Thalamic Neurons in Schizophrenia
Keith A. Young, Kebreten F. Manaye, Chang-Lin Liang, Paul B. Hicks, and
Dwight C. German
Background: The thalamus is a brain region of interest in the study of schizophrenia because it provides critical input to brain regions such as the prefrontal, cingulate, and temporal cortices, where abnormalities have been repeatedly observed in patients with schizophrenia. Post-mortem anatomic studies have rarely investigated the thalamus in this population.
Methods: Postmortem tissue was obtained from the left hemisphere of eight male schizophrenic patients and eight male age-matched control subjects. The optical dissector stereologic procedure was used to count neurons in the mediodorsal (MD) and anteroventral/anteromedial (AV/ AM) nuclei of the thalamus.
Results: The number of neurons and volume of the MD were significantly reduced by 35% and 24%, respectively. The MD cell number reduction was a consistent finding; every control subject had more and every schizophrenic subject had fewer than 3.5 million neurons. Neuron number was also significantly reduced (16%) in the AV/AM nuclei.
Conclusions: The present data indicate that schizophre-nia is associated with robust reductions in nerve cell numbers in nuclei that communicate with the prefrontal cortex and limbic system. These thalamic anatomic deficits may be responsible, in part, for previous reports of such prefrontal cortical abnormalities as reduced synaptic density, reduced volume, and metabolic hypofunction. Biol Psychiatry 2000;47:944 –953 © 2000 Society of Bi-ological Psychiatry
Key Words: Schizophrenia, postmortem brain, thalamus,
mediodorsal nucleus, anterior nucleus, stereology
Introduction
S
chizophrenia is a major mental illness that affects approximately one in every 100 people (Andreasen and Carpenter 1993). Individuals with schizophrenia ex-hibit a lack of motivation, abnormal thought content and process (e.g., delusions, paranoia, hallucinations and dis-continuity of associations), and disturbance of emotion (i.e., inappropriate affect; Andreasen 1997a). Many schizophrenic patients also perform poorly on neuropsy-chologic tasks involving the frontal lobes and exhibit abnormal metabolic activity patterns in the prefrontal cortex (Berman et al 1986; Fletcher et al 1998; Wein-berger and Gallhofer 1997) and anterior cingulate cortex (Haznadar et al 1997). Schizophrenic individuals who exhibit large numbers of errors of perseveration on the Wisconsin Card Sorting Test or who perform poorly on verbal working memory or sustained attention tests lack the normal pattern of metabolic activation in the dorsolat-eral prefrontal cortex (Goldberg et al 1994; Stevens et al 1998). Morphologic abnormalities in the dorsolateral pre-frontal and anterior cingulate cortex have been identified as a possible anatomic basis for the behavioral and metabolic abnormalities of schizophrenia (Arnold and Trojanowski 1996; Buchanan et al 1998; Goldstein et al 1999; Rajkowska et al 1998; Selemon et al 1998).The thalamus, which is an important source of subcor-tical input to the cortex, has become a recent focus of research in schizophrenia (Andreasen 1997b; Jones 1997a; Scheibel 1997). The majority of thalamic studies have utilized magnetic resonance imaging (MRI) (Andreasen et al 1994; Buchsbaum et al 1996; Gur et al 1998; Staal et al 1998). The data indicate that the thalamus of schizo-phrenic subjects is smaller than that of control subjects, although the magnitude of the volume reductions (10%) is relatively small. Quantitative postmortem morphologic studies, on the other hand, have found more substantial thalamic abnormalities in schizophrenic subjects. Using stereologic cell counting procedures, Pakkenberg (1990) reported a 40% reduction in the number of mediodorsal (MD) thalamic neurons in schizophrenic subjects, a find-ing apparently not related to neuroleptic treatment
(Pak-From the Department of Psychiatry and Behavioral Science, Neuropsychiatry Research Program, Texas A&M University System Health Science Center and Central Texas Veterans Health Care System, Temple (KAY), the Department of Psychiatry, University of Texas Southwestern Medical School, Dallas (KFM, C-LL, DCG), and the Department of Psychiatry, Scott and White Memorial Hospital, Temple (PBH), Texas.
Address reprint requests to Keith A. Young, Ph.D., Central Texas Veterans Health Care System, Neuroscience Laboratory, Neuropsychiatry Research Program (151T), 1901 S. 1st Street, Temple TX 76504.
Received June 23, 1999; revised October 25, 1999; revised January 28, 2000; accepted February 8, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
kenberg 1992). Considering the prominent frontal cortical deficits of schizophrenic individuals, an MD neuron num-ber reduction is particularly intriguing because the MD is a major source of subcortical input to the frontal cortex (Giguere and Goldman-Rakic 1988; Ray and Price 1993). There have been few morphologic studies of the thalamus in subjects with schizophrenia. We expected our study to replicate the finding of Pakkenberg (1990), which described a reduction in the number of MD neurons, and also extend the examination of the thalamus by studying another thalamic region that projects to the limbic cortex, the anterior thalamic nuclei. The optical disector stereologic cell counting proce-dure was used to estimate the total number of neurons in control and schizophrenic postmortem brains. Neurons were counted in the MD nucleus, which projects to the dorsolateral prefrontal and orbitofrontal cortex, and the anteroventral-anteromedial (AV/AM) nuclei, which project to the cingulate and entorhinal cortex. We found robust reductions in the number of neurons in both the MD and AV/AM thalamus in schizophrenic subjects.
