Different radiosensitivity of smooth muscle cells and endothelial
cells in vitro as demonstrated by irradiation from a Re-188 filled
balloon catheter
Jo¨rg Kotzerke
a,*, Ralf Gertler
b, Inga Buchmann
a, Regine Baur
b,
Vinzenz Hombach
b, Sven Norbert Reske
a, Rainer Voisard
baDepartment of Nuclear Medicine,Uni6ersity of Ulm,D-89070 Ulm, Germany bDepartment of Internal Medicine II (Cardiology),Uni6ersity of Ulm,D-89070 Ulm, Germany
Received 12 May 1999; received in revised form 4 October 1999; accepted 29 October 1999
Abstract
There is an increasing interest in irradiation to control restenosis after balloon angioplasty by an internal radioactive source. Differences in radiosensitivity of the predominant cells of the human coronary artery (i.e. endothelial cells (HCAEC), smooth muscle cells from the media (HCMSMC) and from plaque material (HCPSMC), are issues of controversal discussion. Therefore, we investigated the graded inhibition of cells by irradiation from a balloon catheter filled with a high-energy beta-emitter (Rhenium-188) in vitro. HCPSMC, HCMSMC and HCAEC were cultured and irradiated with increasing dose from 7.5 to 37.5 Gy at a dose rate of 1.590.3 Gy/min. After irradiation, bromodeoxyuridine (BrdU) was added and cells were fixed 18 h later. In a limited field opposite to the balloon, the number of BrdU-positive cells were analysed in comparison to non-irradiated controls. Significant inhibition was demonstrated in HCPSMC and HCMSMC at 7.5 Gy while HCAEC needed 22.5 Gy for similar effects. The antiproliferative effect was dose dependent in all cell strains. The effect of irradiation with 22.5 Gy on smooth muscle a-actin, vimentin, and a-tubulin of HCPSMC and HCMSMC and on von Willebrand factor (vWF), vimentin, and a-tubulin of HCAEC was investigated by means of indirect immunofluorescence. Within 18 h after irradiation no effect on cytoskeletal components and vWF was documented. This in vitro study demonstrates that irradiation inhibits HCMSMC and HCPSMC at lower dose rates compared to HCAEC. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Re-188; Irradiation; Smooth muscle cell; Balloon catheter; Proliferation
www.elsevier.com/locate/atherosclerosis
1. Introduction
Restenosis is the major drawback of percutaneous transluminal coronary angioplasty (PTCA) and occurs within 6 months in 40 – 60% [1]. Various mechanisms for restenosis have been proposed: migration of my-ocytes from the media to the intima, forming the neointima [2]; damage of the adventitia resulting in proliferation of adventitial fibroblasts and migration of these mesenchymal cells to the media and neointima [3]; organization of a mural thrombus, resulting in a local hypertrophic scar [4]; release of cytokines and growth
factors, e.g. by monocytes/macrophages which mediate the cellular migration and proliferation [5,6]. It was demonstrated in vitro [7 – 9], in animal studies [10 – 12] and more recently in early clinical studies, that irradia-tion can prevent coronary artery restenosis at least in part by b- or g-emitting wires [13 – 16]. A new concept is the use of a liquid-filled balloon containing a beta-emitting radioisotope which combines the advantages of optimal energy transfer to the vessel wall and radia-tion protecradia-tion of the environment [17]. Rhenium-188 (Re-188) is a high-energy b-emitter that is routinely available from a W-188/Re-188 generator in liquid form and that is therefore an ideal b-emitter for this purpose.
The predominant cells of the coronary arteries are the endothelium (HCAEC) and smooth muscle cells in * Corresponding author. Tel.: +49-731-5024507; fax: +
49-731-5024512.
E-mail address: [email protected] (J. Kotzerke).
the media (HCMSMC). A different entity is the smooth muscle cell in plaque material (HCPSMC). Some inves-tigators who have compared the sensitivity of endothe-lial cells with other cell types constituting the vessel wall have concluded that the endothelial cells are the most radiosensitive [7,18]. However, other studies could not demonstrate major differences in survival following ir-radiation [19]. In addition, the experience is limited and restricted to a few tissues, and differences are known from animal and human cells as well as in different individuals [18]. Moreover, data about the radiosensi-tivity of smooth muscle cells isolated from human plaque tissue are very limited [20,21].
