC interneurons, as the same prestimulation of the electrically coupled neuron N3P (central pattern generator) interneurons does not affect the feeding rhythm. The OC interneuron acts as a long term, polycyclic modulator, which peaks several feeding cycles after the OC activity. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Mollusc; Octopamine; Long-term; Polycyclic; Snail; Interneuron
1. Introduction manipulate the cycle in which it is stimulated (intra-cyclic
modulation), or it may have effects that last for many A central feature of the organisation of rhythmically cycles. Such polycyclic modulators are of particular be-active systems is that the pattern generator neurons are havioural interest because they provide mechanisms to influenced by many modulatory neurons. One way to translate a transient stimulus into a persisting response and classify neuromodulators is by their function: they may to allow plasticity of behaviour, for example accelerating trigger the pattern, alter its intensity or rate and even ingestion once a palatable food has been found. Polycyclic reconfigure its phasing. An example from the feeding modulation has not been described for any of the protrac-system of the pond snail Lymnaea is the SO interneuron in tion phase interneurons described in Lymnaea so far; but the buccal ganglia. This can activate fictive feeding in a the objective of this paper is to show that the swallowing quiescent preparation, accelerate and intensify it if fictive phase OC interneurons identified recently [21] have a clear feeding is already occurring [16] and produce a change in polycyclic role.
the phase pattern [3]. However, the SO does not participate Another classification of neuromodulators [9] divides in all the feeding rhythms, for example those driven by them into extrinsic or intrinsic, with the main criterion N1L or CV1 ([14], Fig. 9), ([24], Fig. 9). being that the intrinsic modulators receive rhythmically Neuromodulatory cells may also be separated according patterned inputs from the pattern generator. On this basis, to the time-scale of their effects: an interneuron may the SO can be classified as an intrinsic modulator, since it receives weak excitation and strong inhibition from the central pattern generator circuit, irrespective of whether it is active or not [5]. Other intrinsic modulators of the Lymnaea feeding system include the protraction phase
*Corresponding author. Tel.: 144-190-443-2812; fax:
144-190-443-interneurons CV1 [14] and CBWC [15] in the cerebral 2860.
E-mail address: [email protected] (C.J.H. Elliott). ganglia and the N1L [24] interneurons in the buccal
ganglia. The OC interneurons are also intrinsic neuro- 2. Materials and methods modulators of the feeding system, because they receive
regular inhibitory inputs in the N1 (protraction) and N2 Pond snails, Lymnaea stagnalis were obtained from (rasp) phases of the feeding rhythm [21]. dealers (Blades Biological, Kent, UK) and fed on lettuce. There are just three OC octopamine immunoreactive The circumoesophageal and buccal ganglia were isolated interneurons in the buccal ganglia [2]. They are weakly and neurons impaled as described previously [7,21]. The electrically coupled together and do not fire 1:1 action large buccal motoneurons were visually identified [1] and potentials when one is stimulated. During fictive feeding, the feeding interneurons, SO, N1L, N3p and OC were they tend to fire in the third, swallowing, phase of the identified by probing in their usual positions, and checking rhythm. In some preparations the OC cells rarely fire their identity by firing pattern and interneuronal con-action potentials, but usually their firing rate increases as nections with the motoneurons [4,5,21,24]. The preparation fictive feeding is driven more strongly, with a maximum was bathed in normal Lymnaea saline [6]. Octopamine was firing rate in the isolated central nervous system (CNS) obtained from Sigma (Poole, Dorset, UK).
being 15 Hz. They are always robustly inhibited in the first two phases of the rhythm (protraction and rasp) and these
inputs are always present [20,21]. 3. Experimental design We have previously demonstrated rapidly activating,
short-term intra-cyclic connections to buccal interneurons 3.1. Repetitive OC stimulation and motoneurons. The OC interneurons affect all the cells
tested with the overall effect of these transient connections We used repetitive intracellular stimulation of the OC being to enhance the retraction of the radula and the neurons in these experiments to mimic the way it fires in swallowing movement [20,21]. The inhibitory connections the feeding pattern. Intact Lymnaea rasp at up to 20 to the B3 motoneurons, N2 and N3p interneurons are bites / min (one cycle every 3–4 s) with the third, swallow-known to be octopaminergic since they are blocked by ing, phase lasting about 1 s. Passing suprathreshold antagonists including phentolamine and epinastine [19]. depolarising current pulses at a similar rate and duration The OC interneurons also make electrical connections to into an OC interneuron (Fig. 1A) produces synaptic inputs buccal neurons, including the B4 motoneurons and the (an alternating pattern of inhibition and excitation) in the N3p interneurons (which participate in rhythm generation). N1L and SO neurons. These inputs increase in size with Like the connections between the OC interneurons, these each repetition and eventually the burst of spikes in the electrical synapses are weak and the neurons do not N1L evokes a single cycle of the feeding pattern, as shown normally fire 1:1 action potentials. Although they are by the deep inhibitory N2 (rasp phase) inputs on both the electrically coupled, the OC and N3p interneurons make N1L and SO interneurons.
