Original article
Soil enzymatic activity as affected by long term application
of farm yard manure and mineral fertilizer under
a rainfed soybean–wheat system in N-W Himalaya
Supradip Saha
*
, Ved Prakash, Samaresh Kundu, Narendra Kumar, Banshi Lal Mina
Vivekananda Institute of Hill Agriculture, Indian Council of Agricultural Research, Almora – 263 601, Uttarakhand, Indiaa r t i c l e
i n f o
Article history: Received 8 June 2007 Accepted 29 February 2008 Published online 26 March 2008
Keywords:
Long-term experiment Soil carbohydrate Soil enzymes Nutrient dynamics
a b s t r a c t
Long-term experimental sites are expected to provide important information regarding soil properties as affected by management practices. This study was designed to examine the effects of continuous fertilization, and manuring on the activities of enzymes involved in mineralization of C, N, and P on a long term (33 years) field trial under sub-temperate conditions in India. Treatments at the site included application of recommended doses of nitrogen and phosphorus (NP), nitrogen and potassium (NK), nitrogen, phosphorus and potassium (NPK), farmyard manure (FYM) with N (NþFYM), FYM with NPK (NPKþFYM) and un-amended control (C). The study was done under rainfed soybean– wheat rotation. Manure application increased soil carbohydrate, dehydrogenase, acid and alkaline phosphatases, cellulase, and protease activity significantly. Urease activity was not influenced by the manure treatment and the activity was highest in controls. Both acid and alkaline phosphatase activities were negatively influenced by chemical fertilizer treatment. Almost all the enzymes studied were significantly correlated with soil C content. The results suggest that application of FYM directly or indirectly influences the enzyme activity and it in turn regulates nutrient transformation.
ª2008 Elsevier Masson SAS. All rights reserved.
1.
Introduction
In the effort to achieve sustainable agricultural production while maintaining and preserving the environment, it is crucial that soil biological health be improved or maintained. Agricultural practices that improve soil quality and agricul-tural sustainability have been receiving more attention from researchers and farmers[14]. Inorganic fertilizer, especially N, P and K, not only serve to maintain, but their application directly or indirectly causes changes in chemical, physical
and biological properties of the soil. These changes, in the long term, are believed to have significant influences on the quality and productive capacity of soils.
Soil microorganisms, particularly microbiota, play an essential role in the cycling of elements and stabilization of soil structure[5,32]. They also act as both a source and sink of labile nutrients and C[20,47]. The mineralization of organic matter is carried out by a large community of microorganisms and involves a wide range of metabolic processes. Soil enzymes are believed to be able to discriminate between soil
*Corresponding author. Tel.:þ91 5962 230060. E-mail address:s_supradip@yahoo.com(S. Saha).
a v a i l a b l e a t w w w . s c i e n c e d i r e c t . c o m
j o u r n a l h o m e p a g e : h t t p : / / w w w . e l s e v i e r . c o m / l o c a t e / e j s o b i
management practices probably because they are related to microbial biomass, which is sensitive to such treatments
[32]. Soil enzymes regulate the transformation process of elements required for plant growth in soil[6]. The activity of soil enzyme, whether extracellular or intracellular depends on crop rotation, amendments, tillage and agricultural management[14,24]. Transformation of N in soils includes different processes, which are regulated by a number of extra-cellular degradative enzymes [26,50]. Transformation of organic P through enzymatic reactions and the immobiliza-tion of P in the biomass itself play a fundamental role in P cycling[35,36,46]and are likely to be affected by P amend-ments[10,27].
Although some researchers commented that soil organic matter was not a plant growth requirement in-and-of itself
[38], almost all have realized its ecological significance in overcoming constraints to crop growth, particularly, in the areas of nutrient supply, nutrient and moisture relation, soil structural stability and detoxification [42]. Information is also needed to assess the contribution of the organic matter in maintaining the soil microbial community and which in turn, is related to specific enzymatic reactions, i.e. to biochem-ical processes involved in different nutrient cycling for assess-ing sustainability.
Long-term field treatments provide a means of investigat-ing the influences of organic amendments on soil characteris-tics[8]. However, relatively limited consistent information is available concerning changes in soil biological properties, un-der long-term field conditions of a N-W Himalayan ecosystem. The objective of this study was to ascertain changes in soil enzymatic activity in response to repeated applications of organic amendmentvis-a`-visinorganic nutrients under field conditions over several years. For this reason, soil enzymatic properties, especially those related to the cycles of C, N, and P in the soil, was compared in soils from a long-term field experiment.