Methods and Materials
Brains
The left thalamus was obtained from eight male control subjects with no history of psychiatric disturbance and eight male schizophrenic subjects diagnosed according to DSM-IV criteria (Table 1). The Diagnostic Evaluation after Death was used to obtain postmortem diagnosis of schizophrenic sub-jects. The brains were obtained from Terrell State Hospital, the Waco VA Medical Center, and the Stanley Foundation Neuropathology Consortium. All brains were grossly normal
at autopsy and there was no evidence of ischemic brain damage in the thalamus of any of the subjects. Neuropatho-logic examinations were available on 13 of the brains. There was no histopathologic evidence of neurodegenerative changes in the cortex or hippocampus of any of these brains. The schizophrenic patients were chronically treated with neuroleptic drugs until near the time of death. Schizophrenic and control groups did not differ significantly in age (schizo-phrenic group mean6SD, 65.4611.3 years; control group, 64.9 6 16.7 years), postmortem interval (schizophrenic group, 16.1611.5 hours; control group, 15.369.2 hours), or time in formalin fixative (schizophrenic group, 61.7 6 53 months; control group, 55.3 6 71 months). One poten-tial diagnostic confound in the present study was lack of information about the alcohol or drug abuse history in many of the subjects, particularly during adolescence and early adulthood.
Histology
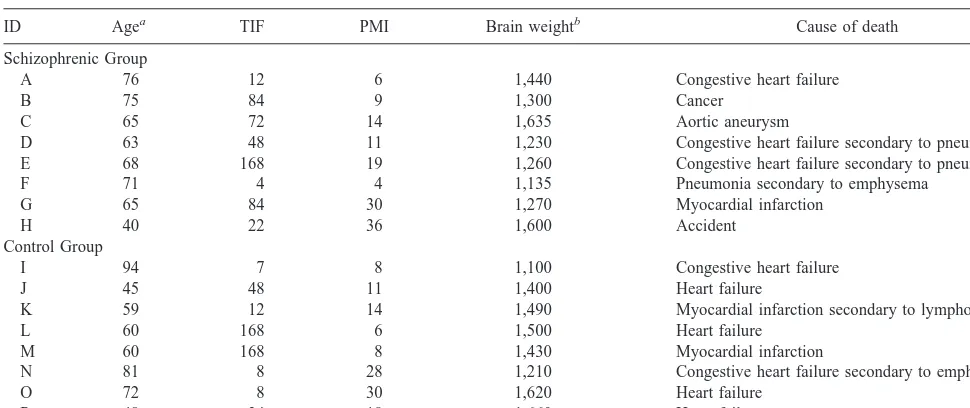
Before sectioning the thalamus, the tissue block was immersed in a solution of 20% sucrose and 10% formalin for 2–3 weeks. A single block containing the entire left thalamus was serially sectioned in the coronal plane on a freezing microtome. Although the microtome thickness was set at 60mm, the actual average distance advanced for each cut was measured to be 56mm. After Nissl staining every 20th section with cresyl violet, the regional boundaries of the two thalamic nuclei (Jones 1997b) were delineated at low magnification with a 2.53objective (Figure 1). The MD begins just caudal and ventral to the AV and ends at the level of the rostral pulvinar. Its medial border is near the lateral wall of the third ventricle except where it merges into the massa intermedia. It is bounded laterally by the fasciculus mammillo-thalamicus anteriorly and by the internal medullary lamina and centromedian nucleus posteriorly. The large Table 1. Specimen Characteristics
ID Agea TIF PMI Brain weightb Cause of death
Schizophrenic Group
A 76 12 6 1,440 Congestive heart failure
B 75 84 9 1,300 Cancer
C 65 72 14 1,635 Aortic aneurysm
D 63 48 11 1,230 Congestive heart failure secondary to pneumonia E 68 168 19 1,260 Congestive heart failure secondary to pneumonia F 71 4 4 1,135 Pneumonia secondary to emphysema
G 65 84 30 1,270 Myocardial infarction
H 40 22 36 1,600 Accident
Control Group
I 94 7 8 1,100 Congestive heart failure
J 45 48 11 1,400 Heart failure
K 59 12 14 1,490 Myocardial infarction secondary to lymphoma
L 60 168 6 1,500 Heart failure
M 60 168 8 1,430 Myocardial infarction
N 81 8 28 1,210 Congestive heart failure secondary to emphysema
O 72 8 30 1,620 Heart failure
P 48 24 18 1,660 Heart failure
TIF, time in formalin (months); PMI, postmortem interval (hours).
cells of the centrolateral nucleus clearly delineate the lateral bound-ary of the MD for most of its rostral-caudal extent. The densocel-lular portion of the MD was included. The lateral ventricle, fornix, anterior nuclei, and laterodorsal nucleus lie on its superior surface and are clearly recognizable. Most posteriorly, the inferior limit merges with the nucleus parafascicularis and centromedian nuclei. The external borders of the AV/AM nuclei are clearly demarcated. The nuclei are encapsulated by the white matter track of the internal medullary lamina; however, the border between the two nuclei often is difficult to identify in Nissl stained coronal sections. We therefore combined the AV nucleus with the AM nucleus to form a single region of interest (AV/AM) for cell counting purposes.