different short-lasting connections to many of their follow-ers. For example the B3 motoneuron is excited by N3p [4] but inhibited by OC [20] suggesting these interneurons do not release the same neurotransmitter.
In our experiments, we demonstrate long-term modula-tion by pre-stimulating an OC interneuron and showing that this facilitates the ability of the SO modulatory interneuron to drive fictive feeding. The SO [16] is an identified buccal interneuron which is excited by sucrose solutions and mechanical stimuli applied to the buccal cavity [11]. In the isolated CNS, when stimulated by steady current, the SO will drive fictive feeding up to the normal feeding rate (mean 16, maximum 20 bites / min) of
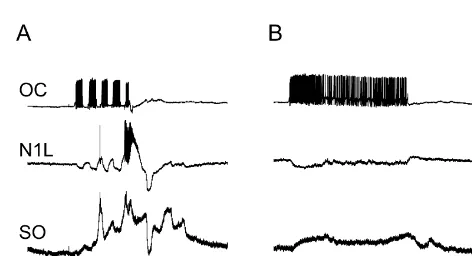
Fig. 1. Comparison of pulse and tonic stimulation of OC interneuron. (A) intact Lymnaea [11,21]. Small currents produce a weak
Suprathreshold pulses of stimuli (five in 10 s) into the OC interneuron rhythm, with the intensity of the synaptic inputs and the
produce short-latency inputs onto the protraction phase interneurons N1L feeding rate increasing with stimulating current. The SO is and SO. With repetition, the size of the potential grows and eventually the electrically coupled to another protraction phase neuro- N1L reaches threshold and fires a burst of action potentials. This activates modulator, the N1L interneuron [24]. Both of these the feeding system and a single feeding cycle occurs, with a strong rasp (N2) input. this is marked by an asterisk. (B) Tonic stimulation of the OC neurons are strongly and reliably inhibited in the rasp (N2)
interneuron with the same current produces maintained, short latency phase, so that their pattern can be used to count the feeding
The effects of tonically stimulating the OC is much min. In the isolated CNS, the fictive feeding pattern was smaller than the repetitive stimulation (Fig. 1B). This monitored and each N2 (rasp phase) input on the SO could be because the OC→SO and OC→N1L connections counted as 1 bite. The N2 inputs are the only central are biphasic, with both inhibitory and excitatory com- pattern generator inputs that inhibit the SO interneuron ponents [20]. In Fig. 1B, the inputs to the SO and N1L do strongly enough to stop it firing.
not lead to activation of the feeding pattern. Note that the firing rate of the OC in the tonic and repetitive stimuli
(Fig. 1A and b) are nearly the same (25–30 spikes / s). 4. Results
3.2. Design of prepulse OC experiment 4.1. Prepulse activation of an OC interneuron modulates the feeding system
Since repetitive OC stimulation has quick outputs on its
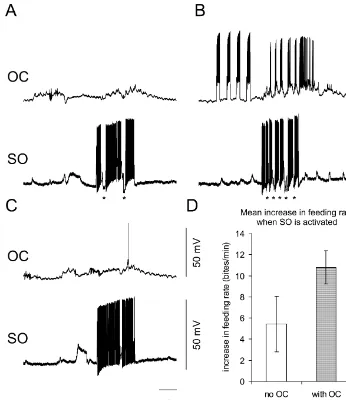
followers in the feeding system (Fig. 1A), we used a SO-driven fictive feeding is enhanced when it is pre-prepulse experiment to separate these intra-cyclic effects ceded by OC prepulse stimuli. Fig. 2A shows a control from the long-term, polycyclic modulation. experiment — in which the stimulus current into the SO We compare the fictive feeding produced by steady protraction phase interneuron triggers SO action potentials stimulation of the SO interneuron with the response to the and two inhibitory rasp (N2) phase inputs, making the same SO current after prepulse stimulation to the OC feeding rate 5.4 bites / min. In the experimental panel (Fig. interneuron. Three aspects of the experiment are important. 2B), a set of prepulse stimuli to the OC interneuron for 20 First, we allowed 4 s between the end of the prepulse s (frequency about 10 cycles / min), is followed 4 s later by stimulation of OC interneuron and the start of the injection the same depolarising current to the SO as in the control. of depolarising current into the SO interneuron. This The feeding rate evoked by SO stimulation is much higher, approach ensures that any intra-cyclic effect will have 17 bites / min and the whole central pattern generator decayed away because 4 s is the average time for a network is much more intensely activated, as indicated by complete feeding cycle. the strong bursts in the OC interneuron. The third panel Secondly, the current injected into the OC neuron during (Fig. 2C) is a second control, 10 min later, without OC the prepulse was set between the level required to evoke stimulation. The feeding rate evoked by the SO is again postsynaptic potentials onto the follower feeding inter- low (3.8 bites / min), and no burst of activity is seen on OC neurons and the level at which the PSPs summated to neuron.