2.
Materials and methods
2.1. Experimental site
A long-term field experiment was initiated in June 1973 on a Typic Haplaquept at the experimental farm of Vivekananda Institute of Hill Agriculture, located in the Indian Himalayan region at Hawalbagh (29360N and 79400E at 1250 m above
mean sea level), in the state of Uttarakhand, India. Soil charac-teristics based on analysis of preserved soil samples taken in 1973 are given in Table 1. The climate is sub-temperate, characterized by a moderate summer (May–Jun), extreme winter (Dec–Jan) and general dryness, except during the southwest monsoon season (Jun–Sep). Based on the records for the period 1973–2006, average kharif (monsoon) season rainfall was 684 mm (e.g. 68% of the average annual rainfall). During soybean growth, average monthly rainfall (mm) was: Jun (146), Jul (257), Aug (187), Sep (119), whereas, during wheat growth the average monthly rainfall (mm) was: Oct (27), Nov (6), Dec (28), Jan (53), Feb (56), Mar (48), Apr (42). The mean monthly temperature ranged from a minimum of 0.4C in
January to a maximum of 31.5C in May.
2.2. Experimental design
The experiment included two crops per year, soybean (Jun– Sep) and wheat (Oct–Apr), with six treatment combinations (in kg ha1): no fertilizer and no manure (un-amended control); 20 Nþ35 P (NP); 20 Nþ33 K (NK); 20 Nþ35 Pþ33 K (NPK), 20 NþFYM at 10 Mg ha1 (NþFYM, commonly used by the local farmers) and NPKþFYM at 10 Mg ha1 (NPKþFYM). The required doses of FYM and fertilizers were given only to the soybean crop every year and the wheat crop was allowed to grow on residual fertility. Treatments were distributed in a randomized block design with six repli-cations over three uniformly level terraces. The net plot size was 5.42.0 m. Fertilizers used were urea for N, single super-phosphate for P and murate of potash for K. Based on the chemical analysis of every fifth year, FYM (C:Nz23.6:1) had 370 g of moisture kg1and contained 7.1–7.5 g N kg1, 2.1–2.4 g P kg1and 5.3–5.8 g K kg1on an oven-dry weight basis. FYM was prepared using cattle dung mixed with urine and bedding material. Oak (Quercus leucotrichophora) leaves (1.1% N, 0.55% P, 0.74% K) were mostly used as bedding mate-rial in cattle sheds. These matemate-rials were thoroughly mixed and stored in a heap under shade. The heap was plastered with a 2-inch thick layer of soil and dung and was left undis-turbed for 45 days. Full doses of N, P, K and FYM were incorpo-rated into the upper 5 cm of soil during final land preparation.
2.3. Soil sampling for chemical and microbial analysis and preparation
Initial soil samples were collected in 1973 prior to the start of the experiment. After harvest of wheat, soil samples were taken from the surface layer (0–15 cm) of six treatments with six replications in May, 2006, before the start of land management for soybean. Random cores were taken from each plot with a 5-cm diameter tube auger and bulked. The moist soil samples were sieved (2 mm) after removing plant material and roots. Half of the soil samples were air-dried and stored at room temperature until chemical analysis. All chemical results are means of triplicate analyses and are expressed on an oven-dry basis. Soil moisture was determined after drying at 105C for 24 h.
The rest of the sieved soil (2 mm) was immediately trans-ferred to the laboratory for microbiological analysis. Soil
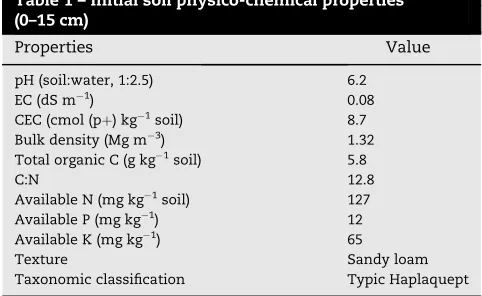
Table 1 – Initial soil physico-chemical properties (0–15 cm)
Properties Value
pH (soil:water, 1:2.5) 6.2
EC (dS m1) 0.08
CEC (cmol (pþ) kg1soil) 8.7
Bulk density (Mg m3) 1.32
Total organic C (g kg1soil) 5.8
C:N 12.8
Available N (mg kg1soil) 127
Available P (mg kg1) 12
Available K (mg kg1) 65
Texture Sandy loam
samples were kept at 4C in plastic bags for a few days to
stabilize the microbiological activity disturbed during soil sampling and handling, and then analysed within 2 weeks. All microbial results reported are means of six replicates and are expressed on a moisture-free basis. Moisture content was determined after drying at 105C for 24 h.