The tissue processing and staining procedures produced ap-proximately 50% tissue shrinkage. The final Z-axis thickness in all specimens measured between 22 and 25mm. Thus, we used a 5-mm upper guard zone, a 7–10-mm lower guard zone and a 10-mm-thick counting frame. The sampling coefficient of error (CE; Scheaffer et al 1996) was estimated with the StereoInves-tigator software (version 1.3; MicroBrightField, Colchester, VT) providing a measure of the variance of the sampling procedure. The biological CE provides an estimate of the variability in neuron counts between specimens (Table 2). The CE estimates in the present study indicate that sampling variances for the MD and AV/AM regions were lower in magnitude than the inherent biological variance, indicating that the stereologic sampling parameters used in the study were sufficient to detect relatively small group differences between schizophrenic and control subjects.
Stereology
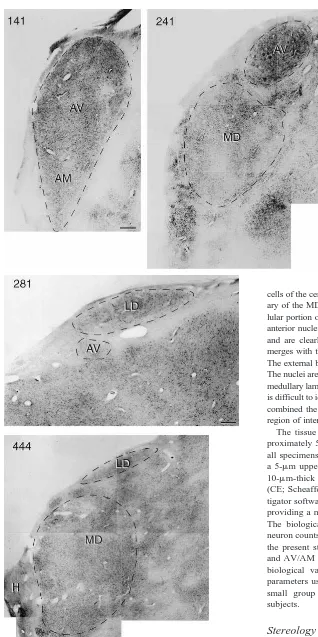
optimal counting procedures. Using a “counting frame” that measured 140380310mm, we varied the number of counting frames (Figure 2) to identify from 100 to 600 neurons in several replicate counts of a set of nine sections through the MD in one case. The results indicated that counting 200 – 600 neurons produced similar estimates of total neuron number. We adjusted the number of counting frame placements used to count cells in the two nuclei based on this result. Outlines of each region of interest were digitized using StereoInvestigator software.
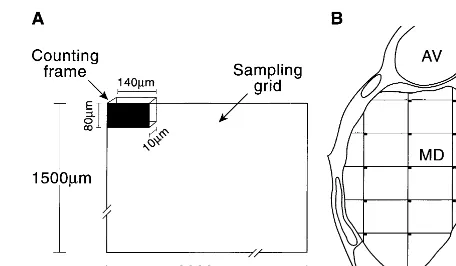
Nine to 11 sections were analyzed in each thalamic region. Using more than 100 counting frames systematically placed throughout the entire rostral-caudal extent of the nucleus, the average density of neurons in the counting frames was deter-mined using a 253oil immersion objective (N.A. 0.75). Neurons were identified as Nissl positive cell bodies containing a nucle-olus that was clearly in focus within the counting frame (Figure 3). Thalamic nuclear volumes were calculated with Cavalieri’s estimate from areas calculated by the StereoInvestigator software using the outlined borders in each tissue section. The total number of neurons in each region was estimated by multiplying the average counting frame neuronal density and the calculated volume of the thalamic region of interest (cell number/mm3
3
total nucleus volume [mm3
]5estimated total cell number).
Statistics
A Student’s t test was used to compare cell number, nuclear volume, and neuron density between the schizophrenic group and control group. The p values reflect Bonferroni correction for two multiple comparisons. Analysis of covariance for age, postmor-tem interval, and time-in-formalin was performed with neuron number as the dependent variable.
Results
Total Cell Number
Anatomic changes were readily apparent in the MD and AV/AM of the schizophrenic group, the most prominent
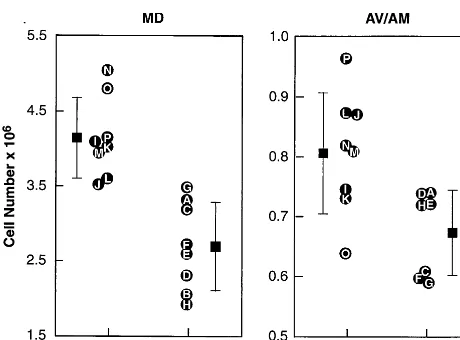
change being a marked reduction in the total number of neurons (Table 3). There was a mean of 4.15 million neurons in the MD from control subjects and a mean of 2.70 million neurons in the MD of schizophrenic subjects (t55.14, p,.001), a 35% reduction. In every control subject, the MD nucleus contained more than 3.5 million neurons; in every schizophrenic subject, it contained less (Figure 4). Values obtained in the current study for control MD neuron numbers are in agreement with two previously published values (Harding et al 1994; Xuereb et al 1991). Analysis of covariance was used to test for effects of several covariates on MD neuron number estimates. These included age [F(1,11) 5 0.75, p..4], time in formalin fixative [F(1,11)50.13, p..7], and postmortem interval [F(1,11)50.4, p..5]. The main effect of diagnosis remained significant after accounting for these covariates [F(1,11)5 24.7, p,.0004]. Neuron numbers were also reduced by an average of 16% in the AV/AM of schizophrenic subjects, from 807,000 to 674,0000 (t 5 2.91, p , .012). Unlike the MD, in the AV/AM there was overlap in the range of neurons for schizophrenic subjects and control subjects.
