Iron content in vegetative and reproductive organs of
nectarine trees in calcareous soils during the development of
chlorosis
Moreno Toselli, Bruno Marangoni, Massimo Tagliavini *
Dipartimento di Colture Arboree,Uni6ersita` degli Studi di Bologna,Via Filippo Re,6-40126Bologna,Italy
Received 18 November 1999; received in revised form 13 April 2000; accepted 28 April 2000
Abstract
We investigated for 2 years (1995 – 1996) the time course development of chlorosis and the variation of iron (Fe) content in vegetative and reproductive organs in two nectarine orchards planted with cv Spring Red and cv Stark Redgold on calcareous soils of the Po valley (Italy) with the final aim to evaluate possible tools for the early prognosis of Fe chlorosis and a more efficient fertilization management. Due to the withdrawal of Fe supply, floral Fe concentration significantly decreased in 1996 as compared with 1995 in cv Spring Red, but not in Stark Redgold. Correlation coefficients between Fe and chlorophyll (Chl) from the same leaves were always higher when Fe was considered as amount present per leaf or per unit of leaf area than as leaf dry weight. The fact that chlorotic and green leaves had similar Fe concentration could be explained by an overestimate of Fe in the chlorotic leaf as a consequence of a reduction of its size. However, the decrease of Chl concentration between 60 and 120 days after full bloom (DAFB) occurred while leaf Fe content generally increased, indicating that even during chlorosis development leaves were supplied with some iron. We therefore suggest that the development of chlorosis was associated with an inactivation of Fe in the leaf apoplast. In 1995, regardless the cultivar, floral Fe concentration and leaf Chl were never correlated. In 1996 floral Fe concentration was linearly related to leaf Chl recorded 60 and 120 DAFB in cv Spring Red only. Floral Fe concentration at full bloom 1996, regardless the variety, was linearly related to leaf Chl determined in spring of the previous year, suggesting that flower Fe concentration might be used for assessing the storage of iron during the previous season. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Calcareous soil; Chlorosis; Flower; Iron;Prunus persica
www.elsevier.com/locate/eja
1. Introduction
Iron (Fe) deficiency-induced leaf chlorosis is the main nutritional disorder of peach orchards
planted on calcareous soil and its occurrence has been reported in several peach production areas, including Italy and others Mediterranean coun-tries (Sanz et al., 1992; Cinelli et al., 1995; Tagli-avini et al., 2000). Susceptibility to lime induced chlorosis of peach is higher when trees are grafted on seedlings (Prunus persica) than almond peach hybrids (Shi and Byrne, 1995; Socias y Company
* Corresponding author. Tel.:+39-51-2091490; fax:+ 39-51-2091500.
E-mail address:[email protected] (M. Tagliavini).
M.Toselli et al./Europ.J.Agronomy13 (2000) 279 – 286
280
et al., 1995), and may cause severe reduction of yields (Almaliotis et al., 1995; Tagliavini et al., 2000). To prevent chlorosis development, in southeastern part of the Po valley in Italy (Emilia Romagna region) for example, more than 3000 ha of peach and nectarine are yearly treated with soil applied iron chelates, at a rate of 4 – 5 kg ha−1of
commercial product with 6% Fe (Sansavini et al., 1999). These Fe fertilizers alleviate chlorosis symptoms and do not produce a permanent cure of Fe deficiency. Iron chelates are often applied according to previous experiences or orchard his-tory and, as a result, potentially chlorotic as well as non-chlorotic trees are fertilized with soil ap-plied Fe chelates before growth resumption in spring, even thought their use might not be re-quired. An early diagnosis of possible occurrence of Fe deficiency-induced chlorosis would be desir-able as the efficiency of both soil and leaf applied Fe fertilizers (mainly chelates) is higher if they are supplied before chlorotic symptoms appear. The effectiveness of Fe-chelate fertilisers (containing mainly Fe3+) depends on an enzymatic reduction
of Fe3+ to Fe2+ before entering the leaf cell
(Bru¨ggemann et al., 1993) which is severely de-pressed in chlorotic leaves (Kosegarten et al., 1999; Rombola` et al., 2000).