2.4. Chemical analyses
Soil was analyzed for pH in a 1:2.5 soil:water suspension[19], soil texture was determined by Bouyoucos hydrometer[4]and available K by 1 N NH4OAc using a flame photometer[19]. Soil was analysed for oxidizable SOC by the method of Walkley and Black[49], Kjeldahl N by FOSS Tecator (Model 2200), and available P following the Olsen method[33]. A core sampler was used for soil bulk density determination. Soil was ana-lysed for total C and N using a CHN analyser (FOSS Heraeus CHNORapid) following a dry combustion method.
Soil carbohydrate was estimated following the modified method of Martens and Loeffelmann [29]. The soil sample (100 mg) was first solubilized and then hydrolysed with 1 M H2SO4to get monosaccharides, which were estimated colori-metrically using the phenol–sulfuric acid method[11].
2.5. Microbiological analyses
Soil dehydrogenase activity was estimated by reducing 2,3,5-triphenyltetrazolium chloride [7]. Five grams of soil sample were mixed with 50 mg of CaCO3and 1 ml of 3% (w/ v) 2,3,5-triphenyltetrazolium chloride (TTC) and incubated for 24 h at 371C. Dehydrogenase enzyme converts TTC to
2,3,5-triphenylformazan (TPF). The TPF formed was extracted with acetone (315 ml), the extracts were filtered through Whatman No. 5 and absorption was measured at 485 nm with a spectrophotometer (Analytik Jena, Germany).
Cellulase activity was determined by estimating the glucose equivalent after mixing the soil sample (10 g) with 15 ml of acetate buffer (2 M, pH 5.5) and 15 ml of 0.7% (w/v) carboxy methyl cellulose and incubated for 24 h at 501C
[39]. The glucose equivalent was estimated following the DNS method[30].
Protease activity was assayed by determining the tyrosine released when 1 g of the oven-dry equivalent of field-moist soil sample was incubated with 5 ml of 50 mM Tris buffer (pH 8.1) and 5 ml of 2% Na-caseinate at 501C for 2 h[25].
The aromatic amino acids released were extracted and the remaining substrate was precipitated with 0.92 M trichloro-acetic acid and measured colorimetrically using Folin–Ciocal-teu reagent at 700 nm. The activity of protease was expressed asmg tyrosine produced g1soil 2 h1.
Urease activity was measured following the method of Tabatabai and Bremner[44]. Five grams of soil were incubated with 5 ml of 0.05 M THAM buffer (pH 9.0) and 1 ml of 0.2% of urea solution at 37C for 2 h. Excess urea was extracted with
KCl–PMA solution and estimated colorimetrically at 527 nm. Phosphomonoesterase (acid and alkaline phosphatase) ac-tivity was assayed using 1 g of soil (wet equivalent), 4 ml of 0.1 M modified universal buffer (pH 11 for alkaline phosphatase and pH 6.5 for acid phosphatase), and 1 ml of 25 mM p-nitro-phenyl phosphate [43]. After incubation for 1 h at 371C
the enzyme reaction was stopped by adding 4 ml of 0.5 M NaOH and 1 ml of 0.5 M CaCl2to prevent dispersion of humic substances. After centrifugation at 4000 rpm for 10 min, the ab-sorbance was measured in the supernatant at 400 nm; enzyme activity was expressed asmgp-nitrophenol released g1soil h1.
2.6. Grain yield
Both soybean and wheat were harvested at 5 cm above the soil surface in the first week of October and the fourth week of April, respectively. Grain yield was expressed on a 12 and 9% moisture basis for wheat and soybean, respectively.
2.7. Statistical analyses
Each sample was analyzed in triplicate and the values were then averaged. Data were assessed by Duncan’s multiple range tests (1955) with a probabilityP0.05[12]. Differences between mean values were evaluated by a one-way analysis of variance (ANOVA) (SPSS version 10.0). Pearson correlation analyses were performed using the SPSS programme.