Nucleus Volume and Cell Density
We also tested for differences in nuclear volume and neuron density between schizophrenic and control sub-jects. In addition to a reduction in neuron number, there was a significant 24% reduction in the volume of the MD in schizophrenics (t53.73, p5 .002; Table 3 and Figure 5). Although there was a 17% reduction in the volume of the AV/AM in the schizophrenic brains, the Figure 2. Stereologic cell counting requires the use of a “count-ing frame” and a “sampl“count-ing grid.” (A) The dimensions of the counting frame used in our study and the dimensions of the sampling grid used to count cells in the mediodorsal nucleus of the thalamus (MD). (B) Neurons whose nucleoli fall within the counting frames are counted and used to calculate an average neuronal density for the nucleus, which is multiplied by the volume of the entire nucleus to determine an estimate of total neuron number. AV, anteroventral nucleus.
Table 2. Stereologic Sampling Parameters
MD AV/AM
Sampling grid size (mm 3103) 2.031.5 1.231.0
Mean # sections counted (6SD)a 11.1 (1.0) 9.5 (1.4) Mean # of neurons counted (6SD)a 345 (101) 204 (33) Sampling CE (Scheaffer et al 1996),
neuron numberb
0.049 0.064
Sampling CE (Gunderson and Jensen 1987), neuron numberc
0.05 0.07
Biological CE, neuron numberd 0.064 0.054
MD, mediodorsal nucleus of the thalamus; AV/AM, anteroventral/anteromedial nuclei of the thalamus.
aFor schizophrenic and control groups combined (n516).
bEstimated coefficient of error for the variance in sampling, given for a
representative brain (Scheaffer et al 1996).
cEstimated coefficient of error for the variance in sampling, nugget corrected
estimator, given for a representative brain (Gunderson and Jensen 1987). dCoefficient of error for the between-subject variance of the control group5
difference between the schizophrenic group and the control group was not statistically significant (t51.92, p 5 .077). The MD was found in the same number of sections along the rostro-caudal length in schizophrenic group (11.25 6 1.2 sections) and the control group (10.88 6 0.9 sections; t5 0.693, p 5 .49). Similarly, the AV/AM was found in the same number of sections along the rostro-caudal length in schizophrenic brains (9.37 6 1.8 sections) and control brains (9.62 6 1.3
sections; t 5 0.283, p 5 .78). Neuronal densities in both the MD and AV/AM nuclei were not signifi-cantly different in the two groups (Table 3); however, six of the eight schizophrenic subjects had lower MD neuron densities than each of the control subjects. There was no evidence for a neuron density reduction in the AV/AM because both subject groups had the same range of density values and virtually identical mean densities.
Figure 3. Examples of cells counted in the anterior nucleus of the thalamus in control case C. (A, B) Single cells, each with a darkly stained nucleolus, in the center of a “counting frame” (80 3 140 mm). Only cells with a clearly visible nucleolus that could be focused within a 10-mm Z depth were counted. These photomicrographs were taken with the 253-oil objective used for cell counting in the present study.
Table 3. Thalamic Neuron Number, Nuclear Volume, and Neuronal Density
Neuron number Volume Neuronal density
MD AV/AM MD AV/AM MD AV/AM
Number 106
6SD (n) mm3
6SD (n) Neurons/mm3
6SD (n) Control group 4.1560.53 (8) 0.80760.101 (8) 248635 (8) 52.367.9 (8) 1,6966256 (8) 1,5586300 (8) Schizophrenic group 2.7060.59 (8) 0.67460.071 (7) 188629 (8) 43.3610.1 (7) 1,4266416 (8) 1,5686330 (7)
t test p , .001 p5 .012 p 5 .002 p5 .077 p 5 .33 p5 .79
Discussion
Thalamic Neuron Number Reductions in Schizophrenia
Our study clearly indicates that there is a marked reduction in the number of MD neurons in the postmortem schizo-phrenic brain. It represents the first replication of an initial report of a reduction in the number of MD neurons in schizophrenic subjects (Pakkenberg 1990). A striking observation of both our study and the earlier report (Pakkenberg 1990) is that there is virtually no overlap in the ranges of the MD neuron numbers between normal control subjects and schizophrenic subjects. In our exper-iment, each of the eight control subjects had more neurons than each of the 8 schizophrenic subjects, whereas in the Pakkenberg study, 11 of the 12 control subjects had more neurons than each of the 12 schizophrenic brains. These findings are also remarkable because of the magnitude of the MD neuron number reductions: on average more than one third of the normal complement of MD neurons is missing from the schizophrenic brain. The large magni-tude of the deficit and the consistency of the reduction from patient to patient are uncommon in schizophrenia research, in which anatomic deficits are not found consis-tently in every patient (Arnold and Trojanowski 1996). The marked reduction in neuron number in the MD is arguably the most robust neuroanatomic deficit in schizo-phrenia research reported to date.
