Possible existence of platelet activation before the onset of cerebral
infarction
Hitoshi Kurabayashi
a,*, Jun’ichi Tamura
b, Takuji Naruse
b, Kazuo Kubota
aaDepartment of Medicine,Kusatsu Branch Hospital,Gunma Uni
6ersity Hospital,627-3Kusatsu,Gunma377-1711,Japan
bThird Department of Medicine,Gunma Uni
6ersity School of Medicine,3-39-15Shouwa,Maebashi,Gunma371-8511,Japan Received 11 January 1999; received in revised form 29 November 1999; accepted 7 January 2000
Abstract
To study the existence of platelet activation before the onset of cerebral infarction, the ultrastructural features of platelets (7-day survival) and coagulation-fibrinolytic markers (70 – 100-min life span) were measured 2 – 12 h (acute phase), 7 days (subacute phase) and 6 months (chronic phase) after onset in 18 patients with cerebral infarction. Seven patients with atherosclerosis but without cerebral infarction and eight healthy subjects were studied as controls. Ultrastructural study included folds, pseudopods, vacuoles and centralization in addition to immunochemical staining such as platelet peroxidase and fibrinogen. Furthermore, b-thromboglobulin, platelet factor-4, thrombin antithrombin complex and a2-plasmin inhibitor plasmin complex
were examined as coagulation-fibrinolytic markers. Ultrastructural study of circulating platelets demonstrated no difference between acute and chronic phases and little difference between cerebral infarction and atherosclerosis, although plasma coagulation-fibrinolytic markers showed an increase in cerebral infarction at the acute phase but no difference among the chronic phase of cerebral infarction, atherosclerosis and normal healthy subjects. It is considered that shape change in circulating platelets was caused by pre-existed atherosclerosis rather than the thrombotic event itself though coagulation-fibrinolytic markers were derived from the thrombotic event. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Platelet activation; Cerebral infarction; Atherosclerosis; Ultrastructure; Platelet peroxidase
www.elsevier.com/locate/atherosclerosis
1. Introduction
Although morphological analysis of platelets in pa-tients with cerebral infarction demonstrated platelet shape change as a result of a thrombotic event [1], little is known about the ultrastructure of platelet in atherosclerosis. It remains controversial whether the shape change observed in platelets obtained from pe-ripheral blood is a result of a thrombotic event or it represents the remaining platelets that escaped the thrombotic event. As injured platelets are removed rapidly by the spleen, ultrastructural shape change ob-served during the subsequent several days seems to be due to pre-existing atherosclerosis rather than the thrombotic event itself. Although it was reported that
shape changes such as folds, pseudopods, ballooning and decreased a-granules were observed in platelets obtained from injured vessels [2,3], the result alone cannot fully explain the question described above. Re-cently, proteins released from platelets,b -thromboglob-ulin (b-TG) and platelet factor-4 (PF-4), were reported to be useful markers for platelet activation [4]. They are removed from circulation with a half life of 70 – 100 min. Some reports demonstrated significant increases in plasma b-TG and PF-4 after the onset of cerebral infarction but others did not [5,6]. As platelet perox-idase (PPO) detected in the dense tubular system is associated with prostaglandin synthesis [7 – 9], PPO is supposed to play a role in platelet activation. Fibrino-gen is localized in thea-granules of resting platelets and released by physical and chemical stimulations [10]. Thus, we examined the ultrastructure of platelets with reference to the localization of PPO and fibrinogen, and the plasma levels of b-TG and PF-4 in patients with cerebral infarction at 3 points: at the acute phase or Abbre6iations: b-TG, b-thromboglobulin; Fbg, fibrinogen; PF-4,
platelet factor-4; PIC, a2-plasmin inhibitor plasmin complex; PPO,
platelet peroxidase; TAT, thrombin antithrombin complex. * Corresponding author. Tel.: +81-279-884011; fax: + 81-279-884000.
immediately after onset, at the subacute phase or 7 days after onset, and at the chronic phase or 6 months after onset, in comparison with that in patients with atherosclerosis and healthy subjects, in order to deduce whether platelet shape change observed in peripheral blood after onset is caused by a thrombotic event itself or by pre-existing atherosclerosis.