The usefulness of determining total leaf Fe concentration to predict Fe chlorosis is quite de-batable for a number of reasons including the fact that leaf analysis is usually performed in summer, when the application of Fe fertilizers have little effect on current fruiting. In addition, it has been
often demonstrated under field conditions that chlorotic leaves of plants grown in calcareous soils have relatively high total Fe concentration, often similar to that of green leaves (Mengel, 1994). The prognosis of Fe deficiency-induced chlorosis has been suggested through the analysis of total leaf Fe concentration (Montan˜e´z and Sanz, 1994) in an earlier period (spring) and through the analysis of Fe concentration in flow-ers at full bloom (Sanz and Montan˜e´z, 1995).
In the present study, we have characterized for 2 years the time course of the development of chlorosis in two nectarine orchards in calcareous soils in relation to the Fe content in vegetative and reproductive organs, with the final aim to evaluate possible tools for the prognosis of Fe chlorosis.
2. Materials and methods
The study was carried out in 1995 and 1996 in two nectarine [P. persica, Batsch var. nectarina
(Ait) Maxim.] orchards located in the southeast-ern part of the Po valley (Italy) and planted on calcareous soils, whose main characteristics are reported in Table 1. Local rainfall averages be-tween 700 and 800 mm per year mostly concen-trated in spring and autumn. In late summer 1994, 50 trees of cv Spring Red from orchard 1 (with a frame of 4.5×3.5 m) and 50 trees of cv Stark Redgold from orchard 2 (with a frame of
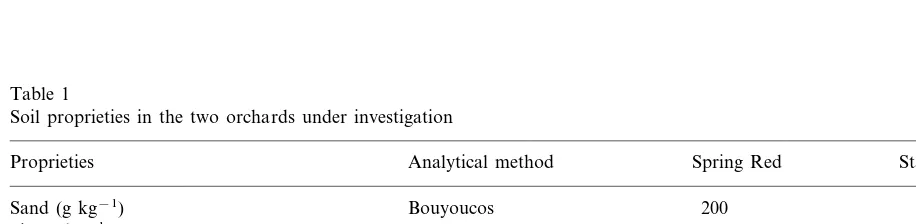
Table 1
Soil proprieties in the two orchards under investigation
Analytical method Stark Redgold
Proprieties Spring Red
Bouyoucos
Sand (g kg−1) 200 60
Silt (g kg−1) Bouyoucos 660 690
Bouyoucos
Clay (g kg−1) 140 250
Ca-carbonate (g kg−1) Gas volumetric 201 230
Droilneau 116
Active lime (g kg−1) 63
25 23
DTPA-extractable Fe (mg kg−1) Lindsay–Norvell
Organic matter (g kg−1) Walkley–Black 13 17
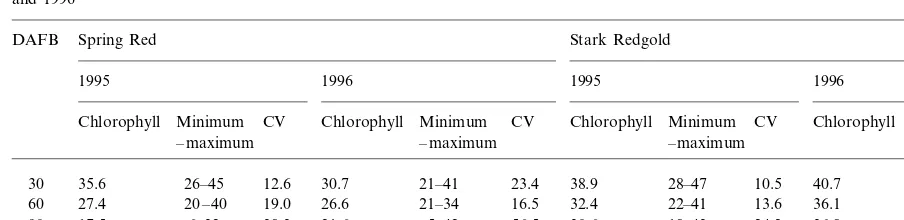
Table 2
Average minimum and maximum value of leaf Chl (mg cm−2
), and coefficient of variation (CV) as observed in Spring Red and Stark Redgold in 1995 and 1996
Chlorophyll Minimum Chlorophyll Minimum CV Chlorophyll Minimum CV Chlorophyll Minimum
– maximum – maximum
– maximum – maximum
7.6 35.6
30 26–45 12.6 30.7 21–41 23.4 38.9 28–47 10.5 40.7 34–44
21–40
22–41 13.6 6.4
19.0 20 – 40 27.4
60 26.6 21–34 16.5 32.4 36.1
17.5 21.2
90 6–32 38.3 21.6 5–42 56.5 30.6 18–43 24.2 36.8 17–49
14.7 7–23 29.2 24.5 5–45 48.6 25.2
120 12–39 23.8 32.3 13–42 24.8
4.0×3.5 m) were chosen to cover a wide range of chlorosis. In both orchards, the soil was completely tilled and drip-irrigated. The two drip emitters per tree, located at 1 m from the trunk on the tree row, ensured a soil moisture, under the emitters, near to the field capacity all summer long. The 8-year-old trees were grafted on seedlings and trained to palmette.