Different investigations have been published on the determination of endothelial cell survival in vitro in-cluding different end-points (e.g. fraction of growth control, endothelial colonies, apoptotic bodies or cy-toskeletal alterations). A prescreening system for poten-tial antiproliferative agents was established by Voisard et al. [22 – 24] which allows the estimation of the amount of proliferating cells and changes of cytoskele-tal cell structure within 48 – 120 h after exposition to various drugs.
Therefore, we investigated whether the proliferation of the predominant cells in coronary artery disease (HCAEC, HCMSMC, HCPSMC) can be reduced by irradiation from ab-emitter (i.e. by liquid Rhenium-188 in a balloon catheter). Furthermore, we wanted to clarify whether this effect is dose dependent and parable or different in the various cell types. To com-pare the antiproliferative effect with cell damage we investigated cytoskeletal components 18 h after irradia-tion by immunofluorescence microscopy.
2. Material and methods
2.1. Radionuclide and dosimetry
Carrier-free Re-188 was obtained from the W-188/
Re-188 generator by elution with saline (generator is available from the Oak Ridge National Laboratory, Oak Ridge, TN). The radiotracer concentration was increased using anion exchange columns [25]. The high-energy b particles (Ebmax=2.12 MeV, mean energy of 764 keV) allow therapeutic uses cintigraphy can be performed from the gamma emission of 155 keV (15% intensity). Radiation absorbed dose from a balloon catheter was measured by means of thermoluminescent dosimetry (TLD) and compared to calculations using the point kernel function of Re-188 [26]. Very good correlation was demonstrated for the absolute radiation absorbed dose and the fast drop of to 50% within 0.5 mm [26]. Assuming a specific volume of 3.7 GBq/ml at the surface of a typical balloon catheter (3.0×20, 135 ml volume) and at 0.5 mm distance (i.e. 2 mm distance
from the center of the balloon) a dose of 7.8 and 3.9 Gy/min could be achieved, respectively [26].
2.2. Cell preparation and cell culture
Smooth musle cells from human coronary plaque material (HCPSMC, plaque material of 52 patients), smooth muscle cells from the human coronary media (HCMSMC, Clonetics-Bio Whittaker, Verviers, Bel-gique) and human coronary endothelial cells (HCAEC, Clonetics-Bio Whittaker) were sucessfully isolated (HCPSMC), identified and cultured (HCPSMC, HCMSMC, HCAEC). HCMSMC and HCAEC of pas-sage three were used in the study presented. For isola-tion of HCPSMC an enzyme mixture of collagenase/elastase (Boehringer Mannheim, Germany) was used [27,28]. Due to the fact that only very few cells could be isolated from coronary plaque material, cells of all 52 patients had to be pooled for the investi-gations. HCPSMC and HCMSMC were identified as smooth muscle cells by positive reaction with antibodies against smooth muscle a-actin (Progen, Heidelberg, Germany) as described elsewhere [27,28]. HCPSMC and HCMSMC of passages two and three were used for the investigations. HCPSMC and HCMSMC were cul-tured in smooth muscle basal medium (SmBM, Clonet-ics-Bio Whittaker), supplemented with 5% fetal calf serum (FCS), insulin (5mg/ml), fibroblast growth factor (2 ng/ml), endothelial growth factor (5 ng/ml), gen-tamycin (50 mg/ml) and amphotericin (50 ng/ml).
HCAEC were cultured in endothelial basal medium (EBM, Clonetics-Bio Whittaker), supplemented with bovine brain extract (12 mg/ml), epidermal growth fac-tor (10 ng/ml), FCS (5%), gentamycin (50mg/ml), am-photericin (50 ng/ml), and hydrocortison (1 mg/ml). HCAEC were subcultured as described [29]; HCAEC of passages three to five were used for the investigations. Identity of HCAEC was confirmed by the typical ‘cob-blestone-growth pattern’ and by positive reaction with antibodies against the von Willebrand Faktor (Dako-patts, Hamburg, Germany). Cell doubling times of HCAEC, HCMSMC and HCPSMC were 3 and 5 days, respectively.
All incubations were performed at 37°C in a humi-dified atmosphere of air containing 5% CO2. Tissues were extracted with the patient’s informed consent and the study was approved by the ethical committee of the University of Ulm.