produce spikes in the follower cells which activate the Our results from 26 trials in five preparations are feeding rhythm. In the experiments analysed here, the summarised in Fig. 2D. This shows the increase in feeding mean firing rate of the OC interneurons is less than in Fig. rate produced by stimulating the SO in the two situations: 1, with a mean of 17 Hz. This rate provides a suitable with and without OC prepulses. Before SO stimulation, the stimulus, because the maximal-firing rate of OC inter- mean feeding rate was 2.060.37 bites / min (mean6S.E.). neurons we have seen in the isolated CNS is 15 Hz. When only the SO is stimulated, the fictive feeding rate Furthermore, we only stimulate one of the OC interneurons more than doubles, increasing on average by 5.462.6 though the microelectrode. Although the other two OC bites / min. However, if the OC stimulus precedes the SO cells are electrically coupled, they are unlikely to fire stimulus the increase in fictive feeding is much bigger, because the electrical coupling is not strong enough to 10.861.6 bites / min. This difference is significant at the evoke action potentials on any of the coupled neurons [21]. 2% level (paired t-test, N55).
Thirdly, we used a small current to depolarize the SO, The histogram in Fig. 3 shows the time course of the so that the feeding rhythm is not activated too strongly in changes in fictive feeding rate before, during and after SO the control traces. Because the SO was less depolarized stimulation. The shaded and open bars show the data with than in many previous publications, e.g. [5,16,23], the N2 and without OC stimulation, respectively. Although the and N3 inputs seen in the SO, N1L and OC interneurons OC stimulation ends 3.861.7 s before the SO stimulation appear smaller. begins, the main increase in fictive feeding rate takes place Taking these aspects together, we can be sure that any during the first 12 s of SO stimulation (Fig. 3). The fastest change in the SO driven fictive feeding is due to the feeding rate recorded following the OC prepulse is 20 prepulse activation of the OC interneuron, prior to SO bites / min and this occurs 6–12 s after the start of SO stimulation. depolarization (i.e. 10–16 s after the end of the OC Twenty-six experiments (14 with, 12 without the OC prepulse stimuli). In the period before SO stimulation, the stimuli), from five preparations were analysed. For statisti- fictive feeding rate is not significantly different between cal tests, the control and OC prepulse feeding rate from control and OC prepulse trials (2.360.55 and 1.860.5 each preparation were averaged, and the means compared bites / min, respectively).
Fig. 2. OC prepulse protocol enhances SO driven fictive feeding (A) Initial control, injection of depolarising current into the SO interneuron evokes a weak fictive feeding pattern, with just cycles as shown by the two inhibitory (N2, rasp) inputs (asterisks). (B) When the OC interneuron is stimulated with four prepulses, and 4 s later the same current is injected into the SO, the SO evokes a faster and stronger feeding pattern, with five feeding cycles (N2 inputs marked by asterisks) and the recruitment of the OC interneuron into the pattern. (C) A repeat control after 10 min showing that the SO alone with the same current cannot activate the feeding system. (D) Summary of 24 experiments. SO stimulation increases the feeding rate by 5.4 bites / min, but when it is preceded by OC stimulation, the SO induced increase is 10.8 bites / min. The bars represent the standard error. The difference is significant at the 2% level (paired t-test on five preparations).
another preparation. This time the stimulus current to the OC and SO interneurons is not required for the long-term SO was set to a level in which the SO fired at a rate too modulatory effect of the OC interneurons.