Since their plantation the two orchards had been regularly treated twice per year, in spring and late summer, with soil-applied Fe-chelates (at a rate of 1.2 g of Fe-EDDHA per tree, per treatment), while in 1995 and 1996, Fe supply was withdrawn in the selected trees. In 1995, at full bloom, 100 intact flowers per tree were collected from the middle portion of twigs; oven dried, milled to pass a 0.5 mm mesh and analyzed for total Fe concentration by atomic absorption spectrophotometry (AAS; Varian SpectrAA 200 HT, Australia). At 30, 60, 90 and 120 days after full bloom (DAFB), leaf chloro-phyll (Chl) was measured by the portable SPAD 502 m (Minolta Co., Ramsey, NJ, USA) on 20 young, healthy, and fully expanded leaves per tree, inserted in the middle portion of shoots. Four SPAD readings in four different sites per leaf were taken. SPAD values were converted into Chl con-centration (mg cm−2) after calibrations performed
separately for each cultivar and sampling dates. For calibration, leaf Chl was extracted from four leaf lamina disks (9 mm of diameter) under dark and low temperature in an acetone:water (80:20 v:v) solution for 72 h and the extract was spec-trophotometrically analyzed according to Arnon (1949).
At 60 and 120 DAFB, after SPAD
measure-ments, leaves were collected, carefully rinsed with deionized water and, after recording their area, oven dried, weighed, ground to pass a 0.5 mm mesh and analyzed for total Fe concentration by AAS, according to the guidelines of the Association of Official Analytical Chemists (AOAC, 1990). Leaf Fe was reported as, (1) concentration per leaf dry weight (mg Fe g−1DW); (2) per leaf area unit (mg
Fe cm−2); and (3) amount per leaf (
mg Fe per leaf).
In 1996, the study continued in both orchards on 15 trees selected, among those used in 1995, for their wide range of chlorosis symptoms.
Correlation analysis between leaf Chl, Fe in flowers and Fe in leaves was performed according to PROC CORR procedure of SAS (SAS Institute, Cary, NC, USA). The absence of significance and the significance of the correlation at 0.05, 0.01, and 0.001 level is indicated as NS, *, **, and ***, respectively. The differences between floral Fe concentration in 1996 and 1995 were assessed according to a repeated measures analysis of vari-ance (SAS Institute, Cary, NC, USA), with year as level of time.
3. Results
M.Toselli et al./Europ.J.Agronomy13 (2000) 279 – 286
282
Fig. 1. Relationship between leaf Chl and leaf dry weight per unit of leaf area (SLW) as detected in 1995, 60 days after full bloom of cv Spring Red.
from 60 to 120 DAFB (Table 4), in spite of a clear decline of their Chl content (Table 2).
In the leaves collected from orchard 1 (Spring Red) in 1995, leaf Chl content was linearly corre-lated to the amount of Fe per leaf at 60 (r=
0.72**) and 120 DAFB (r=0.28*) (data not shown in tables). The following year, linear corre-lation was recorded only at 120 DAFB (r=
0.71**, data not shown in tables). In orchard 2 (Stark Redgold), a significant correlation was recorded at 120 DAFB in 1995 only (r=0.54**, data not shown in tables). Correlation coefficients between Fe and Chl (detected on the same leaf) were always higher when Fe was considered as a values recorded (Table 2). Regardless the
geno-type and the year, the coefficient of variation of Chl content increased over time, indicating that along the season some trees developed severe symptoms and others showed no or little chloro-sis. Positive correlation between leaf Chl concen-tration and leaf dry weight per unit of leaf area (SLW) was recorded in both years in Spring Red, with correlation coefficients ranging from 0.32* (not shown) to 0.88** (at 60 DAFB; Fig. 1). In cv Stark Redgold, a positive relationship was estab-lished only in 1995 at 120 DAFB (r=0.39**, not showed).
