coating agents on microleakage of
conventional and resin modified glass
ionomer cements
S. Bayrak
1, E. Sen Tunc
*
1, N. Tuloglu
1and G. Ceylan
2The authors’ aim was to investigate the effectiveness of self-etch adhesives used as surface
coating agents on the microleakage of conventional and resin modified glass ionomer cements. A
total of 120 class V cavities were restored with either Fuji IX or Fuji II. Specimens were divided into
six groups for each material according to surface coatings: uncoated, G-Coat, Futurabond,
One-Coat, Clearfil S3 and Xeno III. The specimens were immersed in basic fuchsine, sectioned and
scored for microleakage. Results were analysed with Kruskal–Wallis and Mann–Whitney
U
tests.
For Fuji IX, no microleakage was observed at the occlusal margins with any of the coating
materials tested, and microleakage at the gingival margins was lowest with One-Coat. For Fuji II,
no microleakage was observed at either the occlusal or gingival margins for any of the materials
tested. Self-etching adhesives may be used as a surface coating to reduce marginal
microleakage of glass ionomer restorations.
Keywords:Glass ionomer cement, Resin modified glass ionomer cement, Self-etching adhesive, Surface coating
Introduction
The popularity of conventional glass ionomer cements (GICs) has been based on their capacity to release fluoride ions over a prolonged period of time1 and to
form an adhesive bond with enamel and dentin.2Despite these outstanding advantages, GIC suffers from several well known shortcomings, namely, its handling and manipulation difficulties, short working time, poor mechanical properties and sensitivity to moisture con-tamination.3–5 Resin modified GICs (RMGICs), first introduced at the beginning of the 1990s, possess superior physical properties when compared to conven-tional GICs and are relatively easier to use.4–6
The GIC setting mechanism has been described as an acid–base reaction between glass particles and an aqueous solution of polymeric acid in which the glass particles decompose and release Ca and Al ions that form a matrix of cross-linked polyanions.7 Resin modified GICs possess two setting mechanisms: a slow set acid–base chemical reaction that begins when the powder and liquid are mixed together and continues up to 24 h after mixing and a quick set that is activated by light curing.8,9
If GIC comes into contact with intraoral fluid before it has hardened, the matrix forming Ca and Al ions are washed out, resulting in an improperly formed matrix.10 Early exposure to water has been reported to correlate with poor clinical performance, inferior translucency, lower compressive strength and a reduced degree of hydration of the GIC matrix.3,11–13It has been suggested that the application of surface coating agents, such as waterproof varnish, petroleum jelly, cocoa butter and light polymerised adhesive systems, immediately follow-ing the initial set may prevent early water uptake in conventional GICs and RMGICs and maintain the water balance during maturation.14–18
Recently, self-etching adhesive systems have been developed to simplify bonding procedures, and while many studies have evaluated the effects of various surface coating agents on the microleakage of conventional GICs and RMGICs,14–18the effectiveness of self-etch adhesives
used as surface coating agents has yet to be examined. The present study investigated two hypotheses:
(i) the use of self-etch adhesives as surface coating agents decreases the microleakage of GIC and RMGIC
(ii) microleakage values vary among surface coating and restoration materials.
Experimental
Tooth selection
Sixty non-carious extracted human third molars were cleaned with pumice and a scalpel and examined to 1Department of Pediatric Dentistry, Faculty of Dentistry, University of
Ondokuz Mayıs, Samsun, Turkey
2Department of Prosthodontics, Faculty of Dentistry, University of
Ondokuz Mayıs, Samsun, Turkey
ensure that they were free form cracks and fractures, especially in the sites to be restored. Teeth were stored in 0?1% thymol saline solution for 1 week following extraction.
Cavity preparation
Class V cavities were prepared on the buccal and lingual surfaces of each tooth (4 mm mesiodistally, 2 mm deep and 3 mm occlusogingivally, with the occlusal margins in enamel and the gingival margins located 1?5 mm apical to the cemento–enamel junction) using a no. 014 diamond fissure bur (Al Amazonas, 580 Barueni-SP, Industria Brasileria) in a water cooled, high speed handpiece. The bur was changed after every five preparations. No intentional bevels or undercuts for retention were made. A total of 120 cavity preparations were made, all by the same operator.
