Detection of growth factors in the testis of roe deer
(Capreolus capreolus)
Asja Wagener, Steffen Blottner, Frank Göritz, Jörns Fickel
∗Institute for Zoo Biology and Wildlife Research, Alfred-Kowalke-Straße 17, D-10315 Berlin, Germany
Received 15 November 1999; received in revised form 20 June 2000; accepted 18 July 2000
Abstract
Roe deer is a seasonal breeder characterised by a short rutting season in summer. Mature males show synchronised cycles of testicular involution and recrudescence. Therefore, this species is a valuable model to study seasonal regulation of spermatogenesis in ruminants. It is hypothesised that a time-dependent production of testicular growth factors is required to regulate seasonal changes in testis growth and spermatogenesis. To identify potential candidates, total RNA from roe deer testis tissue was extracted at three different seasonal periods (April, August, December), and us-ing RT-PCR the presence of several growth factors (aFGF, bFGF, IGF-I, IGF-II, TGF-a, TGF-b1,
TGF-b3and two isoforms of VEGF) was detected. Sequencing of the growth factor PCR fragments
revealed a high sequence homology between cattle and roe deer. To further explore the expression patterns of the identified growth factors in roe deer their expression levels were standardised using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression. The study demonstrates the expression of several growth factors in roe deer testis and supports the assumption of their sea-sonally diverse regulation. These results provide the basis to investigate the role of growth factors in the regulation of circannual changes of testicular activity. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Growth factors; Gene expression; Testis; Seasonal dynamics; Roe deer
1. Introduction
The reproductive success of male animals requires the production of competent sperm cells. This process includes the proliferation and differentiation of spermatogenic cells in testis tissue as well as programmed cell death (apoptosis). Animal species with a seasonal reproductive activity such as roe deer, undergo cyclic changes of testicular involution and
∗Corresponding author. Tel.:+49-30-5168726; fax:+49-30-5126104. E-mail address: [email protected] (J. Fickel).
recrudescence during the transitions between breeding and non-breeding periods. There-fore, they require mechanisms for the stimulation of cell proliferation and spermatogenesis during testis growth, and also for cell apoptosis during testis involution. These mechanisms require participation of hormones such as testosterone. The production of testosterone by Leydig cells seems to ensure the survival of germ cells (Tapanainen et al., 1993; Thompson, 1994). The hormonal regulation of the changes in testis growth is mediated by paracrine and autocrine effects of different growth factors. Growth factors are key regulator molecules which affect cell proliferation, meiosis and differentiation. Several growth factors such as FGFs, IGFs, TGFs have been isolated from testis tissue and evidence suggests that these factors play a role in the control of spermatogenesis (for review: Lamb, 1993; Spiteri-Grech and Nieschlag, 1993; Kierszenbaum, 1994; Smith and Conti, 1996). These investigations were mostly carried out with non-seasonal laboratory animals or in vitro studies. How-ever, very little is known about the regulation of spermatogenesis in seasonally breeding ruminants. In deer species, so far only involvement of endocrine IGF-I in seasonal growth processes has been shown; for example in red deer by a correlation of the IGF-I secretion to the annual rhythm of antler development (Suttie et al., 1989).
As a prerequisite to test the hypothesis that the above mentioned growth factors are in-volved in processes such as spermatogenesis, cell proliferation and apoptosis in roe deer testes, the expression of the corresponding genes needs to be investigated and was there-fore analysed using the reverse transcriptase polymerase chain reaction (RT-PCR) with the subsequent comparison of results of the periods before, during and after the rut.
2. Materials and methods
2.1. Animals and tissues
Roe deer testis tissue was obtained following castration from six free ranging animals immediately after capture. The animals were immobilised (i.m., using a blow pipe) with xylazin (2 mg/kg body mass (bm)) in combination with ketamin (4 mg/kg bm). Anaesthesia was achieved with 1.5–2% isofluran. After the procedure the anaesthesia was antagonised with atipamezol (0.0875 mg/mg xylazin). After its removal the testis was dissected into small pieces and immediately frozen in liquid nitrogen.
2.2. Reverse transcription (RT)
Total RNA was prepared from 100 mg testis tissue with TRIzol reagent (GIBCO Life Technologies). Isolated RNA was quantified spectrophotometrically at 260 nm. The first
cDNA strand was generated from 1mg of total RNA in a 50ml RT-reaction. RNA was
denaturated together with 0.5mg random hexamer primers (Pharmacia) for 5 min at 70◦C.