In 1995, flower Fe concentration determined at full bloom on the 15 selected trees was equal to 126mg g−1in cv Spring Red and 88mg g−1in cv
Stark Redgold (Table 3). In 1996, flower Fe con-centration significantly decreased as compared with 1995 in Spring Red (65 mg g−1) but not in
Stark Redgold (82mg g−1 (Table 3).
With only few exceptions (i.e. Stark Redgold in 1996), data of minimum leaf Fe concentration and Fe content indicated an increase in leaf Fe
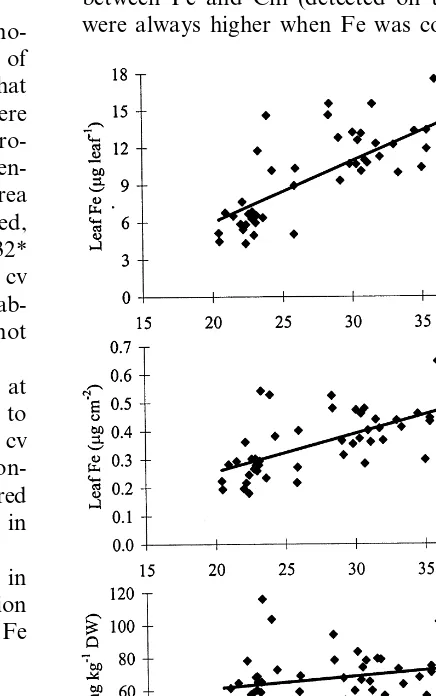
Fig. 2. Correlations between leaf Chl and leaf Fe reported as total amount per leaf (A), concentration per SLW (B), and concentration per leaf dry weight (DW) (C), in cv Spring Red as detected in 1995, 60 days after full bloom.
Table 3
Floral Fe concentration (mg g−1 DW) as measured in 1995
and 1996 on Spring Red and Stark Redgold (n=15) Year Spring Red Stark Redgold
1995 126 88
82 65
1996
M.S.E.a 10 10
Toselli
et
al
.
/
Europ
.
J
.
Agronomy
13
(2000)
279
–
286
283
Table 4
Average, minimum and maximum values of leaf Fe concentration (per dry matter and SLW) and leaf Fe content at 60 and 120 DAFB as observed in Spring Red and Stark Redgold in 1995 (n=50) and 1996 (n=15)
Stark Redgold Spring Red
Stark Redgold Leaf Fe Spring Red
120 DAFB 120 DAFB
60 DAFB 60 DAFB
1996 1995 1996 1995 1996
1996 1995
1995
Average Average Minimum Minimum
Average Minimum Average Minimum Average Minimum Average Minimum Average Minimum Average Minimum –maximum
–maximum –maximum
–maximum –maximum
–maximum –maximum –maximum
28–107 111 37–206 47 30–133 101 60–206
12–201 61
86 52 38–137 100 66–164
mg Fe kg−1DW 73 41–167
0.12–0.49 0.66 0.27–1.14 0.23 0.13–0.59 0.55 0.29–1.13 0.3
mg Fe cm−2 0.36 0.18–0.54 0.54 0.19–1.18 0.24 0.17–0.5 0.62 0.35–1.31
4.4–21.3 24.4 9–45.7 9.2 5.1–29.3 20.2 11.3–46 15.7 9.4–29.4
9.7 19.2
M.Toselli et al./Europ.J.Agronomy13 (2000) 279 – 286
284
Fig. 3. Relationship between flower Fe detected at full bloom of 1996 and leaf Chl as detected in 1995, 60 DAFB in cv Spring Red.