3.
Results
3.1. Soil pH and oxidizable carbon
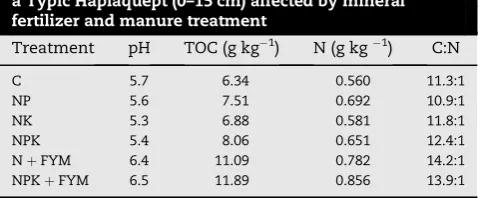
Table 1gives the initial physico-chemical properties of the soil used for the long term experiment. Taxonomically the soil was classified as Typic Haplaquept. The pH values in 0–15 cm of surface soil ranged from 5.3 to 6.5, with that of the untreated control soil around pH 5.7. Manure application increased soil pH significantly, while chemical fertilizer appli-cation resulted in lowering of soil pH (Table 2). NK treatment lowered the soil pH value up to 5.3.
In the surface 0–15 cm, soil oxidizable carbon was less in the different fertilized treatments than in FYM treated plots (Table 2). In different fertilized treatments, oxidizable-C fol-lowed the order NK<NP<NPK. Application of FYM along with NPK contributed significantly to incorporation of C in soil. Addition of FYM at 10 Mg ha1year1 along with NPK for 33 years doubled the organic C content than NPK alone.
3.2. Soil carbohydrate and dehydrogenase activity
Soil total polysaccharide content for six different treatments are tabulated inFig. 1. Carbohydrate content in control and
Table 2 – Soil pH, total SOC and nitrogen, C-to-N ratio of a Typic Haplaquept (0–15 cm) affected by mineral fertilizer and manure treatment
Treatment pH TOC (g kg1) N (g kg1) C:N
C 5.7 6.34 0.560 11.3:1
NP 5.6 7.51 0.692 10.9:1
NK 5.3 6.88 0.581 11.8:1
NPK 5.4 8.06 0.651 12.4:1
NþFYM 6.4 11.09 0.782 14.2:1
NK treatment was at par. NPKþFYM treatment (1.14%) was best for contributing soil carbohydrate content. Values for other treatments increased in the order NPK (0.67%)<Nþ FYM (0.74%)<NP (0.879).
Changes in dehydrogenase activity in differently treated soils are shown to be distributed across a wide range (Fig. 1). Only fertilized treatments along with controls showed signif-icantly lower intracellular metabolism of soil microbes, which depicts dehydrogenase activity. NP, NK and NPK showed similar levels of activity, at par with the controls. FYM applica-tion increased enzymatic activity a few times when it was applied along with N or NPK.
3.3. Soil enzymatic activities
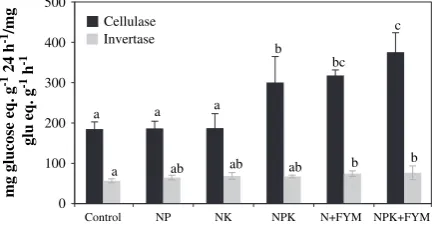
Enzyme activities varied widely among the treatments stud-ied. The treatment with the highest activity was found to vary depending on the enzyme. The highest value for cellulase activity was found in NPKþFYM, followed by NþFYM treat-ments that received organic amendtreat-ments (Fig. 2). Invertase activity did not vary much across treatments and the highest value was found in treatments that received organic manure along with the recommended fertilizer. Protease activity in the soil followed a similar trend and was highest in manure
amended treatments. Unlike other enzymes, urease activity was inhibited in the organic amended soils (Fig. 3). The high-est value was found in non-amended controls followed by NP and NPKþFYM.Fig. 4shows acid and alkaline phospha-tase activities for all the treatments. As the pH of the soil lies within the acidic range, it might be expected that acid phosphatase is the dominant activity in this soil [13]. The highest value was found in NPKþFYM followed by NþFYM. For other treatments, the activity was far below these two treatments. Activity of the manure treated soil was 2–3-fold greater than that in soils from the other treatments. A similar trend was observed in the case of alkaline phosphatase activity. NPK treated soil was least and NPKþFYM was highest for this enzyme activity.