The number of MD neurons in normal subjects has been estimated to be approximately 4 million in two previous
studies (Harding et al 1994; Xuereb et al 1991) and in our study; however, Pakkenberg (1990) reported that control brains have approximately 2 million MD neurons. This disparity is most likely due to differences in defining the borders of the MD (B. Pakkenberg, personal communication, August 1999). Thus, although both Pakkenberg (1990) and Xuereb et al (1991) used similar paraffin embedding tissue processing procedures, MD volumes in the Pakkenberg study were 50% smaller than in the Xuereb et al study.
Our study is the first to quantify the number of neurons in the anterior nucleus of the thalamus in the schizophrenic brain. Neuron numbers in the AV/AM were reduced by 16% on average. Unlike the MD, however, there was overlap between normal and control neuron numbers. These data suggest that AV/AM neuron number reduc-tions may be present in only a subset of schizophrenic individuals, whereas MD reductions are more consistently found in the great majority of patients. It also has been reported that there is a reduction in parvalbumin-positive neurons in the AV nucleus of schizophrenic subjects (Danos et al 1998). It is interesting that the AV/AM is interconnected with medial temporal lobe (hippocampal) circuits of the limbic system, where many morphologic and functional abnormalities have been observed in schizophrenia (see Arnold and Trojanowski 1996 for review).
The thalamic neuron reductions so far revealed are present in nuclei providing input to prefrontal and tem-porolimbic cortex, in which anatomic deficits have been observed in postmortem and MRI studies. In a recent report, for example, Goldstein et al (1999) used MRI to Figure 4. Cell number in schizophrenic and control thalamic
nuclei. Thalamic neuron numbers are reduced in schizophrenia. The mean 6 SD is depicted in the figure. There are fewer neurons in the mediodorsal (MD) and anterior (AV/AM) tha-lamic nuclei of schizophrenic subjects compared with normal control subjects. The identification of individual subjects (letters A–P) corresponds to the identifications given in Table 1. The number of neurons in the schizophrenic group is 35% lower in the MD (p,.001) and 16% lower in the AV/AM (p,.012).
measure the volume of 48 separate regions of the cerebral cortex. The greatest volumetric reductions were observed in the middle frontal gyrus, frontoorbital cortex, the insula and the anterior cingulate cortex. The first three of these brain regions have strong connectivity with the MD nucleus (Giguere and Goldman-Rakic 1988; Ray and Price 1993), whereas the anterior cingulate is a primary cortical target of the AV/AM nuclei (Vogt et al 1987). Our data suggest that thalamic neuron number reductions may contribute to cortical volume deficits. Further quantitative study of all thalamic nuclei is underway to determine the full extent of thalamic abnormalities in schizophrenia.
Thalamic Volume and Density Measurements
In the MD of schizophrenic subjects, a significant reduc-tion in tissue volume was observed. These data support previous MRI studies that have identified modest thalamic volume reductions in schizophrenic individuals (An-dreasen et al 1990; Flaum et al 1995; Gur et al 1998; Staal et al 1998). In one study (Buchsbaum et al 1996), for example, the volume of the left anterior thalamus was selectively reduced by 15%. Because there is a reduction in the volume of the thalamus in never-medicated schizo-phrenic individuals (Buchsbaum et al 1996; Gur et al 1998; Pakkenberg 1992), it appears unlikely that neuro-leptic effects are responsible for such volume reductions. Increased volumes of the lateral ventricles accompany smaller thalamic volumes in schizophrenic subjects (Por-tas et al 1998). These MRI findings raise the possibility that thalamic volume reductions may be associated with the enlargement of the lateral ventricles observed in schizophrenia (Cannon et al 1998; see review by McCar-ley et al 1999). The modest reduction in whole thalamic volume (,10%) reported in some MRI studies of schizo-phrenic individuals (Andreasen et al 1990; Flaum et al 1995) and reports of normal thalamic size in other studies (Wolkin et al 1998; for review, see McCarley et al 1999) suggest that thalamic volume changes in schizophrenia may be limited to select regions of the thalamus such as the AV/AM and MD.
In our study, the density of neurons in the MD and AV/AM nuclei of schizophrenic subjects was normal even though total neuron numbers were significantly reduced in these nuclei. The 15% lower mean density of neurons in the MD was not significantly different from control subjects, although it is possible that a larger sample size would have identified this change as significant. Our data suggest that there is a large effect of nuclear volume reduction on neuron number in the MD of schizophrenics. Nonetheless, the findings do not exclude the possibility that neuron number reductions in this region are the product of both a density and a volume reduction. The
findings in the MD contrast with the pattern observed in the AV/AM, in which neuronal density was virtually identical in both groups. This latter observation replicates a recent study (Danos et al 1998) that identified differ-ences in the density of a select population of thalamocor-tical neurons in the AV but did not find a significant change in the density of AV neurons.
Historically, anatomic studies have relied on ment of neuronal density and regional volume, measure-ments that in our study provided a less robust indication of thalamic neuropathology than total neuron counts. Be-cause postmortem tissue preservation and tissue shrinkage influence volume and density measurements, there is often greater variance associated with these measures compared with neuron number estimates. Variable tissue shrinkage from subject to subject has no net effect on total neuron counts when stereologic procedures are used (West 1994). The observation that neuronal density and nuclear vol-umes are reduced only modestly or are unaffected in the thalamus of schizophrenic individuals provides one expla-nation for why the thalamus has not been previously recognized as a locus of robust anatomic change in schizophrenia.