years of investigation. Factors impairing Fe nutri-tion in calcareous soils include the presence of nitrate as the only form of N available for root uptake (Darrah et al., 1986; Tagliavini et al., 1995a) and the high levels of calcium carbonate. Under these conditions, we speculate that high levels of bicarbonate and nitrate were present under excessive soil moisture provided by rainfall and regular (1 – 2 day intervals) drip irrigation. Bicarbonate (Boxma, 1982) and nitrate (Kosegarten et al., 1999) impairs iron nutrition through several mechanisms (Marschner, 1998), including the uptake of Fe, its transport to the leaves, and its inactivation due to high apoplastic pH (Mengel, 1994; Kosegarten et al., 1999).
In our study, leaf Fe availability was likely insufficient to sustain normal leaf growth, as it is testified by the progressive development of chloro-sis (see Table 2) associated with the reduction of specific leaf weight (SLW) and size as the chloro-sis severity increased (Fig. 1). The growth reduc-tion of chlorotic leaves compensated for the decreased Fe content and this contributed to ex-plaining the better relationship between leaf Chl concentration and amount of Fe per leaf than leaf Fe concentration (Fig. 2). This phenomenon, also described by Morales et al. (1998), suggests that the reliability of leaf Fe determination as a diag-nostic tool would increase when leaf Fe is ex-pressed as amount per leaf or as a concentration per leaf area rather than as concentration per dry matter. Apparently, these findings would suggest that the reason behind the fact that chlorotic and green leaves had similar Fe concentration is mainly due to an overestimate of Fe in the chlorotic leaf as a consequence of a reduction of leaf size. It should be stressed, however that the decrease of Chl content between 60 and 120 DAFB (Table 2) occurred while leaf Fe content generally increased (Table 3), indicating that even during chlorosis development leaves were supplied with some iron. Data therefore suggest that the development of chlorosis was probably associated with an inactivation of Fe in the leaf apoplast, likely due to its alkalinization, which impairs Fe3+ reduction (Kosegarten et al., 1999) a
prereq-uisite for Fe2+ leaf cell uptake (Bru¨ggemann et
al., 1993). The presence of inactive Fe pools concentration per leaf area (mg cm−2) rather than
per dry weight (mg g−1) (Fig. 2); the highest
correlation coefficients were, however, often recorded when Chl was plotted against the amount of Fe per leaf (Fig. 2).
In 1995, regardless the variety, flower Fe con-centration and leaf Chl were never correlated (data not showed). In 1996, floral Fe concentra-tion was linearly related to leaf Chl at both 60 (r=0.60*) and 120 DAFB (r=0.50*) in orchard 1 but not in orchard 2 (data not reported).
Iron concentration in flowers collected in 1996 correlated with leaf Chl measured the previous year at 30 (r=0.59*, not reported) and 60 (r=
0.63*) DAFB in Spring Red (Fig. 3) and at 30 DAFB (r=0.52*) in Stark Redgold (data not reported).
4. Discussion
In the present study, the selected trees did not receive any additional soil Fe supply and leaf chlorosis symptoms developed over the season in both years, although they were more severe in orchard 1 (cv Spring Red). The high variability of chlorosis symptoms among trees under investiga-tion within each orchard has been frequently ob-served on calcareous soils and it is attributed to a spatial variability of soil conditions including CaCO3 content, soil drainage and aeration. This
confined in the leaf apoplast (Mengel, 1994) has been reported by several authors in peach as well as in other Fe chlorosis susceptible species (Kosegarten and Englisch, 1994; Mengel, 1994), which re-greened after being spayed with diluted solutions of sulfuric, citric and ascorbic acid (Tagliavini et al., 1995b, 2000).