RT was performed with 200 units (U) moloney Mouse leukaemia virus reverse transcriptase (M-MLV RT, Promega, Madison, WI, USA). The reaction mixture also contained 25 U ribonuclease inhibitor (Promega), 50 mM Tris–HCl, pH 8.3, 3 mM MgCl, 10 mM DTT, 75 mM KCl and 0.5 mM of each deoxynucleosidetriphosphate (dNTP). RNA was reversely transcribed at 37◦C 30 min, 42◦C 30 min, followed by a 5 min enzyme inactivation period
2.3. PCR-reaction
All RNA samples tested negative for the presence of contaminating DNA in a PCR with 80 ng total RNA (that had not been reversely transcribed) using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) specific primers for 36 cycles (94◦C 30 s, 50◦C 30 s, 72◦C 45 s).
Primer design for aFGF, bFGF, IGF-I and IGF-II was based on sequences of bovine and/or human aFGF (bovine: Halley et al., 1988, human: Chiu et al., 1990), bFGF (bovine: Abraham et al., 1986, human: Prats et al., 1989), IGF-I (bovine: Fotsis et al., 1990, human: Steenbergh et al., 1991), IGF-II (bovine: Brown et al., 1990, human: LeRoith and Roberts, 1993), TGF-b3 (human: ten Dijke et al., 1988) and VEGF (bovine: Leung et al., 1989, human:
Houck et al., 1991). Bovine VEGF reverse primers were from Garrido et al. (1993) and cervine TGF-aand TGF-b1primers from Francis and Suttie (1998). Single PCR fragments
were directly sequenced; in case of multiple PCR fragments all products were cloned into pGemT vector (Promega) according to the manufacturer’s instructions. All inserts were sequenced in order to identify growth factor specific fragments. In the case of aFGF, bFGF, IGF-I, VEGF (Wagener et al., 1999), TGF-aand TGF-b3PCR fragments were first obtained
using bovine primers. Sequencing of these fragments allowed the design of roe deer specific primers. All primers were synthesised by BioTeZ Ltd. (Berlin, Germany).
Either 2.5ml of the RT reaction containing 50 ng of cDNA or 2.5ml of water as negative control, were used in a 50ml PCR reaction. Synthesis of a GAPDH gene fragment served as RT and PCR control. PCRs were carried out as follows: GAPDH: 1.5 U Taq-Polymerase (PAN Systems), 0.2mM of each specific primer, 2 mM MgCl2, 0.2 mM dNTPs; aFGF, bFGF,
IGF-I, IGF-II, TGF-a, TGF-b1, TGF-b3, VEGF: 1.5 U Taq-Polymerase, 0.2mM of each
specific primer, 1.5 mM MgCl2, 0.2 mM dNTPs. All PCRs were initially denaturated for
3 min at 94◦C and had a final extension phase of 10 min at 72◦C. Individual amplification
programmes were applied for GAPDH (27 cycles at 94◦C 30 s, 50◦C 30 s, 72◦C 45 s),
aFGF (30 cycles at 94◦C 30 s, 50◦C 30 s 72◦C 45 s), bFGF (31 cycles at 94◦C 30 s, 55◦C
30 s, 72◦C 45 s), IGF-I, (29 cycles at 94◦C 30 s, 61◦C 30 s, 72◦C 45 s), IGF-II (30 cycles
at 94◦C 30 s, 62◦C 30 s, 72◦C 45 s), TGF-a (35 cycles at 94◦C 30 s, 62◦C 30 s, 72◦C
45 s), TGF-b1(30 cycles at 94◦C 30 s, 61◦C 30 s, 72◦C 45 s), TGF-b3(31 cycles at 94◦C
30 s, 63◦C 30 s, 72◦C 45 s) and VEGF (34 cycles at 94◦C 30 s, 61◦C 30 s, 72◦C 45 s).
One set of PCR fragments (for all 8 GF’s and GAPDH) was always generated from the same cDNA. Primers are listed in Table 1 together with the localisation of the amplified fragments within the corresponding growth factor. Fragments were separated on a 1.5% agarose gel and visualised on an ultraviolet transilluminator by ethidium bromide staining. The optical density of detected fragments was determined using the Video Densitometer with a Cybertech Image Capture Computer and WinCam 2.2. Image Software (Cybertech). The levels of the respective growth factors relative to the level of GAPDH present, were expressed as the appropriate growth factor/GAPDH ratio.