2. Materials and methods
2.1. Patients and controls
Eighteen newly diagnosed patients with cerebral in-farction (11 males and seven females, 63.898.1 [mean9SD] years) presenting hemiplegia, who were treated in our hospital between 1995 and 1996, were included in this study. Patients with consciousness dis-turbance or infectious disease at acute phase were ex-cluded from this study. There were no patients demonstrating any episode of myocardial or pulmonary infarction, primary platelet or coagulation disorder, or any evidence that the cerebral infarction was due to cardiac embolism. No patients were given anticoagulant or antiplatelet drugs during the period of this study. Repeated CT scans demonstrated no evidence of post-infarction hemorrhage in any patients. An age-matched control group consisted of eight normal healthy volun-teers (five males and three females, 61.3910.9 years). These eight volunteers had no diseases that could be detected by physical, urinary, fecal and blood examina-tions, chest radiography, echocardiogram, and brain CT scan. A second control group consisted of seven patients with atherosclerosis (five males and two fe-males, 73.796.8 years), which was diagnosed based on either moderate or severe hypertension in stage 2 [11] and arteriosclerotic retinopathy of grade II or III by Keith-Wagener-Barker classification [12]. These pa-tients had been all treated with calcium antagonist alone and had not demonstrated any evidence of coro-nary heart disease, cerebrovascular disease or primary platelet disorder. Informed consent was obtained from all patients and control subjects. This study was ap-proved by the ethical committee of Kusatsu Branch Hospital, Gunma University Hospital.
2.2. Ultrastructural study
Venous blood was carefully collected without a tourniquet into a plastic syringe containing acid citrate dextrose solution at three points: at the acute phase or 2 – 12 h after the onset of cerebral infarction, at the subacute phase or 7 days later, and at the chronic phase or 6 months later in patients with cerebral infarction. Control blood samples were obtained from atheroscle-rotic patients and normal healthy subjects at 08:00 h.
The blood sample was gently transferred into a plastic tube and centrifuged at 190×g for 15 min to obtain platelet-rich plasma. The platelet-rich plasma was trans-ferred into another plastic tube containing acid citrate dextrose solution and further centrifuged at 800 g for 15 min. The pellet was used for ultrastructural study by the conventional method using an electron microscope (JEM 200CX, JEOL, Japan). Two hundred platelets were observed to evaluate the frequency of folds, pseu-dopods, vacuoles and centralization. PPO reaction was performed by the method of Breton-Gorius et al. [8]. Immunoelectron gold staining was performed by the method of Cramer et al. [10]. Anti-fibrinogen poly-clonal antibody and goat antirabbit immunoglobulin fractions coupled to colloidal gold particles were pur-chased from Dakopatts (Copenhagen) and Janssen Pharmaceutica (Belgium), respectively.
The degrees of PPO staining and immunogold ing were tentatively expressed as follows [13]: full stain-ing of PPO or immunogold was scored as 3, while intermediate, weak and no stainings were scored as 2, 1 and 0, respectively. The total score was calculated as the sum after counting 100 platelets at random. Ultra-structural analysis was performed by an electron micro-scopist who did not know the patients’ information.
2.3. Hematological 6ariables
Plasma levels ofb-TG, PF-4, thrombin antithrombin complex (TAT) and a2-plasmin inhibitor plasmin
com-plex (PIC) were measured by the enzyme immunoassays in patients with cerebral infarction and atherosclerosis and in normal healthy subjects.
2.4. Statistical analysis
All data are presented as mean9SD. Unpaired Stu-dent’s t-test was used to evaluate the significance of differences between the two categorical variables. Dif-ferences were considered significant at PB0.05.