2.3. Application of irradiation
Before irradiation HCAEC, HCPSMC and HCMSMC were seeded in a density of 2 – 5×103
cells cm−2
placed into a distance holder parallel to and above the cell sheet with a reproducible distance of 0.22 mm (Fig. 1). The specific volume of Re-188 was 8909172 MBq/
ml causing a mean dose at balloon surface of 2.090.4 Gy/min which dropped to 1.590.3 Gy/min at the level of the cell sheet opposite the balloon. These radiation doses rule out the possibility that repair of sublethal damage could occur during exposure. Predicted dose to the cells increased from 7.5 to 37.5 Gy by prolonging irradiation time from 5.190.9 to 25.794.4 mins. Ev-ery irradiation time was compared with its own control to avoid errors by changes in pH due to CO2deficiency during irradiation. The filling condition of the balloon catheter was estimated at the end of each experiment by gamma camera imaging [26]. Each experiment was re-peated three times.
2.4. Cell proliferation
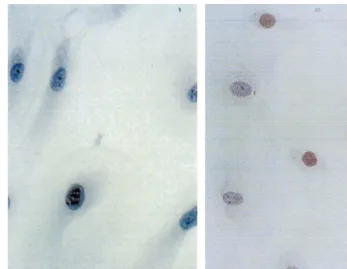
18 h before fixation bromodeoxyuridine and d-cy-tidine (BrdU and d-cyt, 20 mM, Serva, Heidelberg, Germany) was added, immediately after irradiation, to the cultures of HCPSMC, HCMSMC and HCAEC. After fixation with methanol (−20°C), the cells were stained with the avidin – biotin method. Anti-BrdU-an-tibodies were used as primary anAnti-BrdU-an-tibodies (Dakopatts, Hamburg, Germany), biotinylated horse anti mouse secondary antibodies were purchased from Camon (Wiesbaden, Germany). 3-Amino-ethyl-carbacote was used as substrate. The number of BrdU-positive cells (Fig. 2) was analysed in a strictly limited field directly
opposite to the Re-188 filled balloon catheter (500 cells for each single assay). This cell number was compared with the cell number of untreated controls (=100%).
2.5. Cytoskeletal components and 6on Willebrand
factor (6WF)
To characterize the effect of irradiation with rhe-nium-188 on cytoskeletal components of HCPSMC, HCMSMC and HCAEC (passage two and three) these cells were seeded at a density of 3 – 5×103
cells cm−2 in four-well dishes. One day after seeding the culture medium was exchanged and cells were treated with rhenium-188 (30 Gy at balloon surface, respectively, 22.5 Gy) as already described. After 18 h cells were fixed in methanol for 6 min at−20°C and indirect immunofluorescence was carried out as previously de-scribed [30,31]. The following primary antibodies were used in a concentration of 10 mg/ml: (1) monoclonal anti-smooth musclea-actin (Progen Biotechnik, Heidel-berg, Germany); (2) monoclonal anti-vimentin (Camon, Wiesbaden, Germany); (3) monoclonal anti-a-tubulin (Amersham Buchler, Braunschweig, Germany); (4) polyclonal von Willebrand factor (Dakopatts, Ham-burg, Germany). Tetramethylrhodaminisothiocyanate (TRITC)-labelled secondary antibodies (goat anti-mouse IgG) and fluorescein-isothiocyanate (FITC) goat anti-rabbit were purchased from Dianova (Hamburg, Germany).
All cells were mounted in Mowiol 4-88 [30,31] and examined with a Nikon Optiphot microscope (Nikon,
Fig. 2. BrdU-positive cell, indicating mitotic activity within the last 18 h (magnification: 650) in irradiated smooth muscle cells (22.5 Gy) (A); and untreated control (B). The different number of BrdU-positive cells is clearly demonstrated.
Du¨sseldorf, Germany) equipped with appropriate filter sets. The destruction of cytoskeletal components was quantified in percent in comparison with untreated controls. For each investigation 100 cells were exam-ined according to the following criteria: cytoskeletal structure ‘intact’ if the localisaton of smooth muscle-actin, vimentin, and a-tubulin was completely normal and ‘changed’, if the structures were little or severely destroyed.
2.6. Statistical e6aluation
Results are expressed as mean9standard deviation. Statistical significance of differences between controls and irradiated cells was determined by paired t-test. For comparisons between HCAEC and HCMSMC re-spectively HCAEC and HCPSMC the unpaired t-test was used. Differences were considered significant at a value of PB0.05.