slow to produce rhythmic feeding activity in any of the
neurons recorded (Fig. 4A). After the OC prepulse with 4.2. Specificity of the modulation to the OC interneuron parameters similar to those in the previous experiment (12
Fig. 3. A histogram showing the time course of the effect of the OC prestimulus on SO driven fictive feeding. Summary of 26 trials (14 with OC, 12 without) in five preparations. Each bar shows the mean feeding rate in each 6-s period (the minimum time for two feeding cycles) with time measured relative to the start of SO stimulation. The open bars represent control trials, with no OC stimulation, while the filled bars indicate the trials in which the OC prepulse stimulation was applied. The dotted line under the graph represents the average time of OC stimulation, while the solid bar shows the average period of SO stimuli. The OC stimulation ended 3.7 s before SO stimulation. During the SO depolarization, the trials with an OC prepulse gave a significantly higher feeding rate, but there is no significant difference in feeding rate beforehand.
facilitatory modulation of OC neuron cannot be replicated presence of octopamine facilitates the SO-activation of by stimulating the N3p interneurons in the same way. In feeding pattern.
Fig. 4B, the same prepulse protocol is used, but now stimulating the N3p interneuron (instead of the OC
inter-neuron) before depolarizing the SO interneuron. The 5. Discussion repetitive stimulation of the N3p interneuron for 20 s
evokes synaptic responses on both OC and SO neurons, 5.1. OC interneurons produce a polycyclic modulatory but fails to enhance the subsequent response to SO effect on the feeding network
depolarization. This experiment was replicated in a second
preparation. Our prepulse experiments show that the ability of the SO to drive fictive feeding is enhanced if the OC inter-neuron fires beforehand. In this case, the time between the 4.3. Octopamine mimics the modulatory effect of OC end of the OC prepulse and start of SO current was 3.7 s. stimulation The enhancement peaks about 12 s after the end of OC stimulation. As feeding cycles normally take 3–5 s in the Applying a low concentration of octopamine mimics the intact Lymnaea and in the isolated CNS, this means that facilitatory effect of OC stimulation. In the control panel the prepulse stimulation of the OC interneuron is having an (Fig. 5A), weak current injection into SO interneuron effect, which lasts three to four times longer than the elicits a slow feeding pattern with two inhibitory inputs in feeding cycle, i.e. the OC is a polycyclic neuromodulator. 22 s (which corresponds to the feeding rate of 5.4 bites / The effect that we have observed is quite powerful, as the min). In contrast, in the presence of 5mM octopamine in OC prepulse enhances the SO-driven rhythm nearly 4-fold, the bath, passing the same current into the SO produces a up to a peak of 20 bites / min, the maximum feeding rate of feeding rhythm nearly twice as fast (four inhibitory inputs Lymnaea in sucrose [11,21].
the feeding rhythm. Secondly, the short term, intra-cycle action of the OC interneuron is a biphasic effect (inhibition then excitation) on all known protraction phase inter-neurons. This inhibitory component is very effective in preventing the SO, N1L or N1M from firing [20].
The facilitatory neuromodulation by the OC does not depend on reinforcement cycle by cycle: even when the SO is weakly stimulated and the OC is not recruited into the feeding pattern facilitation still occurs (Fig. 4). How-ever, in the intact snail, or in rhythms driven by the SO or other modulatory interneurons, the OC will fire in a rhythmic manner because of the strong N1 and N2 phase inhibitory inputs [20], and thus the OC interneurons will influence both the current cycle and several subsequent ones.
5.2. Behavioural role of polycyclic neuromodulation
Polycyclic facilitation may be important behaviourally, as molluscs usually accelerate their feeding rate during a feeding episode. This is true of the intact animal [18], and also sometimes of fictive feeding in the isolated CNS, when the rhythm is driven by tonic current into SO, CV1 or N1L (see e.g. [23], Fig. 9A or 10A). This acceleration could be accounted for by the recruitment of the OC to the third, swallowing phase of the first cycle and its facilitatory effect on subsequent cycles of the feeding pattern. In many molluscs (including Lymnaea, Helix and Aplysia) the Fig. 4. Stimulation of the OC interneuron, but not the N3p interneuron,
behavioural acceleration of feeding has been partially enhances the SO-driven fictive feeding. (A) A small control current to the
accounted for by the action of an extrinsic modulatory SO evokes no feeding rhythm. (B) Prepulse stimulation of the N3P
interneuron (electrically coupled to the OC) does not facilitate the interneuron, the serotonergic giant cell in the cerebral SO-activation of fictive feeding 4 s later. (C) OC prestimulation followed ganglia [8,12,13,22]. However, in Aplysia, ablation of this by the same the SO depolarizing current 4 s later elicits a rhythmic
cell shows that intrinsic interneurons have a major role in feeding pattern with the N2 (rasp) inputs indicated by asterisks. (D) A
the control of feeding latency [17]. The properties of second control with no OC or N3P activation.
neurons like OC, enhancing the responsiveness of central networks, could account for these observations.