2.4. Sequencing
(Sanger et al., 1977). The PCR products were sequenced bidirectionally using an A310 DNA Sequence Analyzer (Applied Biosystems, USA).
3. Results
The expression of eight different growth factors in roe deer testis was detected using a RT-PCR approach. Primers were synthesised according to the corresponding bovine or human growth factors. Primer sequences, expected fragment sizes and amplified regions within the targeted growth factor are described in Table 1. VEGF primers were designed to detect all four VEGF isoforms (Houck et al., 1991) although VEGF165is reported to be
the most common form (Houck et al., 1992). In case of bFGF the three possible isoforms differ at their N-terminus, therefore, a single PCR to detect all isoforms was not possible. After optimisation, PCRs targeting IGF-II, TGF-a, TGF-b1and TGF-b3generated single
fragments that were directly sequenced. Thus, their identity as growth factor sequences was confirmed. In the case of aFGF, bFGF, IGF-I and VEGF the bovine primers generated multiple PCR products which were all cloned and subsequently sequenced. In each case a growth factor derived fragment could be identified. Sequence information from the cloned fragments was used for the design of roe deer specific primer pairs. Since the PCRs for IGF-II, TGF-aand TGF-b3 with either bovine or cervine primers were not optimal, roe
deer specific primers were derived from the sequenced fragment.
All PCR products generated with bovine primers are listed in Table 2 together with their nucleotide and amino acid homologies in comparison to the respective bovine and human growth factor cDNA and protein sequences. In the case of the vascular endothelial growth factor two fragments were generated, corresponding to two of the four known isoforms (Houck et al., 1991). Sequence comparison showed that the shorter VEGF fragment corre-sponded to the VEGF121isoform whereas the longer one corresponded to VEGF165(Houck
et al., 1991). Fig. 1 shows the PCR fragments obtained from different roe deer testicular growth factors. According to our knowledge this is the first study reporting the detection of aFGF, bFGF, IGF-I, IGF-II, TGF-a, TGF-b1, TGF-b3and VEGF gene expression in
roe deer testes. The consensus sequences (obtained from six animals) for roe deer aFGF, bFGF, IGF-I and IGF-II, TGF-a, TGF-b1, TGF-b3and VEGF were submitted to Genbank
and have been assigned accession numbers AF152586–AF152594, respectively (Table 2). Homology percentage data are based on the fragments that were originally obtained with bovine derived primers. All roe deer growth factor fragments showed a higher degree of similarity to their bovine homologs than to the corresponding human growth factors.
Compared to their bovine homologs, two roe deer growth factor fragments (TGF-b1,
bFGF) had no amino acid (aa) exchanges, three differed in one aa (IGF-I: S118A, TGF-b3:
A354S, VEGF: G113S) and one had two aa exchanges (TGF-a: L63V, T106S, compared to
sheep). A larger number of exchanges were seen in IGF-II (five positions: D104A, V105L, Q115R, I118T, A136V) and in the aFGF fragment (eight positions: S32R, Y36H, C62S, L104I, I113T, H117Y, H121N, R131S).
Table 2
Homologies between DNA and protein sequences of roe deer, bovine and human growth factors
Growth factor (GF)
Total fragment length (bp)
Length of roe deer specific sequence (w/o primers) (bp)
Homology (%) between roe deer and bovine sequences (nucleotides/ amino acids)
Homology (%) between roe deer and human sequences (nucleotides/ amino acids)
Genbank accession number
aFGF 364 320 96,3/92 91,3/91 AF152586
bFGF 360 325 97,2/100 94,5/98 AF152587
IGF-I 239 199 96,5/98 90/98 AF152588
IGF-II 379 340 96,8/95 86,7/85 AF152589
TGF-a 258 223 96,4/97a 94,1/97a AF152590
TGF-b1 200 152 96,7/100 90,2/100 AF152591
TGF-b3 280 244 n.e. 94,3/98 AF152592
VEGF121 278 236 98,3/98 93,6/94 AF152593
VEGF165 411 369 98,9/99 95,9/96 AF152594
aHomology to ovine nucleotide and protein sequences; n.e.: no entry (no Genbank entry available regarding
bovine or ovine TGF-b3sequences).