Treatments
Two different restorative materials (Fuji IX, a GIC, and Fuji II LC, an RMGIC), one new surface coating agent [G-Coat plus (GC)] and four self-etching adhesives [Futurabond NR (FNR), One-Coat self-etching bond (OC), Clearfil S3 bond (CS3) and Xeno III (XIII)] were used in the present study (Table 1).
Cavity conditioner was applied to all cavity prepara-tions, and the restorative materials were mixed accord-ing to the manufacturer’s instructions (Table 1). Sixty specimens were restored with Fuji IX, and 60 were restored with Fuji II LC. Specimens were then divided into 12 groups of equal size (n510), and surface coating agents were applied with a brush on all surfaces of the specimens, as follows:
Group 1: (Fuji IX) uncoated (control)
Table 1 Materials used in this study*
Material Composition Manufacturers’ instructions Manufacturer
Fuji IX Fluoroaluminium silicate
glass, polyacrylic acid, polybasic
Mix powder with liquid for 15–20 s GC Corp.,
Tokyo, Japan Apply in the cavity; wait until set
Fuji II LC Fluoroaluminium silicate
glass, polyacrylic acid, HEMA, water
Apply 10% of polyacrylic acid solution for 20 s using a light scrubbing motion
GC Corp., Tokyo, Japan
Rinse thoroughly and gently dry with absorbent paper
Hand mix
Insert into cavity using a syringe injector in a single increment
Light cure{for 20 s
Apply finishing gloss and light cure for 20 s GC cavity
conditioner
Water, polyacrylic acid, aluminum chloride
Apply GC cavity conditioner for 10 s using a cotton pellet
GC Corp., Tokyo, Japan Rinse thoroughly with water for
20 s and dry with absorbent paper G-Coat plus
Apply on the surface and margins of the restoration using a microtip applicator
GC Corp., Tokyo, Japan
Dry with a gentle air stream
Light cure{for 20 s
Futurabond NR (FNR)
Bottle A and B: BIS-GMA, HEMA, phosphate methacrylates,
BHT, ethanol,£uorides, CQ,
silicium dioxide nanoparticles
Mix liquids A and B in equal amounts for 5 s Voco, Cuxhaven,
Germany Apply a layer of adhesive, massage
over the restoration surface for 20 s Dry with a gentle air stream for at least 5 s
Light cure{for 10 s
One-Coat SE bond (OC)
Primer: water, HEMA, acrylamidosulphonic acid, glycerol mono- and dimethacrylate, methacrylised polyalkenoate bond: HEMA, glycerol mono- and dimethacrylate, UDMA,
methacrylised polyalkenoate, CQ
Apply primer to restoration surface for 20 s Coltene/Accord,
Alsta¨tten, Switzerland Dry with a gentle air stream for 2 s
With a new microbrush, apply to restoration surface for 20 s Dry with a gentle air stream for 2 s
Light cure{for 10 s
Apply adhesive under pressure for 20 s Kuraray Corp.,
Osaka, Japan Dry with a gentle air stream for 5 s
Light cure{for 10 s
Xeno III (XIII)
Bottle A: HEMA, ethanol, water, BHT, nanofiller Bottle B: Pyro-EMA, PEM-F, UDMA, CQ, BHT,
ethyl-4-dimethylaminobenzoate (co-initiator)
Mix liquids A and B in equal amounts for 5 s
Dentsply de Trey, Konstanz, Germany Apply one thick coat of the adhesive
under pressure for 20 s
Dry with a gentle air stream for 2 s
Light cure{for 10 s
*Bis-GMA: bis-phenol A diglycidylmethacrylate; BHT: butylated hydroxy toluene; CQ: camphorquinone or camphoroquinone or 1?7?
7-trimethylbicyclo-[2,2,1]-hepta-2,3-dione (photo-initiator); HEMA: 2-hydroyethylmethacrylate; HPMA: hydroxypropylmethacrylate; MDP: methacryloyloxydecyl dihydrogen phosphate; PEM-F: pentamethacryloyloxyethylcyclohexaphosphazene mono£uoride; Pyro-EMA: tetramethacryloyloxyethyl pyrophosphate; UDMA: urethane dimethacrylate.
Group 2: (Fuji II LC) uncoated (control) Group 3: (Fuji IX) GC
Group 4: (Fuji II LC) GC Group 5: (Fuji IX) FNR Group 6: (Fuji II LC) FNR Group 7: (Fuji IX) OC Group 8: (Fuji II LC) OC Group 9: (Fuji IX) CS3 Group 10: (Fuji II LC) CS3 Group 11: (Fuji IX) XIII Group 12: (Fuji II LC) XIII.