3. Results
3.1. Cell proliferation after irradiation
Proliferation of HCAEC was not significantly inhib-ited 18 h after irradiation with 7.5 and 15 Gy. After irradiation with 22.5, 30, and 37.5 Gy proliferation of HCAEC was significantly inhibited in a dose dependent
manner (Table 1). HCPSMC reacted with a significant, dose dependent reduction of cell proliferation after irradiation with 7.5 – 37.5 Gy (Table 1). After irradia-tion with 7.5 Gy the inhibitory effect was more than 45% (PB0.05; paired t-test), the maximal inhibitory effect of 75% was found after irradiation with 37.5 Gy (P=0.004; paired t-test; Table 1). Proliferation of HCMSMC was significantly inhibited in a dose
depen-Table 1
Comparison of BrdU-positive HCAEC, HCPSMC, and HCMSMC 18 h after irradiation to untreated controls (mean9SD; paired t-test)a
HCAEC HCMSMC
Mean absorbed dose HCPSMC
82.9910.3
7.5 Gy 53.2913.6 58.897.4
n.s. PB0.05 PB0.01
n=3 n=3 n=4
71.9911.7 53.795.9
15 Gy 37.0912.8
PB0.001 P=0.01
n.s.
n=3 n=3 n=3
60.5911.7
22.5 Gy 32.6912.7 48.396.7
PB0.001 PB0.001
PB0.001
n=6 n=6 n=8
31.890.8 46.795.3
30 Gy 51.193.5
P=0.002 PB0.001 PB0.001
n=3 n=3 n=4
46.791.8 26.897.6 40.494.7 37.5 Gy
PB0.001 PB0.001 PB0.001
n=3 n=3 n=3
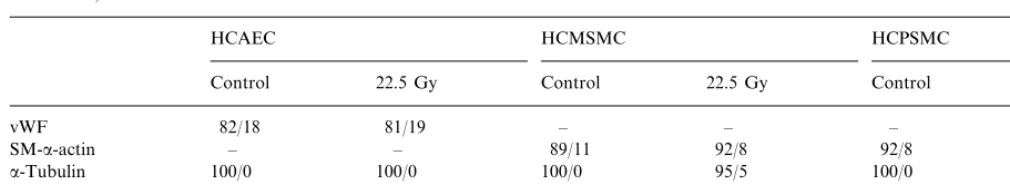
Table 2
Effect of irradation with rhenium-188 to the cytoskeletal structures of smooth muscle-a-actin, vimentin,a-tubulin, and the von vWF in HCPSMC, HCMSMC, and HCAECa
HCMSMC HCPSMC
HCAEC
22.5 Gy Control
Control 22.5 Gy Control 22.5 Gy
81/19 – – – –
aA total of 100 cells per structure were evaluated regarding damage (values given as unaffected/damaged).
dent manner after irradition with 7.5 – 37.5 Gy (Table 1, Fig. 2). After irradiation with 7.5 Gy an inhibitory effect of more than 40% was found (P=0.002; paired
t-test), the maximal inhibition of almost 60% was seen after irradiation with 37.5 Gy (PB0.001; pairedt-test; Table 1). HCAEC were significantly (PB0.05) less radiosensitive in comparison to HCPSMC and HCMSMC after irradiation with 7.5, 15, and 22.5 Gy. After irradiation with 30 and 37.5 Gy a significantly decreased effect (PB0.001 and PB0.01, respectively) of irradiation on HCAEC was seen in comparison to HCPSMC, differences in comparison to HCMSMC were not statistically significant.
3.2. Alterations after irradiation of cytoskeleton and the 6on Willebrand factor
The HCAEC were identified by detection of vWF, the HCPSMC and HCSMSC by binding of the anti-body against smooth muscle a-actin. Eighteen hours after Re-188-irradiation with 22.5 Gy the localisation of smooth musclea-actin, vimentin anda-tubulin did not show any alteration in comparison to non-irradiated cells (Fig. 3; Table 2).