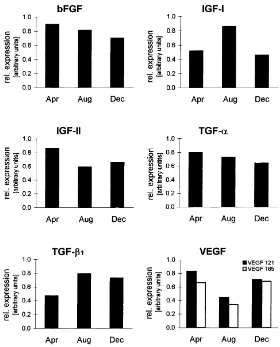
post-rutting (December) season. Fig. 2 shows the approximate course of the gene expression from six representative growth factors. Expression of IGF-I and TGF-b1seem to reach their
peak during rutting season with TGF-b1having a prolonged high expression. The expression
of IGF-II and VEGF, however, appear to be down regulated during rutting season. The expression of bFGF and TGF-a seem to remain relatively uninfluenced throughout the year although a timespan of 4 month between the sample yielding might obscure actual changes. Since only two animals were available for each month, statistical analysis was not performed.
Fig. 2. Relative expression levels of different growth factors during pre-rutting (April), rutting (August) and post-rutting (December) season. Values (arbitrary units) represent the ratio of mean GF/mean GAPDH expression, measured video densitometrically.
4. Discussion
This study was launched based on the fact that many growth factors are important reg-ulators of growth and differentiation in general and that their occurrence and regulatory function has been demonstrated in the testicular tissues of several species (for reviews: Spiteri-Grech and Nieschlag, 1993; Smith and Conti, 1996; Schlatt et al., 1997). There is however, a lack of information about the course of gene expression and the implications growth factors may have on the differential growth in testes of seasonal species.
Fibroblast growth factors and insulin-like growth factors have positive effects on Sertoli cells, in which DNA and protein synthesis is stimulated, as well as on Leydig cells, where they act as enhancers for steroid hormone synthesis and secretion. In germ cells they activate the expression of cellular genes and trigger cellular differentiation and most likely also stimulate DNA synthesis (Tajima et al., 1995; Lahr et al., 1992). Their actions are mediated through their corresponding receptors which have been found on the surface of somatic and germ cells (Han et al., 1993; Schlatt et al., 1997).
The presence of fibroblast growth factors and insulin-like growth factors in roe deer testicular tissue and their expression peaks during pre-rutting season in April (bFGF, IGF-II) and during rutting season in August (IGF-I), respectively, very likely reflects the importance of these factors in regulating DNA-, RNA- and protein synthesizing processes coinciding with developmental changes taking place during the annual cycle.
The differences between the deduced amino acid sequences of roe deer aFGF and IGF-II fragments and the corresponding bovine sequences are within the variability be-tween species, even though some of the exchanged amino acids differ considerably in their physico-chemical properties.
TGF-a, which is secreted by Leydig and Sertoli cells, stimulates DNA synthesis and cell proliferation in peritubular cells. The TGF-a/EGF receptor was found on Sertoli cells but also on germ cells (Caussanel et al., 1996). EGF expression could not be detected in roe deer testes, whereas TGF-amRNA was present. Since TGF-awas found in testicular tissue as well (Mullaney and Skinner, 1992), it probably exerts the same functions as in somatic cells. In addition, TGF-apossibly interacts with FGFs and IGFs in a synergistic manner. Although IGFs, FGFs and TGF-aall act as cell mitogens, there is, however, very likely an underlaying selectivity, for differing cell types may recognise each growth factor with different relative affinity. This may be indicated by the seasonally differing relative expression levels of bFGF, IGF-I, IGF-II and TGF-ain roe deer. Additionally, particular responsibilities of each growth factor within the cell cycle must also be considered. Opposing these effects is another growth factor, which very potently counteracts mitogenic stimulation in many somatic cells: TGF-b
(Moses et al., 1990). Not only does TGF-binhibit the proliferation of somatic cells, it also induces apoptosis (for review: Haufel et al., 1999). Such an induction of apoptosis is seen in germ cells too, but without mitosis inhibition (Olaso et al., 1998). Watrin et al. (1991) have demonstrated, that TGF-b1(1.8 kb mRNA) only occurs in male germ cells, whereas
the larger form of TGF-b1(2.4 kb mRNA) and TGF-b3are both present in somatic cells.
This is supported by the fact that in roe deer the TGF-b1expression increases during the
end of rutting season (August) and is still high in the non-rutting season (December), when apoptosis occurs. This results in the involution of the roe deer testis (Blottner et al., 1996). By comparison, TGF-b3has a more consistent expression level and might not be as important
as TGF-b1in the event of apoptosis in roe deer testis. Nevertheless, the presence of TGF-b1
and TGF-b3in roe deer testes is seen as an indicator for their importance during the onset
of processes involved in testes mass reduction and functional down regulation.
neovascularisation, the presence of VEGF in roe deer testes — not just with one but two isoforms — is of special interest, especially in terms of an energy saving strategy during the non-rutting season and additional energy provision during the rut. This is reflected by the different expression levels of both VEGF isoforms at the examined seasons. The data indicates that the production of VEGF is stimulated in December explaining the increased testicular microvascularisation in early spring (unpublished results). Until the testis volume has almost reached its maximum in June, the expression level of VEGFs is relatively high, whereas in the beginning of the mating season capillarisation of testicular tissue has been completed and less VEGF is required.