5.3. Comparison with other molluscan neuromodulatory interneurons
This long-term, polycyclic effectiveness of the OC interneuron is an important difference from the properties of the other intrinsic modulators in the Lymnaea feeding system which accelerate or (in a quiet preparation) initiate feeding (SO [16]; N1L [24]; CV1 [14]; and CBWC [15]). All these typically have their effects on the cycle of feeding in which they are activated, either affecting the current phase or else the current and next phases. Once the stimulus is turned off, the control situation is quickly restored. These interneurons lack a polycyclic facilitatory Fig. 5. The response of the feeding system is enhanced by 5 mM effect like that of the OC interneuron.
octopamine. (A) Control depolarization of the SO drives weak fictive There are some similarities with the serotonergic inter-feeding with one weak and one strong inhibitory input (N2, rasp, asterisk) neuron DSI in the swimming network of Tritonia. Like the to the N1L interneuron. (B) in 5mM octopamine, the same depolarizing
OC, the DSI interneuron is an intrinsic neuromodulator current evokes a faster and stronger fictive feeding rhythm, with deeper
with fast, intra-cyclic and slower, polycyclic actions [10]. activation of the N2 inhibitory inputs (asterisks) and recruitment of the
[5] C.J.H. Elliott, P.R. Benjamin, Interactions of the slow oscillator C2 transiently. The polycyclic, longer-term modulatory
interneuron with feeding pattern-generating interneurons in Lymnaea effects include enhancement of the synaptic output of the
stagnalis, J. Neurophysiol. 54 (1985 b) 1412–1421.
C2 interneuron and reduction in its spike frequency [6] C.J.H. Elliott, P.R. Benjamin, Esophageal mechanoreceptors in the adaptation. Both for DSI and OC interneurons the poly- feeding system of the pond snail, Lymnaea stagnalis, J. Neuro-cyclic effects are behaviourally important. For DSI they physiol. 61 (1989) 727–736.
[7] C.J.H. Elliott, R.A. Stow, C. Hastwell, Cholinergic interneurons in enable a swim episode to be initiated and supported while
the feeding system of the pond snail Lymnaea stagnalis. 1. Choliner-for OC only the polycyclic component enhances the
SO-gic receptors on feeding neurons, Philos. Trans. Royal Soc. Lond. B driven fictive feeding. As noted above, the short term 336 (1992) 157–166.
effects are to inhibit many of the central pattern generator [8] L. Hernadi, L. Hiripi, A. Vehovszky, G. Kemenes, K.S. Rozsa,´ ´ ´ interneurons and this short-term inhibition may explain the Ultrastructural, biochemical and electrophysiological changes in-duced by 5,6-dihydroxytryptamine in the CNS of the snail Helix fact that when fictive feeding is strongly active, OC
pomatia L., Brain Res. 578 (1992) 221–234. stimulation produces a transient slowing in the rate [20].
[9] P.S. Katz, W.N. Frost, Intrinsic neuromodulation — altering neuronal circuits from within, Trends Neurosci. 19 (1996) 54–61.
5.4. Specificity of the long-term effect [10] P.S. Katz, W.N. Frost, Removal of spike frequency adaptation via neuromodulation intrinsic to the Tritonia escape swim central pattern generator, J. Neurosci. 17 (1997) 7703–7713.
Only the OC interneuron and not its electrically coupled
[11] G. Kemenes, C.J.H. Elliott, P.R. Benjamin, Chemical and tactile counterpart the N3p interneuron, can produce the
long-inputs to the Lymnaea feeding system — effects on behavior and term facilitation of the SO-driven feeding pattern. This neural circuitry, J. Exp. Biol. 122 (1986) 113–137.
experiment is important, for three reasons. First, it shows [12] G. Kemenes, L. Hiripi, P.R. Benjamin, Behavioral and biochemical-that the effects of stimulating an OC interneuron were not changes in the feeding system of Lymnaea induced by the dopamine and serotonin neurotoxins 6-hydroxydopamine and 5,6-dihydroxy-mediated through the electrically coupled interneuronal
tryptamine, Philos. Trans. Royal Soc. Lond. B 329 (1990) 243–255. network. Second, both the N3p and OC interneurons
[13] I. Kupfermann, K.R. Weiss, Activity of an identified serotonergic produce a weak depolarization of the SO, which decays neuron in free moving Aplysia correlates with behavioral arousal, rapidly. This shows that this short-term depolarization Brain Res. 241 (1982) 334–337.