Floral Fe concentration has been suggested as a valuable tool for the prognosis of Fe chlorosis in peach (Sanz et al., 1997a) as well as in pear orchards (Sanz and Montan˜e´z, 1995). Our find-ings indicate that withdrawal of Fe supply in 1995 caused a decrease of flowers Fe concentration in 1996 in cv Spring Red. In a long term study, Sanz et al. (1997b) proposed that leaf chlorosis likely occurs when flower Fe concentration is less than 160 mg kg−1 DW. Our results agree with this
threshold value since our nectarine flowers hardly reached this concentration; however under our experimental conditions, floral Fe concentration predicted leaf chlorosis development in one of the two orchards (cv Spring Red) and in the second year only. The absence of any relationship be-tween floral Fe and leaf Chl in 1995 might be explained considering that the entire process of flower bud differentiation was completed before the beginning of the trial, when all the trees were regularly supplied with Fe-chelates. Flower bud differentiation in peach occurs during the summer previous to the blossom, it lasts 54 – 65 days on average (Faust, 1989). Nectarine trees blossom very early in spring, just after flower buds open and before leaves appear. As reported by Abadı´a et al. (2000) most if not all the Fe present in the flower at blossom is already present in the peach tree during its dormancy (Abadı´a et al., 2000). In this context it is not surprising that, regardless the genotype, as leaf chlorosis in 1995 progressively increased, flower Fe concentration in 1996 de-creased. This suggests that flower Fe concentra-tion (and possibly Fe in winter buds) might be used for assessing the storage of Fe during the previous season. The internal cycling of mineral elements, which includes winter storage and spring remobilization of nutrients, is an important strategy developed by deciduous trees to sustain growth in spring before root uptake occurs (Mil-lard, 1996). Although winter storage and spring
remobilization of iron has not been studied in details in deciduous trees (Tagliavini et al., 2000) the knowledge of Fe reserve pools would be of practical importance in early spring to schedule an appropriate Fe supply in orchards.
Acknowledgements
We thank G.L. Spada and F. Folli for their support in collection of data. Dipartimento di Colture Arboree publication no. 1563. This study was supported by EU AIR3-CT94-1973, and MURST ex 40% (Roma, grant Cofin 1998) projects funding to B. Marangoni.
References
Abadı´a, J., Tagliavini, M., Grasa, R., Belkhodja, R., Abadı´a, A., Sanz, M., Faria, E.A., Tsipouridis, C., Marangoni, B., 2000. Using the flower Fe concentration for estimating chlorosis status in fruit tree orchards: a summary report. J. Plant Nutr., in press.
Almaliotis, D.D., Manganaris, A.G., Simonis, A.D., Bladenopoulou, S.B., 1995. Rootstock effect on yield and mineral nutrition of ‘Maycrest’ peach trees under condi-tions of lime-induced chlorosis. In: Abadı´a, J. (Ed.), Iron Nutrition in Soils and Plants. Kluwer Academic Publish-ers, Dordrecht, pp. 301 – 306.
AOAC, 1990. In: Helrich, K. (Ed.), Official Methods of Anal-ysis of the Association of Official Analytical Chemists. Washington, DC.
Arnon, D.I., 1949. Copper enzymes in isolated chloroplasts polyphenoloxidase inBeta6ulgaris. Plant Physiol. 24, 1 – 5.
Boxma, R., 1982. Bicarbonate as the most important soil factor in lime-induced chlorosis in the Netherlands. Plant Soil 37, 233 – 243.
Bru¨ggemann, W., Maas-Kantel, K., Moog, P.R., 1993. Iron uptake by leaf mesophyll cells: the role of the plasma membrane-bound ferric-chelate reductase. Planta 190, 151 – 155.
Cinelli, F., Viti, R., Byrne, D.H., Reed, D.W., 1995. Physio-logical characterization of two peach seedling rootstocks in bicarbonate nutrient solution. I. Root iron reduction and iron uptake. In: Abadı´a, J. (Ed.), Iron Nutrition in Soils and Plants. Kluwer Academic Publishers, Dordrecht, pp. 323 – 328.
M.Toselli et al./Europ.J.Agronomy13 (2000) 279 – 286
286
Faust, M., 1989. Physiology of Temperate Zone Fruit Trees. Wiley, New York.