Although an essential influence of some growth factors on somatic and germ cell de-velopment in the testis of several species has been shown in previous studies, effects on the seasonal reproduction of wild animals have not been sufficiently investigated yet. To clarify the role of the examined growth factors for the photoperiodically driven involution and recrudescence of roe deer testes throughout the year and their necessity for the respec-tive reproducrespec-tive stages, further studies are needed. Investigating the fluctuation of these growth factors on both mRNA and protein levels during the course of the year may supply additional information regarding their importance for seasonal animals. The detection of the above mentioned growth factors in roe deer testes is a prerequisite to investigate their role in the regulation of circannual changes of testicular activity. Future investigations will include the measurement of the expression of each growth factor on a monthly basis with more samples for stastical analysis in order to investigate the annual course of GF gene expression in a much more narrow time frame. In addition those results could provide in-formation as to whether certain GF’s operate by themselves or rather through their ratio to other GF’s in order to induce such drastic changes as seen in testicular growth (including functional changes) before the mating season and during the following testicular regression.
Acknowledgements
The authors would like to thank Dr. Ralph Einspanier for his support during this study and Dr. Mark Kelly for carefully reading the manuscript.
References
Abraham, J.A., Mergia, A., Whang, J.L., Tumolo, A., Friedman, J., Hjerrild, K.A., Gospodarowicz, D., Fiddes, J.C., 1986. Nucleotide sequence of a bovine clone encoding the angiogenic protein basic fibroblast growth factor. Science 233, 545–548.
Blottner, S., Hingst, O., Meyer, H.H.D., 1996. Seasonal spermatogenesis and testosterone production in roe deer (Capreolus capreolus). J. Reprod. Fertil. 108, 299–305.
Brown, W.M., Dziegielewska, K.M., Foreman, R.C., Saunders, N.R., 1990. The nucleotide and deduced amino acid sequences of insulin-like growth factor II cDNAs from adult bovine and fetal sheep liver. Nucleic Acids Res. 18, 4614.
Caussanel, V., Tabone, E., Mauduit, C., Dacheux, F., Benahmed, M., 1996. Cellular distribution of EGF, TGF alpha and their receptor during postnatal development and spermatogenesis of the boar testis. Mol. Cell. Endocrinol. 123, 61–69.
Derynck, R., Rhee, L., Chen, E.Y., Van Tilburg, A., 1987. Intron-exon structure of the human transforming growth factor-beta precursor gene. Nucleic Acids Res. 15, 3188–3189.
Ergün, S., Kilic, N., Fiedler, W., Mukhopadhyay, A.K., 1997. Vascular endothelial growth factor and its receptors in normal human testicular tissue. Mol. Cell. Endocrinol. 131, 9–20.
Fotsis, T., Murphy, C., Gannon, F., 1990. Nucleotide sequence of the bovine insulin-like growth factor 1 (IGF-1) and its IGF-1A precursor. Nucleic Acids Res. 18, 676.
Francis, S.M., Suttie, J.M., 1998. Detection of growth factors and proto-oncogene mRNA in the growing tip of red deer (Cervus elaphus) antler using reverse-transcriptase polymerase chain reaction. J. Exp. Zool. 281, 36–42. Garrido, C., Saule, S., Gospodarowicz, D., 1993. Transcriptional regulation of vascular endothelial growth factor
gene expression in ovarian bovine granulosa cells. Growth factors 8, 109–117.
Halley, C., Courtois, Y., Laurent, M., 1988. Nucleotide sequence of bovine acidic fibroblast growth factor cDNA. Nucleic Acids Res. 16, 10913.
Han, I.S., Sylvester, S.R., Kim, K.H., Schelling, M.E., Venkateswaran, S., Blanckaert, V.D., McGuinness, M.P., Griswold, M.D., 1993. Basic fibroblast growth factor is a testicuar germ cell product which may regulate sertoli cell function. Mol. Endocrinol. 7, 889–897.