3. Results
Fig. 1. Typical ultrastructure of platelets. (A) Peroxidase reaction in a platelet obtained from a normal subject. Scale bar=0.5mm. (B) Peroxidase reaction in a platelet obtained from a stroke patient (5 h after the onset of stroke). Scale bar=1.0 mm. (C) Immunoelectron gold staining for fibrinogen in a platelet obtained from a normal subject. Scale bar=0.5 mm. (D) Immunoelectron gold staining for fibrinogen in a platelet obtained from a stroke patient (3 h after the onset of stroke). Scale bar=0.5mm.
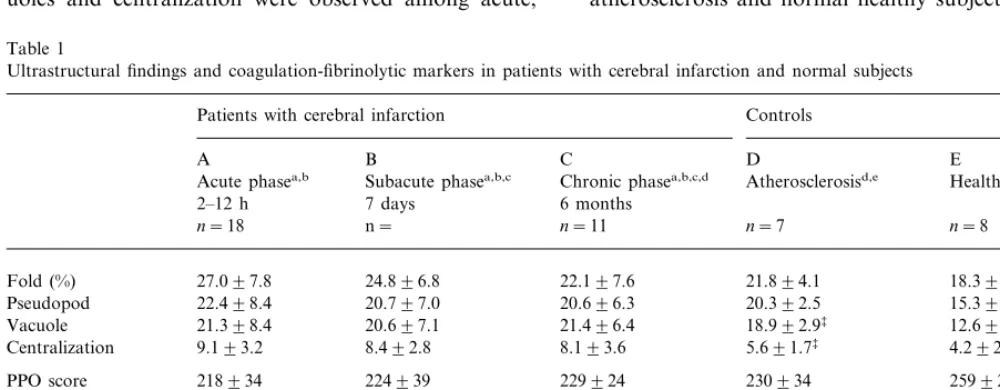
subacute and chronic phases of cerebral infarction (Table 1). The frequency of pseudopods in patients with atherosclerosis was similar to that in patients with cerebral infarction at the chronic phase but significantly higher than that in normal healthy subjects. The fre-quency of vacuoles in cerebral infarction at the chronic phase was significantly higher than that in patients with atherosclerosis which was significantly higher than that in normal healthy subjects. The frequency of centraliza-tion in patients with cerebral infarccentraliza-tion at the chronic phase was significantly higher than that both in atherosclerosis and in normal healthy subjects. Strong PPO activity was detected only in the dark tubular system in healthy subjects but weak PPO reaction was scarcely observed in patients with cerebral infarction at the acute, subacute and chronic phases (Fig. 1). Simi-larly, although fibrinogen was detected exclusively in a-granules in normal healthy subjects, less fibrinogen was observed in patients with cerebral infarction at the acute, subacute and chronic phases. Table 1 shows the total scores of PPO and fibrinogen content of platelets in patients with cerebral infarction and atherosclerosis and healthy subjects. PPO and fibrinogen scores in patients with cerebral infarction at the chronic phase were significantly decreased compared with those in normal healthy subjects (Table 1). However, there was no significant difference between patients with atherosclerosis and normal healthy subjects.
fold, pseudopods, vacuoles and centralization obtained from a patient with cerebral infarction. No significant differences in the frequencies of fold, pseudopods, vac-uoles and centralization were observed among acute,
Table 1
Ultrastructural findings and coagulation-fibrinolytic markers in patients with cerebral infarction and normal subjects
Controls Patients with cerebral infarction
A B C D E
Healthy subjectsd,e
Acute phasea,b Subacute phasea,b,c Chronic phasea,b,c,d Atherosclerosisd,e
7 days
2–12 h 6 months
n= n=8
n=18 n=11 n=7
18.396.1 27.097.8
Fold (%) 24.896.8 22.197.6 21.894.1
22.498.4 20.797.0
Pseudopod 20.696.3 20.392.5 15.393.1‡ ¶
12.695.8‡‡ ¶¶
18.992.9‡
21.398.4 21.496.4
Vacuole 20.697.1
9.193.2 8.492.8 8.193.6
Centralization 5.691.7‡ 4.292.4‡
259928‡
PPO score 218934 224939 229924 230934
142936 145933
Fbg score 146934 153933 184933‡
74.8924.6 51.3914.8**§§
b-TG (ng/ml) 32.999.9** 3197.3 38.8914.1
23.6915.6*
40.1923.2 16.797.4
PF-4 (ng/ml) 14.893.4** 13.193.2
2.8491.13 TAT (mg/ml) 5.2892.60 2.9891.75** 3.5391.19* 2.991.7
0.8090.26 0.6590.22
PIC (mg/ml) 0.7190.18 0.4990.31 0.6190.31
aAcute, subacute and chronic phases show results 2–12 h, 7 days and 6 months after the onset of cerebral infarction, respectively. Data are
presented by mean 9SD.