Relationship between Thalamic Deficits in
Schizophrenia and Cortical Anatomy and Function
cortex, there are changes in lamina II and III GABA receptors (Benes 1993) and other subtle findings (Benes 1993; Heckers 1997; Lahti et al 1998). Schizophrenic individuals have abnormalities in the distribution of “guidepost” interstitial white matter neurons (Akbarian et al 1996) in the frontal cortex. Because thalamocortical axons project into lamina III and IV in the frontal cortex (Giguere and Goldman-Rakic 1988), a reduction in the number of thalamocortical axons innervating the frontal lobes could contribute to the observed reductions in neuropil and spine density. Our findings of neuron number reductions in the thalamus of schizophrenic subjects sug-gests that perturbation of thalamocortical connections may contribute to reduced neuropil and other cortical deficits in schizophrenia.
Thalamic cell number reductions in schizophrenia may also contribute to metabolic activation deficits observed in the frontal cortex. Thalamocortical projections provide excitatory input to the frontal cortex, which influences the level of cortical metabolism and blood flow. For instance, animal studies have shown that inhibition of MD neuron activity selectively depresses metabolic activity in the frontal cortex (Young et al 1994); in humans, thalamic vascular accidents often induce frontal lobe metabolic hypoactivity (Casselli et al 1991; Szelies et al 1991). In schizophrenic individuals, a reduction in the number of thalamic neurons available to activate or sustain frontal cortex neural activity may impair metabolic activation during performance of verbal working memory and sus-tained attention tests, resulting in frontal cortical metabolic hypoactivity (Berman et al 1986; Fletcher et al 1998; Goldberg et al 1994; Stevens et al 1998; Weinberger and Gallhofer 1997).
Reductions in MD and AV/AM neuron number, through interactions with frontal circuits, have the potential to influence behaviors relevant to schizophrenia. MD lesions in rats produce increased perseverative behaviors (Hunt and Aggleton 1998), and perseverative behaviors are observed in schizophrenic subjects performing the Wis-consin Card Sorting Task (Berman et al 1986; Goldberg et al 1994). Strokes that selectively affect the MD and AV/AM can induce a schizophrenialike thought disorder characterized by bizarre, disconnected, and incoherent speech patterns (Chatterjee et al 1997). Accidental tha-lamic lesions that affect the MD and anterior nuclei are particularly prone to create disturbances in verbal memory and language (Casselli et al 1991; Squire and Moore 1979; Szelies et al 1991). Finally, the thalamus is metabolically activated during episodic hallucinations (Silbersweig et al 1995), and elevated thalamic, frontal, and cingulate met-abolic activity is associated with delusions and thought disorder (Erkwoh et al 1997; Sabri et al 1997). Because of the important role played by the thalamus in support of
cortical processing, it is clear that the substantial reduction in the number of neurons in the MD and anterior nuclei of schizophrenics could have profound effects on cortical metabolic activation deficits and behavioral signs and symptoms of schizophrenia.
Mechanisms Involved in Thalamocortical Pathology in Schizophrenia
There are a variety of mechanisms that could potentially account for the observed pattern of MD and AV/AM neuron number reductions in schizophrenia. Broadly de-fined, the mechanisms fall into two categories: develop-mental and degenerative. First, fewer MD and anterior thalamic neurons may have been generated, or they may have died during the development and maturation of the brain. A developmental mechanism promoting thalamic neuron number reductions would include a failure to develop or maintain appropriate synaptic connections with cortical targets. Developmental processes have been widely implicated as factors in producing anatomic defi-cits in schizophrenia (Andreasen 1997b; Hyde and Wein-berger 1990; Jones 1997a; Raedler et al 1998; Stefen and Murray 1997). Alternatively, a degenerative process could be involved in MD and AV/AM nerve cell number reductions. Thalamic neurons may develop normally but subsequently die because of a necrotic degenerative pro-cess. This later process usually produces alterations of glial cells (gliosis) in the regions where neuronal degen-eration has occurred. As an example, following dorsolat-eral prefrontal cortical lesions in rats, a secondary neuro-nal degeneration occurs in the MD. Thalamic gliosis is observable for at least 40 days after the lesion placement (van Eden et al 1998); however, in schizophrenic individ-uals there are no increases in the number of glial cells in the MD nucleus (Pakkenberg 1990). The lack of a gliotic reaction in the schizophrenic brain has been interpreted as evidence against a necrotic involvement in the disease. Further investigation is needed to determine why there are
fewer neurons in the thalamus of patients with
schizophrenia.
has been difficult to find marked abnormalities in the schizophrenic brain that parallel those found in diseases such as Huntington’s disease and Parkinson’s disease, making the study of schizophrenia difficult to approach from a mechanistic level; however, the finding of robust neuroanatomic deficits in the thalamus provides an oppor-tunity to better focus anatomic research in schizophrenia. It is likely that further study of the thalamus in schizo-phrenia will reveal important clues about the etiology of this debilitating mental illness.