Kosegarten, H., Englisch, G., 1994. Effect of various nitrogen forms on the pH in leaf apoplast and on iron chlorosis of
Glycine maxL.Z. Pflanzenerna¨hr. Bodenk. 157, 401 – 405. Kosegarten, H., Hoffmann, B., Mengel, K., 1999. Apoplastic
pH and Fe3+ reduction in intact sunflower leaves. Plant Physiol. 121, 1069 – 1079.
Marschner, H., 1998. Mineral Nutrition of Higher Plants. Academic Press, London.
Mengel, K., 1994. Iron availability in plant tissues-iron chloro-sis on calcareous soils. Plant Soil 165, 275 – 283.
Millard, P., 1996. Ecophysiology of the internal cycling of nitrogen for tree growth. Journal of Plant Nutrition and Soil Science 159, 1 – 10.
Montan˜e´z, L., Sanz, M., 1994. Prediction of reference values for early leaf analysis for peach trees. J. Plant Nutr. 17, 1647 – 1657.
Morales, F., Grasa, R., Abadı´a, A., Abadı´a, J., 1998. Iron chlorosis paradox in fruit trees. J. Plant Nutr. 21, 815 – 825. Rombola`, D.A., Bru¨ggemann, W., Tagliavini, M., Marangoni, B., Moog, P.R., 2000. Iron source affects Fe-reduction and re-greening of kiwifruit (Actinidia deliciosa) leaves. J. Plant Nutr., in press.
Sansavini, S., Corelli Grappadelli, L., Costa, G., Lugli, S., Marangoni, B., Tagliavini, M., Ventura, M., 1999. Ri-costruzione degli impianti e revisione degli indirizzi produt-tivi della peschicoltura romagnola. Frutticoltura 3, 8 – 20. Sanz, M., Cavero, J., Abadı´a, J., 1992. Iron chlorosis in the
Ebro river basin, Spain. J. Plant Nutr. 15, 1971 – 1981. Sanz, M., Montan˜e´z, L., 1995. Flower analysis as a new
approach to diagnosing the nutritional status of the peach tree. J. Plant Nutr. 18, 1667 – 1675.
Sanz, M., Belkhodja, R., Toselli, M., Montan˜e´z, L., Abadı´a, A., Tagliavini, M., Marangoni, B., Abadı´a, J., 1997a. Floral analysis as a possible tool for the prognosis of iron deficiency in peach. Acta Hort. 448, 241 – 245.
Sanz, M., Pascual, J., Machı`n, J., 1997b. Prognosis and corre-lation of iron chlorosis in peach trees: influence on fruit quality. J. Plant Nutr. 20 (11), 1567 – 1572.
Shi, Y., Byrne, D.H., 1995. Tolerance ofPrunusrootstocks to potassium carbonate-induced chlorosis. J. Am. Soc. Hort. Sci. 120, 283 – 285.
Socias y Company, R., Gomez Aparisi, J., Felipe, A.J., 1995. A genetic approach to iron chlorosis in deciduous fruit trees. In: Abadı´a, J. (Ed.), Iron Nutrition in Soils and Plants. Kluwer Academic Publishers, Dordrecht, pp. 167 – 174.
Tagliavini, M., Masia, A., Quartieri, M., 1995a. Bulk soil pH and rhizosphere pH of peach trees in calcareous and alkaline soils as affected by the form of nitrogen fertilizers, 1995. Plant Soil 176 (2), 263 – 271.
Tagliavini, M., Scudellari, D., Marangoni, B., Toselli, M., 1995b. Acid-spray regreening of kiwifruit leaves affected by lime-induced iron chlorosis. In: Abadia, J. (Ed.), Iron Nutrition in Soils and Plants. Kluwer Academic Publish-ers, Dordrecht, pp. 191 – 196.
Tagliavini, M., Abadı´a, J., Rombola`, A. D., Abadı´a, A., Tsipuoridis, C., Marangoni, B., 2000. Agronomic means for the control of iron deficiency and chlorosis in decidu-ous fruit plants. J. Plant Nutr., in press.