bThe values at the chronic and subacute phases are compared with those at the acute phase, (A vs B, A vs C); *PB0.05, **PB0.01 by unpaired
Student’st-test.
cThe values at the subacute phase are compared with those at chronic phase, (B vs C);§§PB0.01 by unpaired Student’st-test.
dThe values in patients with atherosclerosis and healthy subjects are compared with those in patients with cerebral infarction at the chronic
phase, (C vs D, C vs E);‡PB0.05,‡‡PB0.01 by unpaired Student’st-test.
Plasma levels of b-TG, PF-4 and TAT at the acute phase were significantly higher than those both at the subacute and at the chronic phases. In addition, the plasma level ofb-TG at the subacute phase was signifi-cantly higher than that at the chronic phase and the plasma level of PF-4 at the subacute phase showed a tendency to increase compared with that at the chronic phase. However, there were no significant differences among patients with cerebral infarction at the chronic phase, patients with atherosclerosis, and normal healthy subjects. The plasma level of PIC did not change.
4. Discussion
In the acute phase of cerebral infarction, the plasma levels of b-TG, PF-4 and TAT were increased when compared with those in normal healthy subjects, sug-gesting the existence of platelets being destroyed. The frequencies of pseudopods, vacuoles and centralization were increased. In the subacute phase, the plasma level of b-TG remained significantly increased when com-pared with those in normal healthy subjects and those of PF-4 and TAT were decreased to normal levels, suggesting that platelets were still being destroyed or had been destroyed. The increase in platelet shape change persisted. In the chronic phase, the plasma levels of b-TG, PF-4 and TAT were decreased to normal levels. The frequencies of pseudopods, vacuoles and centralization remained significantly increased when compared with those in normal healthy subjects. The frequency of pseudopods in cerebral infarction at the chronic phase was equal to that in atherosclerosis and higher than that in normal healthy subjects. The frequency of vacuoles in cerebral infarction at the chronic phase was higher than that in atherosclerosis which was higher than that in the normal healthy subjects. The frequency of centralization in cerebral infarction at the chronic phase was higher than that in atherosclerosis which was equal to that in the normal healthy subjects. In contrast, the platelet shape change, and PPO and fibrinogen scores were equal among acute, subacute and chronic phases of cerebral infarc-tion. In atherosclerotic patients, the frequencies of pseudopods and vacuoles were higher than those of normal healthy subjects, though the serum levels of b-TG, PF-4 and TAT were equal between the two groups. Therefore, platelet shape changes or platelet activation existed in atherosclerotic patients and fur-thermore, platelet activation may have existed before the onset of cerebral infarction, considering that b-TG and PF-4 have a 70 – 100-min life span and increase transiently after the onset of cerebral infarction [4 – 6], platelet survival is about 7 days and injured platelets are rapidly trapped by the spleen. In addition, this study deduced that centralization might be
characteris-tic for cerebral infarction or unstable atherosclerosis. Though the transient increase inb-TG, PF-4 and TAT may be attributable to the thrombotic event itself, the consistent increase in pseudopods, vacuoles and central-ization and the coincidental decrease in PPO and Fbg may be caused by preexisting atherosclerosis.