4. Discussion
The earliest observations that blood vessels in irradi-ated tissues show specific changes were made 100 years ago [32]. The radiation-induced effects in the various tissues and the multitude of changes of the blood vessels in these tissues have been repeatedly reported and have been recently reviewed by Fajardo [33]. Dose dependent gain and loss of tissue irradiation are issues of constant interest, especially since Bo¨ttcher et al. [34] reported that irradiation with 12 Gy after angioplasty in peripheral arteries can reduce the risk of restenosis. The response of mammalian cells to ionizing radia-tion has been extensively studied since the development of techniques and growth media to sustain growth in vitro [19]. At high doses (e.g.\10 Gy) the dominant cellular response is cell death. The predominant
mecha-nism of radiation induced cell death results from chro-mosomal damage by misrepair of pairs of double stand breaks either by a single electron track or by two independent electron tracks. Following a dose of 8 Gy, the surviving cell fraction after 10 days is 1% which means that only one in a 100 cells remains viable, in the sense that it can proliferate indefinitely [19]. This can be demonstrated with a colony-forming assay [9]. How-ever, prerequisite for this assay is a homogeneous irra-diation of the dishes which can be easily obtained by X-ray but not by irradiation from a balloon catheter filled with a b-emitter. In this geometry, only a small field opposite to the catheter is irradiated with equal dose and would be increasingly falsified by migrating cells during prolonged incubation time. However, if radiation energy is delivered to a dividing cell, its effects are independent of the source used. That is, cell division should be equally inhibited by X-ray,gandbenergy as long as the energy is brought to the intended target. We wanted to investigate the cellular effects of irradiation by the same technique of a radioactive filled balloon catheter which is as well applied in vivo.
While in our experiments, 18 h after irradiation the proliferation rate decreased dose dependently, the struc-ture of smooth musclea-actin, vimentin, anda-tubulin remained unchanged with no hints of destruction. As mentioned above, early apoptotic death may occur 3 – 6 h following irradiation [35,36]. Cells undergoing apop-tosis may completely disappear within 4 h [38]. Eigh-teen hours after irradiation, a few early reacting cells might have already completed apoptosis. However, in our experiments this could not be proved definitely. Moreover, most of the cells die in late apoptosis caused by chromosomal aberrations after two or more divi-sions [37]. Cell doubling times of 3 and 5 days require investigations after 10 – 15 days following irradiation. This corresponds to observations made by Han et al. [39] and Bochaton-Piallat et al. [40] that the number of apoptotic smooth muscle cells becomes important be-tween 9 and 15 days after denudation of the rat aorta. It has been reported that the proliferative activity of HCMSMC is higher than that of HCPSMC with popu-lation doubling per day of 0.28 and 0.15, respectively [24]. The lower proliferative activity and the higher amount of extracellular matrix and debris in cultures of HCPSMC seemed to be responsible for the higher resistance of HCPSMC against diltiazem compared to HCMSMC [24]. However, in this study no difference in radiosensitivity could be stated in both strains of smooth muscle cells. In addition, smooth muscle cells were more radiosensitive than endothelial cells. These findings are supported by observations by Fischell et al. [8] who found that proliferating bovine endothelial cells are more radioresistant after low-dose irradiation from radioactive wires than HCSMC. Fischer-Dzoga et al. [7] observed some difference in radiosensitivity between aortic medial and intimal cells as well. Nevertheless, Brenner et al. [19] could not demonstrate any difference in radiosensitivity between endothelial and smooth muscle cells by survival data from a colony-forming assay. It is always difficult to compare in vitro findings with the in vivo situation. However, if HCSMCs are significantly more radiosensitive than endothelial cells, the radiation might inactivate all of the HCSMCs and allow the endothelial cells to re-populate and re-line the artery after the trauma of angioplasty.
The very steep energy loss of theb-energy of Re-188 of 50% within 0.5 mm stresses the importance of identi-cal irradiation conditions. Even a filling pressure of 3 atm instead of 6 atm would increase the distance from the balloon surface to the cell layer by 0.07 mm or decrease the dose by approximately 10%. However, g camera imaging revealed a balloon filling of 131914ml corresponding to a mean balloon diameter of 2.96 mm. Balloon volumes for irradiation of HCAEC were not different from irradiation of HCSMCs (13498 and 130917 ml, respectively).
5. Conclusion
We could demonstrate that irradiation of various cell strains in vitro (HCAEC, HCMSMC and HCPSMC) is feasable by a Re-188 filled balloon catheter. Eighteen hours after irradiation a significant antiproliferative effect was detectable in all three cell strains, dependent on the applied dose. Endothelial cells were less ra-diosensitive than both strains of smooth muscle cells. For the first time, it was demonstrated that smooth muscle cells isolated from plaque material are as ra-diosensitive as smooth muscle cells derived from the media. The cytoskeleton demonstrated no alterations at this early timepoint. Therefore, for distinguishing sub-lethal but proliferation reducing damage from apopto-sis inducing damage, longer kinetic studies are necessary.