Restorations were finished with a diamond bur (Accurata, GzK Mahnhardt Dental, Germany), polished (Sof Lex Pop On discs, 3M ESPE, USA) and recoated.
Microleakage assessment
After finishing and polishing, specimens were stored for 24 h in deionised water at 37¡1uC and then thermo-cycled between 5 and 55uC for 1500 cycles using a dwell time of 10 s and a transfer time of 30 s between each bath. Following thermocycling, specimen apices were sealed with sticky wax, and all surfaces were coated with two coats of nail varnish to withiny1 mm of the tooth restoration margin. Teeth were soaked in 0?5% basic fuchsine dye for 24 h, rinsed and dried. Using a slow speed saw, the roots were cut from the crown of each specimen. Specimens were then sectioned mesiodistally and buccolingually. Each section was examined under a stereomicroscope (Nikon SMZ-1500, Osaka, Japan) at
630 magnification and scored for dye penetration by a single examiner blinded to the treatment procedure. Specimens were randomly examined and scored as follows:
05no dye penetration
15dye penetration up to one-third of the cavity depth
25dye penetration up to two-thirds of the cavity depth
35dye penetration onto the axial wall of the cavity.
Statistical analysis
Statistical analysis was performed using the Minitab software program (Minitab V. 13?20, 2000, Minitab Statistical Software, Release 13?20, Minitab Inc., State College, PA, USA). The Wilcoxon signed rank test was used to compare microleakage at the occlusal and gingival margins for each group. The Kruskal–Wallis test (H test), corrected for ties, was used to compare microleakage values of the Fuji IX and Fuji II specimens. The Mann–Whitney U test was used to compare microleakage values between Fuji IX and Fuji II LC specimens for each subgroup.
Results
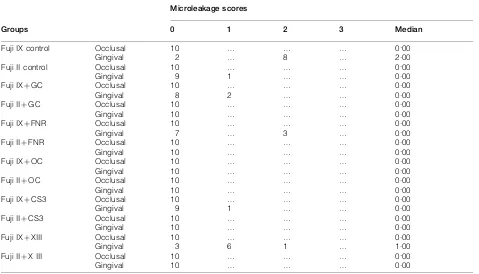
Table 2 shows the microleakage scores for the occlusal and gingival margins by group. No microleakage was observed at the occlusal margins in any of the groups, whereas microleakage scores of 1 and 2 were observed at the gingival margins. Except for the Fuji IX control and XIII groups, no significant differences in microleakage was observed between the occlusal and gingival margins for any of the groups (p.0?05).
When microleakage values of the Fuji IX specimens were examined according to surface coating, no sig-nificant differences were found in microleakage at the occlusal margins among the groups. At the gingival margins, microleakage was lowest in the OC group, followed by the CS3, GC, FNR, XIII and control groups. Microleakage scores of the control group were significantly higher than those of all other groups except for the XIII group (p,0?05). Microleakage scores of the XIII group were also statistical higher than those of the OC, CS3 and FNR groups (p,0?05).
When microleakage values of the Fuji II LC speci-mens were examined according to surface coating, no
Table 2 Microleakage scores at occlusal and gingival margins
Groups
Microleakage scores
0 1 2 3 Median
Fuji IX control Occlusal 10 … … … 0.00
Gingival 2 … 8 … 2.00
Fuji II control Occlusal 10 … … … 0.00
Gingival 9 1 … … 0.00
Fuji IXzGC Occlusal 10 … … … 0.00
Gingival 8 2 … … 0.00
Fuji IIzGC Occlusal 10 … … … 0.00
Gingival 10 … … … 0.00
Fuji IXzFNR Occlusal 10 … … … 0.00
Gingival 7 … 3 … 0.00
Fuji IIzFNR Occlusal 10 … … … 0.00
Gingival 10 … … … 0.00
Fuji IXzOC Occlusal 10 … … … 0.00
Gingival 10 … … … 0.00
Fuji IIzOC Occlusal 10 … … … 0.00
Gingival 10 … … … 0.00
Fuji IXzCS3 Occlusal 10 … … … 0.00
Gingival 9 1 … … 0.00
Fuji IIzCS3 Occlusal 10 … … … 0.00
Gingival 10 … … … 0.00
Fuji IXzXIII Occlusal 10 … … … 0.00
Gingival 3 6 1 … 1.00
Fuji IIzX III Occlusal 10 … … … 0.00
significant differences were found at either the occlusal or gingival margins among any of the groups tested (p.0?05).