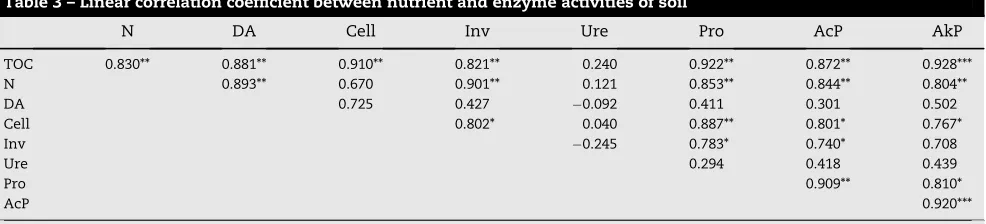
A correlation matrix (Table 3) shows some significant rela-tionships among the enzymes studied. There was positive correlation between cellulase and invertase, which are involved in C transformation. Total organic carbon was corre-lated with all enzymes assayed except urease and dehydroge-nase. Cellulase also showed a strong positive correlation with protease and phosphatase. Acid phosphatase shared a strong correlation with protease and alkaline phosphatase. Protease activity was strongly correlated (r¼0.953,P<0.001) with the N content of the soil. Cellulase activity was also correlated with invertase activity (r¼0.802,P<0.01).
0
Control NP NK NPK N+FYM NPK+FYM
0.0
Fig. 1 – Dehydrogenase activity and carbohydrate content in soil with different treatments. Bars sharing the same letter are not significantly different (P<0.05). Error bars represent standard deviation. acand adrepresent
carbohydrate and dehydrogenase respectively.
Control NP NK NPK N+FYM NPK+FYM
mg glucose eq. g
Fig. 2 – Cellulase and invertase activities in soil with different treatments. Bars sharing the same letter are not significantly different (P<0.05). Error bars represent standard deviation.
Control NP NK NPK N+FYM NPK+FYM
Urease
Fig. 3 – Urease and protease activities in soil with different treatments. Bars sharing the same letter are not
significantly different (P<0.05). Error bars represent standard deviation.
Control NP NK NPK N+FYM NPK+FYM
g p-nitrophenol produced g
3.4. Grain yield
Mean yield of soybean and wheat after 33 years of cropping is presented inTable 4. Soybean yield varied from 0.56 Mg ha1 for C to 2.93 Mg ha1for NPKþFYM. In the case of wheat, it ranged from 0.72 to 1.91 Mg ha1, highest for NPKþFYM and lowest for C.
4.
Discussion
Soil pH ranged from 5.3 in the NK plots, which is significantly lower than for all other treatments, to 6.5 in the NPKþFYM plots. The pH of the N treated plots was significantly lower than that of the C and manured plots. Long term applications, especially N, therefore had acidifying effects resulting in lowering of pH. This confirms earlier findings that most N-containing fertilizers tend to acidify soil[1,2]. This is mainly due to the fact that most fertilizers supply N as NH4þfirst, which upon oxidation releases Hþions[28].
Manure treated plots showed significantly greater net C build up than fertilized treatments. Recommended fertilizer application has been shown to result in modest increases in labile organic matter content. Total SOC in NPK treatment was higher than NP and NK treatments. This is attributable to higher yields under fertilized conditions, which result in increased inputs of organic matter to the soil in the form of both root turnover and crop residues (Table 4). Furthermore,
in a soybean–wheat cropping system, a significant amount of leaf fall from soybean contributes to a greater amount of added organic matter[23].
Soil invertase catalyses the hydrolysis of sucrose to glucose and fructose, and is linked to the soil microbial biomass
[15,21,37]. In our experiment, organic amendment did not contribute much to the hydrolysis of sucrose. The activity was correlated with cellulase (P<0.05). Cellulases play an important role as a group of enzymes in global recycling of the most abundant polymer, cellulose in nature. Though the rate of cellulase activity was highest in NPKþFYM followed by NþFYM and NPK, no clear effect of manures was visible. Cellulase activity was positively correlated with protease (P<0.01), invertase (P<0.05) and phosphatases (P<0.05).
The activity of assayed enzymes was generally well correlated with the organic C content because all of these parameters were increased substantially by increasing returns of organic residues. Indeed, in general, the activity of soil enzymes is significantly correlated with organic C content
[17,40]. Being the substrate of enzymes, soil organic matter plays a vital role in protecting soil enzymes since they form complexes with clay and humus[45].