Acknowledgements
The authors wish to acknowledge that plaque mate-rial from coronary arteries was supplied by O. Ickrath and A. Both, Katharinen Hospital, Stuttgart, Germany.
References
[1] Califf RM, Fortin DF, Frid DJ, et al. Restenosis after coronary angioplasty: an overview. J Am Coll Cardiol B 1991;17:2B – 13. [2] Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recur-rent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1985;6:369 – 75. [3] Wilcox JN, Waksman R, King SB, Scott NA. The role of the
adventitia in the arterial response to angioplasty: the effect of intravascular radiation. Int J Radiat Oncol Biol Phys 1996;36:789 – 96.
[4] Schwartz RS, Holmes Jr DR, Topol EJ. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms (edito-rial). J Am Coll Cardiol 1992;20:1284 – 93.
[5] Libby P, Schwartz D, Brogi E, Tanaka H, Clinton SK. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation 1992;86:47 – 52.
[6] Rubin P, Williams JP, Riggs PN, et al. Cellular and molecular mechanisms of radiation inhibition of restenosis. Part I: role of the macrophage and platelet-derived growth factor. Int J Radiat Oncol Biol Phys 1998;40:929 – 41.
[7] Fischer Dzoga K, Dimitrievich GS, Griem ML. Radiosensitivity of vascular tissue. II. Differential radiosensitivity of aortic cells in vitro. Radiat Res 1984;99:536 – 46.
[8] Fischell TA, Kharma BK, Fischell DR, et al. Low-dose,g -parti-cle emission from ‘stent’ wire results in complete, localized inhibition of smooth muscle cell proliferation. Circulation 1994;90:2956 – 63.
[9] Gajdusek CM, Tian H, London S, Zhou D, Rasey J, Mayberg MR. Gamma radiation effect on vascular smooth muscle cells in culture. Int J Radiat Oncol Biol Phys 1996;36:821 – 8.
[11] Waksman R, Robinson KA, Crocker IR, et al. Intracoronary radiation before stent implantation inhibits neointima formation in stented porcine coronary arteries. Circulation 1995;92:1383 – 6. [12] Wiedermann JG, Marboe C, Amols H, Schwartz A, Weinberger J. Intracoronary irradiation markedly reduces neointimal prolif-eration after balloon angioplasty in swine: persistent benefit. A 6-month follow-up. J Am Coll Cardiol 1995;25:1451 – 6. [13] Condado JA, Waksman R, Gurdiel O, et al. Long-term
angio-graphic and clinical outcome after percutaneous transluminal coronary angioplasty and intracoronary radiation therapy in humans. Circulation 1997;96:727 – 32.
[14] King SBr, Williams DO, Chougule P, et al. Endovascular beta-radiation to reduce restenosis after coronary balloon angio-plasty: results of the beta energy restenosis trial (BERT). Circulation 1998;97:2025 – 30.
[15] Teirstein PS, Massullo V, Jani S, et al. Catheter-based radiother-apy to inhibit restenosis after coronary stenting. New Engl J Med 1997;336:1697 – 703.
[16] Verin V, Urban P, Popowski Y, et al. Feasibility of intracoro-naryb-irradiation to reduce restenosis after balloon angioplasty. A clinical pilot study. Circulation 1997;95:1138 – 44.
[17] Amols HI, Reinstein LE, Weinberger J. Dosimetry of a radioac-tive coronary balloon dilatation catheter for treatment of neointimal hyperplasia. Med Phys 1996;23:1783 – 8.
[18] Reinhold HS, Hopewell JW, Calvo W, Keyeux A, Reyners H. Vasculoconnective tissue, In: Scherer E, Streffer, Trott, editors. Radiopathology of organs and tissues. Berlin: Springer, 1991. p. 243 – 68.
[19] Brenner DJ, Miller RC, Hall EJ. The radiobiology of intravascu-lar irradiation. Int J Radiat Oncol Biol Phys 1996;36:805 – 10. [20] Voisard R, Schneider E, Baur R, Hannekum A, Ho¨her M,
Ro¨ttinger EM. Die Auswirkung von Ionisierenden Strahlen auf die Proliferation und das Zytoskelett von Glatten Muskelzellen aus Koronarem Plaquegewebe des Menschen. Z Kardiol 1994;83:139.