cannot account for the polycyclic effects. Thirdly, we [14] C.R. McCrohan, Initiation of feeding motor output by an identified interneurone in the snail Lymnaea stagnalis, J. Exp. Biol. 113 know that the OC interneuron — but not the N3p
(1984) 351–366. interneuron is octopamine immunoreactive [21]. Our
ex-[15] C.R. McCrohan, R.P. Croll, Characterization of an identified cere-periments here suggest that the OC and N3p interneurons brobuccal neuron containing the neuropeptide APGWamide (Ala-differ in their ability to induce polycyclic modulation as Pro-Gly-Trp-NH2) in the snail Lymnaea stagnalis, Invert. Neurosci. well as in their intra-cycle modulation [20]. Further, the 2 (1997) 273–282.
[16] R.M. Rose, P.R. Benjamin, Interneuronal control of feeding in the effect of prepulse stimulation of OC neurons (Fig. 2) is
pond snail Lymnaea stagnalis. 1. Initiation of feeding cycles by a mimicked by a concentration of bath octopamine which is
single buccal interneurone, J. Exp. Biol. 92 (1981) 187–201. 10 times less than the concentration required to activate the [17] S.C. Rosen, I. Kupfermann, R. Goldstein, K.R. Weiss, Lesion of a feeding rhythm [21]. This suggests that octopamine (which serotonergic modulatory neuron in Aplysia produces a specific is actually localised to the cell body of the OC inter- defect in feeding-behavior, Brain Res. 260 (1983) 151–155.
[18] A.J. Susswein, K.R. Weiss, I. Kupfermann, The effects of food neurons [21]) and which is used as a short-term transmitter
arousal on the latency of biting in Aplysia, J. Comp. Physiol. A 123 [19] might be the long-term modulator.
(1978) 31–41. ´
[19] A. Vehovszky, L. Hiripi, C.J.H. Elliott, Octopamine is the synaptic transmitter between identified neurons in the buccal feeding network
Acknowledgements of the pond snail Lymnaea stagnalis, Brain Res. 867 (2000a)
188–199. ´
[20] A. Vehovszky, C.J.H. Elliott, The octopamine-containing buccal We would like to thank the BBSRC for their support.
neurons are a new group of feeding interneurons in the pond snail Lymnaea stagnalis, Acta Biol. Hung. 51 (2000) 165–176.
´
[21] A. Vehovszky, C.J.H. Elliott, E.E. Voronezhskaya, L. Hiripi, K.
References Elekes, Octopamine: A new feeding modulator in Lymnaea, Philos.
Trans. Royal Soc. Lond. B 353 (1998) 1631–1643.
[22] K.R. Weiss, J.L. Cohen, I. Kupfermann, Modulatory control of [1] P.R. Benjamin, R.M. Rose, C.T. Slade, M.G. Lacy, Morphology of
buccal musculature by a serotonergic neuron (metacerebral cell) in identified neurones in the buccal ganglia of Lymnaea stagnalis, J.
Aplysia, J. Neurophysiol. 41 (1978) 181–203. Exp. Biol. 80 (1979) 119–135.
[23] M.S. Yeoman, D.C. Parish, P.R. Benjamin, A cholinergic modulatory [2] K. Elekes, E. Voronezhskaya, L. Hiripi, M. Eckert, J. Rapus,
interneuron in the feeding system of the snail Lymnaea, J. Neuro-Octopamine in the developing nervous system of the pond snail
physiol. 70 (1993) 37–50. Lymnaea stagnalis L., Acta Biol. Hung. 47 (1996) 73–87.
´
[24] M.S. Yeoman, A. Vehovszky, G. Kemenes, C.J.H. Elliott, P.R. [3] C.J.H. Elliott, T. Andrew, Temporal analysis of snail feeding
Benjamin, Novel interneuron having hybrid modulatory-central rhythms —a 3-phase relaxation-oscillator, J. Exp. Biol. 157 (1991)
pattern generator properties in the feeding system of the snail 391–408.
Lymnaea stagnalis, J. Neurophysiol. 73 (1995) 112–124. [4] C.J.H. Elliott, P.R. Benjamin, Interactions of pattern-generating