When microleakage values of the GIC and RMGIC restorations were compared, no significant differences were found in the microleakage at the occlusal margins of the Fuji IX and Fuji II LC specimens for any of the surface coatings tested (p.0?05). Microleakage at the gingival margins was significantly higher in the Fuji IX control group compared to that in the Fuji II LC control group (p,0?001) and in the Fuji IX XIII group compared to that in the Fuji II LC XIII group (p,0?01) (Table 3).
Discussion
The marginal sealing capacities of restorative materials play an essential role in the longevity of a restoration. Microleakage, i.e. the passage of bacteria, fluids, chemical substances, molecules and ions between the tooth and restoration, is an intrinsic problem with traditional restorative materials.19 The presence of microleakage may lead to postoperative problems, such as bacterial accumulation, fluid flow in the gap and detachment of the restoration.19 Therefore, dentistry continues to seek improved sealing capacity at the restoration margins. The use of surface coating agents has been suggested to improve the marginal integrity of glass ionomer based materials. Various studies have evaluated the effects of different surface coating agents in reducing the marginal microleakage of GICs and RMGICs.14–18However, no studies have evaluated the use of self-etch adhesives as surface coating agents. The purpose of the present study was to investigate the effects of self-etch adhesive systems used as surface coating agents on the microleakage of GIC and RMGIC restorations.
The present study evaluated the sealing performance of surface coatings in terms of their ability to prevent penetration of basic fuchsine dye. While no microleak-age was noted in the occlusal margins of either Fuji IX or Fuji II LC restorations, some microleakage was observed at the gingival margins. These findings are in general agreement with the literature.20–22
In the present study, the dye uptake of uncoated (control) Fuji IX specimens immersed in basic fuchsine was significantly higher than surface coated Fuji IX specimens (p,0?05). This suggests that uncoated mate-rials can take up oral fluid and that the application of a surface coating is able to provide sufficient early protection to prevent water gain or loss from the restorative material by preserving the water balance within the system.23–26Cocoa butter, waterproof varnish and even nail varnish have been recommended as surface coating agents.12,14,26
Light polymerised adhesive systems have also been reported to be effective agents in limiting water move-ment across the surface of conventional GIC,16,24 and light polymerised, low viscosity resins have been found to prevent water penetration better than conventional varnishes.14,24The present study also found GC, a new nanofilled surface coating agent, to exhibit results similar to that of previously tested self-etch adhesives. Interestingly, microleakage of Fuji IX restorations coated with OC, CS3 and GC was significantly lower
when compared to FNR and XIII coated specimens. Table
It may be possible to ascribe the disparity in the performance of self-etch adhesives containing the same solvents to differences in pH values and filler ratios. In line with an earlier study,27the present study found self-etch adhesives with low pH values [XIII (pH,1) and FNR (pH51?4)] to have higher microleakage values than adhesives with higher pHs [OC (primer pH52?0, bond pH54?0) and CS3 (pH52?7)]. Although CS3 (5– 10%) and FNR (5%) have higher amounts of filler than OC (2?5%) and XIII (2?4%), the present study found no correlation between filler ratios and microleakage values.
Resin modified GICs are generally believed to be less affected by moisture than conventional GICs,28 and RMGIC manufacturers claim that they can be used with or without surface protection. Although earlier studies demonstrated that RMGICs require surface protection,18,29,30the present study found no significant differences between the uncoated and coated Fuji II LC specimens. Furthermore, no significant differences were observed among the sealing abilities of the different agents tested.
Although no significant differences were found in the microleakage of the Fuji IX and Fuji II LC specimens at the occlusal margins (p.0?05), microleakage values at the gingival margins were significantly different, with microleakage significantly higher for the Fuji IX control and XIII groups compared to the Fuji II LC control and XIII groups (p,0?001 andp,0?01).
Choet al.28found that RMGICs were less adversely affected by water than GICs. This is consistent with a setting mechanism dominated by resinous polymerisation compared to one dominated by acid–base reactions.31,32
The present study has the same limitations associated with allin vitroresearches. Clinical studies are needed to examine thein vivo performance of self-etch adhesives when used as surface coating agents over conventional GIC and RMGIC restorations.