The ultrastructural platelet shape change at the acute phase in this study was consistent with a study reported previously [1], which did not describe platelet shape change at the chronic phase. Ultrastructural platelet shape change caused by mechanical or electrical dam-age [2,3] also resembles the results of our study. Our study revealed that the decrease in PPO and fibrinogen concentrations coincided with the increase in platelet shape change. Peroxidase in platelets is reported to play a role in the synthesis of prostaglandins associated with hemostasis, thrombosis and aggregation [14]. Fibrino-gen is synthesized only in megakaryocytes and cannot be taken up by platelets from plasma [15]. In addition, it was reported that the fibrinogen content ina-granules decreased after platelet activation. Thus, increased pseudopods, vacuoles and centralization, and decreased peroxidase activity and fibrinogen content observed in patients with cerebral infarction at the chronic phase may have been partly caused by atherosclerosis or endothelial injury. It is interesting that centralization was increased in patients with cerebral infarction but not in patients with atherosclerosis. The centralization was reported to be a final process of morphological change in platelet activation in contrast to folds and pseudopods which might be reversible and were sepa-rated events from centralization [16]. Since atheroscle-rotic patients included in this study had no episodes of thrombosis or ischemia, they seemed to have had stable atherosclerosis or plaque. Unstable atherosclerosis or plaque might be associated with centralization, while the stable forms might be associated with pseudopods and vacuoles. Platelet activation observed in hyperten-sive patients was reversible change and platelets might not be completely activated by stable atherosclerosis alone. Thus, centralization might be a possible marker of unstable plaque or infarction.
may be a useful addition to their results. Comparing outcomes among cerebral infarction, atherosclerosis and healthy subjects, circulating platelets might begin to be activated in atherosclerosis before a thrombotic event occurrs. Although thrombosis is caused by ulcer-ated plaque, increased aggregability of platelets, and endothelial injury by platelets [26 – 30], it is suggested that morphological platelet activation is associated with the increasing risk of cerebral infarction in atheroscle-rotic patients.
References
[1] Joseph R, Riddle JM, Welch KMA, D’Andrea G. Platelet ultrastructure and secretion in acute ischemic stroke. Stroke 1989;20:1316 – 9.
[2] Honour AJ, Pickering GW, Sheppard BL. Ultrastructure and behavior of platelet thrombi in injured arteries. Br J Exp Pathol 1971;52:482 – 4.
[3] Harrison P, Cramer EM. Platelet alpha granules. Blood Rev 1993;7:52 – 62.
[4] Kaplan KL, Owen J. Plasma levels ofb-thromboglobulin and platelet factor 4 as indices of platelet activation in vivo. Blood 1981;57:199 – 202.
[5] De Bohr AC, Turpie AGG, Butt RW, Duke RJ. Plasma be-tathromboglobulin and serum fragment E in acute partial stroke. Br J Haematol 1982;50:327 – 34.
[6] Lane DA, Wolff S, Ireland H, Gawel M, Foadi M. Activation of coagulation and fibrinolytic systems following stroke. Br J Haematol 1983;53:655 – 8.
[7] Breton-Gorius J, Guichard J. Ultrastructural localization of peroxidase activity in human platelets and megakaryocytes. Am J Pathol 1972;66:277 – 86.
[8] Breton-Gorius J, Vanhaeke D, Pryzwansky KB, et al. Simulta-neous detection of membrane markers with monoclonal antibod-ies and peroxidase activitantibod-ies in leukemia: ultrastructural analysis using a new method of fixation preserving the platelet perox-idase. Br J Haematol 1984;58:447 – 58.
[9] Gerrard JM, White JG, Rao GM, Townsend D. Localization of platelet prostaglandin production in the platelet dense tubular system. Am J Pathol 1976;83:283 – 98.