This work was supported by NIMH (Grant No. MH55879), the Scott, Sherwood and Brindley Foundation, Veterans Affairs VISN 17 New Investigator Award, the Theodore and Vada Stanley Foundation, and the John Shermerhorn Fund. The authors thank S. Bauserman, C. Conley, E. Johnson, C. Cook, and E. Rachut for assistance in obtaining postmortem material and Dr. E. G. Jones for assistance in validating our definition of the mediodorsal thalamic nucleus.
References
Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr, et al (1995): Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 52:258 –266.
Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE Jr, Jones EG (1996): Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic pa-tients. Arch Gen Psychiatry 53:425– 436.
Andreasen NC (1997a): The evolving concept of schizophrenia: From Kraepelin to the present and future. Schizophr Res 28:105–109.
Andreasen NC (1997b): The role of the thalamus in schizophre-nia. Can J Psychiatry 42:27–33.
Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O’Leary D, et al (1994): Thalamic abnormalities in schizo-phrenia visualized through magnetic resonance image aver-aging. Science 266:294 –298.
Andreasen NC, Carpenter WT (1993): Diagnosis and classifica-tion of schizophrenia. Schizophr Bull 19:199 –214.
Andreasen NC, Ehrhardt JC, Swayze VW, Alliger RJ, Yuh WTC, Cohen G, et al (1990): Magnetic resonance imaging of the brain in schizophrenia: The pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 47:35– 44. Arnold SE, Trojanowski JQ (1996): Recent advances in defining
the neuropathology of schizophrenia. Acta Neuropathol
(Berl) 92:217–231.
Benes FM (1993): Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull 19:537–549. Berman KF, Zec RF, Weinberger DR (1986): Physiological
dysfunction of dorsolateral prefrontal cortex in schizophrenia.
Arch Gen Psychiatry 43:126 –135.
Buchanan RW, Vladar K, Barta PE, Pearlson GD (1998): Structural evaluation of the prefrontal cortex in schizophre-nia. Am J Psychiatry 155:1049 –1055.
Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A,
et al (1996): PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 153:191–199.
Burnet PW, Eastwood SL, Harrison PJ (1996): 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia.
Neuropsychopharma-cology 15:442– 455.
Cannon TD, Van Erp GM, Huttunen M, Lonnqvist J, Salonen O, Valanne L, et al (1998): Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic pa-tients, their siblings and controls. Arch Gen Psychiatry 55:1084 –1091.
Casselli RJ, Graff-Radford NR, Rezai K (1991): Thalamocortical diaschesis: Single photon emission tomographic study of cortical blood flow changes after focal thalamic infarction.
Neuropsychiatry Neuropsychol Behav Neurol 4:193–214.
Chatterjee A, Yapundich R, Mennemeier M, Mountz JM, In-ampudi C, Pan JW, et al (1997): Thalamic thought disorder: On being “a bit addled.” Cortex 33:419 – 440.
Danos P, Baumann B, Bernstein H-G, Franz M, Stauch R, Northoff G, et al (1998): Schizophrenia and anteroventral thalamic nucleus: Selective decrease of parvalbumin-immu-noreactive thalamocortical projection neurons. Psychiatry
Res 82:1–10.
Daviss SR, Lewis DA (1995): Local circuit neurons of the prefrontal cortex in schizophrenia: Selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Res 59:81–96.
Erkwoh R, Sabri O, Steinmeyer EM, Bull U, Sass H (1997): Psychopathological and SPECT findings in never-treated schizophrenia. Acta Psychiatr Scand 96:51–57.
Flaum MA, Swayze VW, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, et al (1995): Effects of diagnosis, laterality and gender on brain morphology in schizophrenia. Am J
Psychi-atry 152:704 –714.
Fletcher PC, McKenna PJ, Friston KJ, Dolan RJ (1998): Brain activations in schizophrenia during a graded memory task studied with functional imaging. Arch Gen Psychiatry 55: 1001–1008.
Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al (1998): Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol
Neu-rosurg Psychiatry 65:446 – 453.
Giguere M, Goldman-Rakic PS (1988): Mediodorsal nucleus: Arial, laminar, and tangential distribution of afferents and efferents in the frontal lobe of Rhesus monkeys. J Comp
Neurol 277:195–213.
Glantz LA, Lewis DA (1997): Reduction of synaptophysin immunoreactivity in the prefrontal cortex of schizophrenic subjects: Regional and diagnostic specificity. Arch Gen
Psy-chiatry 54:660 – 669.
Goldberg TE, Torrey EF, Berman KF, Weinberger DR (1994): Relations between neuropsychological performance and brain morphological and physiological measures in monozygotic twins discordant for schizophrenia. Psychiatry Res 55:51– 61. Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, et al (1999): Cortical abnormalities in schizophre-nia identified by structural magnetic resonance imaging. Arch
Gunderson HJG, Jensen EB (1987): The efficiency of sampling in stereology and its prediction. J Microsc 147:229 –263. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC
(1998): Subcortical MRI volumes in neuroleptic naive and treated patients with schizophrenia. Am J Psychiatry 155: 1711–1717.
Harding AJ, Halliday GM, Cullen K (1994): Practical consider-ations for the use of the optical disector in estimating neuronal number. J Neurosci Methods 51:83– 89.