[21] Voisard R, Schneider E, Baur R, et al. Niedrig Dosierte Ion-isierende Bestrahlung Inhibiert die Proliferation und das Zy-toskelett von Glatten Muskelzellen aus Koronarem Plaquegewebe des Menschen: Perspektive fu¨r eine Lokale App-likation zur Restenose-Prophylaxe Nach Koronarer Angioplas-tie? In: Heinle H, Schulte H, Breddin HK, editors. Endothelfunktion und Arteriosklerose. Stuttgart: Kohlhammer, 1994:298 – 303.
[22] Voisard R, Seitzer U, Baur R, et al. A prescreening system for potential antiproliferative agents: implications for local treat-ment strategies of postangioplasty restenosis. Int J Cardiol 1995;51:15 – 28.
[23] Voisard R, Dartsch PC, Seitzer U, et al. The in-vitro effect of antineoplastic agents on proliferative activity and cytoskeletal components of plaque-derived smooth-muscle cells from human coronary arteries. Coron Artery Dis 1993;4:935 – 42.
[24] Voisard R, Koschnick S, Baur R, et al. High-dose diltiazem prevents migration and proliferation of vascular smooth muscle cells in various in-vitro models of human coronary restenosis.
Coron Artery Dis 1997;8:189 – 201.
[25] Knapp Jr FF, Beets AL, Guhlke S, et al. Availability of rhe-nium-188 from the alumina-based tungsten-188/rhenium-188 generator for preparation of rhenium-188-labeled radiopharma-ceuticals for cancer treatment. Anticancer Res 1997;17:1783 – 95. [26] Kotzerke J, Rentschler M, Glatting G, et al. Dosimetric funda-mentals of endovascular brachytherapy using Re-188 to prevent restenosis after angioplasty. Nuklearmedizin 1998;37:68 – 72. [27] Dartsch PC, Voisard R, Bauriedel G, Hofling B, Betz E. Growth
characteristics and cytoskeletal organization of cultured smooth muscle cells from human primary stenosing and restenosing lesions. Arteriosclerosis 1990;10:62 – 75.
[28] Dartsch PC, Voisard R, Betz E. In vitro growth characteristics of human atherosclerotic plaque cells: comparison of cells from primary stenosing and restenosing lesions of peripheral and coronary arteries. Res Exp Med Berl 1990;190:77 – 87.
[29] Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identifica-tion by morphologic and immunologic criteria. J Clin Invest 1973;52:2745 – 56.
[30] Osborn M, Weber K. Immunofluorescence and immunocyto-chemical procedures with affinity purified antibodies: tubulin-containing structures. Methods Cell Biol 1982;24:97 – 132. [31] Osborn M, Caselitz J, Puschel K, Weber K. Intermediate
filament expression in human vascular smooth muscle and in arteriosclerotic plaques. Virchows Arch A, Pathol Anat Histo-pathol 1987;411:449 – 58.
[32] Gassmann A. Zur Histologie der Roentgenulcera. Fortschr Roentgenstr 1899;2:199 – 207.
[33] Fajardo LF. Pathology of Radiation Injury. New York: Masson, 1982.
[34] Bo¨ttcher HD, Schopohl B, Liermann D, Kollath J, Adamietz IA. Endovascular irradiation-a new method to avoid recurrent steno-sis after stent implantation in peripheral arteries: technique and preliminary results. Int J Radiat Oncol Biol Phys 1994;29:183 – 6. [35] Hendry JH, Potten CS. Intestinal cell radiosensitivity: a compari-son for cell death assayed by apoptosis or by a loss of clono-genicity. Int J Radiat Biol Relat Stud Phys Chem Med 1982;42:621 – 8.
[36] Potten CS, Merritt A, Hickman J, Hall P, Faranda A. Charac-terization of radiation-induced apoptosis in the small intestine and its biological implications. Int J Radiat Biol 1994;65:71 – 8. [37] Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys 1995;33:781 – 96.
[38] Bosman FT, Visser BC, van Oeveren J. Apoptosis: pathophysiol-ogy of programmed cell death. Pathol Res Pract 1996;192:676 – 83.
[39] Han DK, Haudenschild CC, Hong MK, Tinkle BT, Leon MB, Liau G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol 1995;147:267 – 77. [40] Bochaton Piallat ML, Gabbiani F, Redard M, Desmouliere A,
Gabbiani G. Apoptosis participates in cellularity regulation dur-ing rat aortic intimal thickendur-ing. Am J Pathol 1995;146:1059 – 64.