[10] Cramer EM, Vainchenker W, Vinci G, Guichard J, Breton-Gorius J. Gray platelet syndrome: Immunoelectron microscopic localization of fibrinogen and von Willebrand factor in platelets and megakaryocytes. Blood 1985;66:1309 – 16.
[11] 1993 Guidelines for the management of mild hypertension: Memorandum from a WHO/ISH meeting. Bull WHO 1993;71:503 – 17.
[12] Williams GH. Hypertensive vascular disease. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DS, editors. Harrison’s Principles of Internal Medicine, 13th. New York: McGraw-Hill, 1994.
[13] Kurabayashi H, Kubota K, Take H, Tamura K, Shirakura T. Effects of hyperthermal stress on the ultrastructure of platelets with reference to the localization of platelet peroxidase and fibrinogen in vivo. Am J Hematol 1997;56:244 – 7.
[14] Smith JB, Silver MJ. Prostaglandin synthesis by platelets and its biological significance. In: Gordon JL, editor. Platelets in Biol-ogy and PatholBiol-ogy. Amsterdam: Elsevier, 1976.
[15] Leven RM, Schick PK, Budzynski AZ. Fibrinogen biosynthesis in isolated guinea pig megakaryocytes. Blood 1985;65:501 – 4. [16] White JG. Current concepts of platelet structure. Am J Clin
Pathol 1979;741:363 – 78.
[17] Kubota K, Sakurai T, Tamura J, Shirakura T. Is the circadian change in hematocrit and blood viscosity a factor triggering cerebral and myocardial infarction? Stroke 1987;18:812 – 3. [18] Turton MB, Deegan T. Circadian variations of plasma
cate-cholamine, cortisol, and immunoreactive insulin concentrations in supine subjects. Clin Chim Acta 1974;55:389 – 97.
[19] Davis MJ, Thomas AC. Plaque-fissuring the cause of acute myocardial infarction, sudden ischemic death, and crescendo angina. Br Heart J 1985;53:363 – 73.
[20] Falke E. Plaque rupture with severe pre-existing stenosis precip-itating coronary thrombosis. Brit Heart J 1993;750:31 – 4. [21] Rao GH, Rao AT. Pharmacology of platelet
activation-in-hibitory drugs. Ind J Physiol Pharmacol 1994;38:69 – 84. [22] Kooten VF, Ciabattoni G, Koudstaal PJ, Dippel DW, Patrono
C. Increased platelet activation in the chronic phase after cere-bral ischemia and intracerebral hemorrhage. Stroke 1999;30:546 – 9.
[23] Grau AJ, Ruf A, Vogt A, et al. Increased fraction of circulating activated platelets in acute and previous cerebrovascular is-chemia. Thromb Haemost 1998;80:298 – 301.
[24] Lip GY, Blann AD, Jones AF, Lip PL. Beevers DG. Relation of endothelium, thrombogenesis, and hemorheology in systemic hypertension to ethnicity and left ventricular hypertrophy. Am J Cardiol 1997;80:1566 – 71.
[25] Blann AD, Lip GY, Islim IF, Beevers DG. Evidence of platelet activation in hypertention. J Int Hypertens 1997;11:607 – 9. [26] White JG. Platelets and atherosclerosis. Eur J Clin Invest
1994;24(Suppl. 1):25 – 9.
[27] Futrell N, Riddle JM, et al. The ultrastructure of photochemi-cally induced thrombi with embolization in a rat model. Stroke 1993;24:1983 – 91.
[28] Knight CJ, Panesar M, Wright C, et al. Altered platelet function detected by flow cytometry. Effects of coronary artery disease and age. Arterioscler Thromb Vasc Biol 1997;17:2044 – 53. [29] Boudoulas H. Factors determining and prevention of
atheroscle-rotic plaque rupture. Postgrad Med J 1994;70(Suppl 1):S43 – 5. [30] Kaul S, Heistad DD, Mugge A, et al. Vascular responses to
platelet activation in normal and atherosclerotic primates in vivo. Arterioscler Thromb Vasc Biol 1991;11:1745 – 51.