Haznadar MM, Buchsbaum MS, Lu C, Hazlett EA, Seigel BV, Lohr J, et al (1997): Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 154:682– 684.
Heckers S (1997): Neuropathology of schizophrenia: Cortex, thalamus, basal ganglia and neurotransmitter-specific projec-tion systems. Schizophr Bull 23:401– 421.
Hunt PR, Aggleton JP (1998): Neurotoxic lesions of the dorso-medial thalamus impair the acquisition but not the perfor-mance of delayed matching to place by rats; a deficit in the shifting response rules. J Neurosci 181:9099 –9111. Hyde TM, Weinberger DR (1990): The brain in schizophrenia.
Semin Neurol 10:276 –286.
Jones EG (1997a): Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 23:483–501.
Jones EG (1997b): A description of the human thalamus. In: Steriade M, Jones EG, McCormick DA, editors. Thalamus, Vol II. New York: Elsevier, 425– 499.
Lahti RA, Roberts RC, Cochrane EV, Primus RJ, Gallager DW, Conley RR, et al (1998): Direct determination of dopamine D4 receptors in normal and schizophrenic postmortem brain tissue: A [3H]NGD-94-1 study. Mol Psychiatry 6:528 –533. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JL,
Fischer IA, et al (1999): MRI anatomy of schizophrenia. Biol
Psychiatry 45:1099 –1119.
Mufson EJ, Mesulam MM (1984): Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol 227:109 –120.
Pakkenberg B (1990): Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accum-bens in schizophrenics. Arch Gen Psychiatry 47:1023–1028. Pakkenberg B (1992): The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr
Res 7:95–100.
Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, et al (1998): Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry 43:649 – 659.
Raedler TJ, Knable MB, Weinberger DR (1998): Schizophrenia as a developmental disorder. Curr Opin Neurobiol 8:157– 161.
Rajkowska G, Selemon LD, Goldman-Rakic PS (1998): Neuro-nal and glial somal size in the prefrontal cortex: A postmor-tem morphometric study of schizophrenia and Huntington’s disease. Arch Gen Psychiatry 55:215–224.
Ray JP, Price JL (1993): The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial
prefrontal cortex in macaque monkeys. J Comp Neurol 337:1–31.
Sabri O, Erkwoh R, Scheckenberger M, Owega A, Sass H, Buell U (1997): Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-medicated schizophrenics. Lancet 349:1735–1739.
Scheaffer RL, Mendenhall W, Ott L (1996): Elementary Survey
Sampling, 5th ed. Boston: PWS-Kent.
Scheibel AB (1997): The thalamus and neuropsychiatric illness.
J Neuropsychiatry 9:342–353.
Selemon LD, Goldman-Rakic PS (1999): The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol
Psychiatry 45:17–25.
Selemon LD, Rajkowska G, Goldman-Rakic PS (1998): Elevated neuronal density in prefrontal area 46 in brains from schizo-phrenic patients: Application of a three-dimensional, stereo-logic counting method. J Comp Neurol 392:402– 412. Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A,
Grootoonk S, et al (1995): A functional neuroanatomy of hallucinations in schizophrenia. Nature 378:176 –179. Squire LR, Moore RY (1979): Dorsal thalamic lesion in a noted
case of human memory dysfunction. Ann Neurol 6:503–506. Staal WG, Hulshoff HE, Schnack H, Van der Schot AC, Kahn RS (1998): Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry 155:1784 –1786.
Stefen MD, Murray RM (1997): Schizophrenia: Developmental disturbance of brain and mind. Acta Paediatr 442:112–116. Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RE, Wexler
BE (1998): Cortical dysfunction in schizophrenia during audi-tory word and tone working memory demonstrated by functional resonance imaging. Arch Gen Psychiatry 55:1097–1103. Szelies B, Herholz K, Pawlik G, Karbe H, Hebold I, Wolf-Dieter
H (1991): Widespread functional effects of discrete thalamic infarction. Arch Neurol 48:178 –182.
van Eden CG, Rinkins A, Uylings HMB (1998): Retrograde degeneration of thalamic neurons in the mediodorsal nucleus after neonatal and adult aspiration lesions of the medial prefrontal cortex in the rat. Implications for mechanisms of functional recovery. Eur J Neurosci 10:1581–1589. Vogt BA, Pandya DN, Sosene DL (1987): Cingulate cortex of
the rhesus monkey: I. Cytoarchitecture and thalamic affer-ents. J Comp Neurol 262:256 –270.
Weinberger DR, Gallhofer B (1997): Cognitive function in schizophrenia. Int Clin Psychopharmacol 12(suppl 4):S29 – S36.
West MJ (1994): New stereological methods for counting neu-rons. Neurobiol Aging 14:275–285.
Wolkin A, Rusinek H, Vaid G, Arena L, Lafargue T, Sanfilipo M, et al (1998): Structural magnetic image averaging in schizophrenia. Am J Psychiatry 155:1064 –1073.
Xuereb JH, Perry RH, Candy JM, Perry EK, Marshall E, Bonham JR (1991): Nerve cell loss in the thalamus in Alzheimer’s disease and Parkinson’s disease. Brain 114:1363–1379. Young KA, Hicks PB, Randall PK, Wilcox RE (1994):