Haufel, T., Dormann, S., Hanusch, J., Schwieger, A., Bauer, G., 1999. Three distinct roles for TGF-beta during intercellular induction of apoptosis: a review. Anticancer Res. 19, 105–111.
Houck, K.A., Ferrara, N., Winer, J., Cachianes, G., Li, B., Leung, D.W., 1991. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 5, 1806–1814.
Houck, K.A., Leung, D.W., Rowland, A.M., Winer, J., Ferrara, N., 1992. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 267, 26031–26037. Kierszenbaum, A.L., 1994. Mammalian spermatogenesis in vivo and in vitro: a partnership of spermatogenesis
and somatic cell lineages. Endocr. Rev. 15, 116–134.
Lahr, G., Mayerhofer, A., Seidl, K., Bucher, S., Grothe, C., Knochel, W., Gratzl, M., 1992. Basic fibroblast growth factor (bFGF) in rodent testis. Presence of bFGF mRNA and of a 30 kDa bFGF protein in pachytene spermatocytes. FEBS. Lett. 302, 43–46.
Lamb, D.J., 1993. Growth factors and testicular development. J. Urol. 150, 583–592.
LeRoith, D., Roberts Jr., C.T., 1993. Insulin-like growth factors. Ann. N. Y. Acad. Sci. 692, 1–9.
Leung, D.W., Cachianes, G., Kuang, W.J., Goeddel, D.V., Ferrara, N., 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309.
Moses, H.L., Yang, E.Y., Pietenpol, J.A., 1990. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 63, 245–247.
Mullaney, B.P., Skinner, M.K., 1992. Transforming growth factor-alpha and epidermal growth factor receptor gene expression and action during pubertal development of the seminiferous tubule. Mol. Endocrinol. 6, 2103–2113. Olaso, R., Pairault, C., Boulogne, B., Durand, P., Habert, R., 1998. Transforming growth factor beta1 and beta2
reduce the number of gonocytes by increasing apoptosis. Endocrinology 139, 733–740.
Prats, H., Kaghad, M., Prats, A.C., Klagsbrun, M., Lelias, J.M., Liauzun, P., Chalon, P., Tauber, J.P., Amalric, F., Smith, J.A., Caput, D., 1989. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc. Natl. Acad. Sci. U. S. A. 86, 1836–1840.
Sanger, F., Nicklen, S., Coulson, A.R., 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74, 5463–5467.
Schlatt, S., Meinhardt, A., Nieschlag, E., 1997. Paracrine regulation of cellular interactions in the testis: factors in search of a function. Eur. J. Endocrinol. 137, 107–117.
Smith, E.P., Conti, M., 1996. Growth factors and testicular function: relevance to disorders of spermatogenesis in humans. Semin. Reprod. Endocrinol. 14, 209–217.
Spiteri-Grech, J., Nieschlag, E., 1993. Paracrine factors relevant to the regulation of spermatogenesis — a review. J. Reprod. Fertil. 98, 1–14.
Steenbergh, P.H., Koonen-Reemst, A.M., Cleutjens, C.B., Sussenbach, J.S., 1991. Complete nucleotide sequence of the high molecular weight human IGF-I mRNA. Biochem. Biophys. Res. Commun. 175, 507–514. Suttie, J.M., Fennessy, P.F., Corson, I.D., Laas, F.J., Crosbie, S.F., Butler, J.H., Gluckman, P.D., 1989. Pulsatile
Tajima, Y., Watanabe, D., Koshimizu, U., Matsuzawa, T., Nishimune, Y., 1995. Insulin-like growth factor-I and transforming growth factor-alpha stimulate differentiation of type A spermatogonia in organ culture of adult mouse cryptorchid testes. Int. J. Androl. 18, 8–12.
Tapanainen, J.S., Tilly, J.L., Vihko, K.K., Hsueh, A.J., 1993. Hormonal control of apoptotic cell death in the testis: gonadotropins and androgens as testicular cell survival factors. Mol. Endocrinol. 7, 643–650.
ten Dijke, P., Hansen, P., Iwata, K.K., Pieler, C., Foulkes, J.G., 1988. Identification of another member of the transforming growth factor type beta gene family. Proc. Natl. Acad. Sci. U. S. A. 85, 4715–4719.
Thompson, E.B., 1994. Apoptosis and steroid hormones. Mol. Endocrinol. 8, 665–673.
Wagener, A., Blottner, S., Einspanier, R., Fickel J., 1999. Testicular growth factor expression in seasonal and non-seasonal ruminants. Reprod. Dom. Anim. 34, V30.