An increase in soil polysaccharide content in manure treat-ment has been interpreted because of more activity of soil microflora, which can be correlated with dehydrogenase activ-ity. There was an increasing trend of soil carbohydrate in NP treatment, which is not explainable with the present state of knowledge. Our results show that the activities of dehydroge-nase, acid and alkaline phosphatases, cellulase, and protease were highest in FYM treated soil. This maximum activity might be linked to more substrate availability in these soils. This reflects the greater biological activity in these plots and the sta-bilization of extracellular enzymes through complexation with humic substances[6,9,16,22,31]. In contrast, urease and inver-tase activity followed a different pattern, where organic manure had very little effect on the activity. Dehydrogenase activity basically depends on the metabolic state of the soil biota. A significant increase in dehydrogenase activity occurred in the plots with organic treatments, especially with NPK.
Activity of urease was significantly higher in plots under control followed by NPKþFYM and NP treated plots. Dick et al.[10]showed that urease activity decreased with increasing application of NH3based-N fertilizers. It was hypothesized that the addition of the end product of the enzymatic reaction (NH4þ)
Table 3 – Linear correlation coefficient between nutrient and enzyme activities of soil
N DA Cell Inv Ure Pro AcP AkP
TOC 0.830** 0.881** 0.910** 0.821** 0.240 0.922** 0.872** 0.928***
N 0.893** 0.670 0.901** 0.121 0.853** 0.844** 0.804**
DA 0.725 0.427 0.092 0.411 0.301 0.502
Cell 0.802* 0.040 0.887** 0.801* 0.767*
Inv 0.245 0.783* 0.740* 0.708
Ure 0.294 0.418 0.439
Pro 0.909** 0.810*
AcP 0.920***
TOC, total organic carbon; N, total N; DA, dehydrogenase; Cell., cellulase; Inv, invertase; Ure, urease; Pro, protease; AcP, acid phosphatase;
AkP, alkaline phosphatase. *P<0.1, **P<0.05, ***P<0.001 respectively.
Table 4 – Mean grain yield as influenced by fertilization over 33 years of rainfed soybean–wheat cropping
Treatment Mean grain yield (Mg ha1)
Soybean Wheat
Control 0.56a 0.72a
NP 0.88c 0.92c
NK 0.64b 0.81b
NPK 1.42d 1.10d
NþFYM 2.42e 1.60e
NPKþFYM 2.93f 1.91f
Columns sharing the same letter are not significantly different
suppressed urease synthesis. The N mineralization in soil was characterized by measuring extracellular protease activity. The rates of protease activity were higher with the organic amend-ments than in the other treatamend-ments. The high activity of manure treated soil might be due to an increased growth of the microbial community and it was supported by the study of Kandeler et al.[22], which showed that biomass-specific protease activities of different soils were not related to the organic content or texture. More opinion was available for the greater enzyme activity and it was suggested that strong bind-ing of the enzyme to soil colloids protects from denaturation
[6]. Activity of protease was significantly and positively correlated with cellulase (P<0.01) and invertase (P<0.05).
Acid phosphatase activity in NPK treatment was more than 4-fold lower than NPKþFYM treatment and it was supported by Haynes and Swift[18], who found that acid phosphatase activity of soils generally decreases in response to fertilizer application. Phosphatases play an important role in P cycling where organic P is more due to limited biological mineraliza-tion of SOM as a result of formamineraliza-tion of complexes of organic P with active Al and Fe[48]and the amount of available P is low. P transformation and cycle also depend on soil reaction. Acid phosphatase activities in different treatments were greatly regulated by soil pH values. In general, FYM applied to soil has long been employed to enhance favourable soil conditions in terms of pH and availability of P. In this study, higher acid phosphatase activity was shown in soils with pH of 6.5. The observed effect could be due to quantity, specific activity, or stability of the enzyme at that particular soil pH. Acid phosphatase activity was correlated with cellulase (P<0.05), invertase (P<0.05) and protease (P<0.01). The significantly greater activities of alkaline phosphatase in the manure treated soil might be attributed to enhanced microbial activity and diversity due to manure input over the years. Manure addition to a soil may have resulted in changes in origin, states, and/or persistence of enzymes in the soil[34]. The inhibition of activities with mineral fertilizer could be seen as well. Control soils exhibited higher activities than the NPK and NK. This can be explained by the inhibition of phosphatase synthesis by mineral fertilization. In slight contrast to our results, the literature reported that this inhibi-tion was due to mineral-P[3,41]. Alkaline phosphatase was affected and was negatively correlated to soil P concentrations and microbial biomass C and P[51].