Conclusions
Within the context of thisin vitro study, the following conclusions can be made.
1. Microleakage at the occlusal margins of teeth restored with GIC and RMGIC is lower than that at the gingival margins.
2. Self-etch adhesives can be used as a surface coating agent to improve marginal sealing of GIC restorations. 3. Resin modified GIC restorations do not require the application of a surface coating.
References
1. M. L. Swartz, R. W. Phillips and H. E. Clark:J. Dent. Res., 1984,
63, 158–160.
2. D. R. Powis, T. Follera˚s, S. A. Merson and A. D. Wilson:J. Dent. Res., 1982,61, 1416–1422.
3. S. Phillips and B. M. Bishop:Quintessence Int., 1985,16, 175–177. 4. S. K. Sidhu and T. F. Watson:Am. J. Dent., 1995,8, 59–67. 5. J. W. Nicholson:Biomaterials, 1998,19, 485–494.
6. V. J. Setien, S. R. Armstrong and J. S. Wefel:Dent. Mater., 2005,
21, 498–504.
7. P. Crisp, M. A. Pringuer, D. Wardleworth and A. D. Wilson:
J. Dent. Res., 1974,53, 1414–1419.
8. A. Lin, N. S. Mcintyre and R. D. Davidson:J. Dent. Res., 1992,71, 1836–1841.
9. A. M. Bourke, A. W. Walls and J. F. McCabe:J. Dent. Res., 1992,
20, 115–120.
10. H. M. Anstice and J. W. Nicholson:J. Mater. Sci., Mater. Med., 1992,3, 447–451.
11. A. D. Wilson and J. W. McLean: ‘Glass ionomer cement’, 1st edn, 136–137; 1988, London, Quintessence Publishing.
12. E. Asmussen:Acta Odontol. Scand., 1983,41, 155–157. 13. B. E. Causton:Biomaterials, 1981,2, 112–115.
14. M. S. Earl, W. R. Hume and G. J. Mount:Aust. Dent. J., 1985,30, 298–301.
15. R. C. Garcia, M. F. de Goes and A. A. Del Bel Cury:Am. J. Dent., 1995,8, 294–296.
16. M. Hotta, H. Hirukawa and K. Yamamoto:Oper. Dent., 1992,17, 57–61.
17. A. P. Ribeiro, M. C. Serra, L. A. Paulillo and A. L. Rodrigues Ju´nior:Quintessence Int., 1999,30, 427–431.
18. M. Miyazaki, B. K. Moore and H. Onose:Eur. J. Oral Sci., 1996,
104, 600–604.
19. E. A. Kidd:J. Dent., 1976,4, 199–206.
20. D. A. Gerdolle, E. Mortier and D. Droz:J. Dent. Child., 2008,75, 125–133.
21. K. I. Delme´, P. J. Deman, M. A. de Bruyne and R. J. de Moor:
Photomed. Laser Surg.,26, 541–549
22. K. B. Hallett and F. Garcia-Godoy:Dent. Mater., 1993,9, 306– 311.
23. J. W. McLean:Oper. Dent., 1992, (Suppl 5), 184–190.
24. M. S. Earl, G. J. Mount and W. R. Hume:Aust. Dent. J., 1989,34, 326–329.
25. T. Watson and A. Banerjee: Eur. J. Prosthodont. Restor. Dent., 1993,2, 85–90.
26. R. C. Rodrigues Garcia, M. F. de Goes and A. A. Del Bel Cury:
Am. J. Dent., 1995,8, 294–296.
27. S. Inoue, M. A. Vargas, Y. Abe, Y. Yoshida, P. Lambrechts, G. Vanherle, H. Sano and B. van Meerbeek:J. Adhes. Dent., 2001,
3, 237–245.
28. E. Cho, H. Kopel and S. N. White:Quintessence Int., 1995,26, 351–358.
29. D. F. Cefaly, B. G. Seabra, C. M. Tapety, E. M. Taga, F. Valera and M. F. Navarro:Oper. Dent., 2001,26, 401–405.
30. S. Karaog˘lanog˘lu, N. Akgu¨l, H. N. Ozdabak and H. M. Akgu¨l:
Dent. Mater. J., 2009,28, 96–101.
31. A. D. Wilson and B. E. Kent:Br. Dent. J., 1972,132, 133–135. 32. A. D. Wilson, J. M. Paddon and S. Crisp:J. Dent. Res., 1979,58,