Grain yield of soybean and wheat were significantly influ-enced by amendments. All the fertilized treatments signifi-cantly improved soybean yields relative to un-amended control (Table 4). Wheat yields were also significantly influ-enced by the residual effect of the treatments. Application of organic amendments along with inorganic nutrients (NPKþ FYM) yielded 2.07 and 1.73 times more than inorganic nutrient (NPK) only in the case of soybean and wheat respectively. The yield data clearly demonstrate the superiority of the inte-grated use of FYM and chemical fertilizers, which provided greater stability in crop production in comparison to NPK treatment. The beneficial effect of integrated use of NPK and FYM was more pronounced and effective in enhancing the productivity with the advancement of year of cultivation. This is attributed to the maintenance and improvement of soil nutrient status as well as soil biological activity especially
soil enzymatic activity. Application of FYM along with NPK improved soil organic carbon by 47.5 and 87.5% over NPK alone and un-amended control. It also increased dehydroge-nase, cellulase, protease and phosphatases by several fold over inorganic nutrient alone. Improvement in soil dehydro-genase activity was more than five times in NPKþFYM over C treatment. Substantial improvement in soil chemical and biological activity due to application of FYM must contribute in sustaining the productivity and soil health.
5.
Conclusions
It is concluded that the accumulation of organic matter and recycling of C have substantial effects on the activity of enzymes involved in mineralization of C, N and P. Activities of these studied enzymes are correlated with each other and further correlated with soil organic matter content. Farm yard manure along with recommended fertilizer improved soil biological activity, depicted from several fold increase in soil enzymatic activity. Improvement in soil organic mat-ter and biological activity upon application of organic amendments along with inorganic nutrients resulted in sub-stantial increase in crop yield, which can be sustainable for years.
r e f e r e n c e s
[1] S. Aref, M.M. Wander, Long term trends of corn yield and soil organic matter in different crop sequences and soil fertility treatments on the morrow plots, Adv. Agron. 62 (1998) 153–161.
[2] A. Belay, A.S. Claassens, F.C. Wehner, Effect of direct nitrogen and potassium and residual phosphorous fertilizers on soil chemical properties, microbial components and maize yield under long term crop rotation, Biol. Fertil. Soil 35 (2002) 420–427. [3] L. Bohme, F. Bohme, Soil microbiological and biochemical
properties affected by plant growth and different long-term fertilization, Eur. J. Soil Biol. 42 (2006) 1–12.
[4] G.J. Bouyoucos, Hydrometer method improved for making particle size analysis of soils, Agron. J. 54 (1962) 464. [5] M. Brzezinska, Significance of soil enzymes in nutrient
transformations, Acta Agrophys. 63 (2002) 5–23.
[6] R.G. Burns, Enzyme activity in soil: location and a possible role in microbial ecology, Soil Biol. Biochem. 14 (1982) 423–427. [7] L.E. Casida, D. Klein, T. Santoro, Soil dehydrogenase activity,
Soil Sci. 98 (1964) 371–376.
[8] H.P. Collins, P.E. Rasmussen, C.L. Douglas Jr., Crop rotation and residue management effect on soil carbon and microbial dynamics, Soil Sci. Soc. Am. J. 56 (1992) 783–788.
[9] S.R. Colvan, J.K. Syers, A.G. O’Donnell, Effect of long term fertilizer use on acid and alkaline phosphomonoesterase and phosphodiesterase activities in managed grassland, Biol. Fertil. Soils 34 (2001) 258–263.
[10] R.P. Dick, P.E. Rasmussen, E.A. Kerle, Influence of long term residue management on soil enzymatic activities in relation to soil chemical properties of a wheat fallow system, Biol. Fertil. Soils 6 (1988) 159–164.
[11] M. Dubois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem. 28 (1956) 350–356.
[13] J. Eivazi, M.A. Tabatabai, Phosphatases in soil, Soil Biol. Biochem. 27 (1977) 1011–1016.
[14] F. Eivazi, M.R. Bayan, K. Schmidt, Selected soil enzyme activities in the historic sanborn field as affected by long term cropping systems, Commun. Soil Sci. Plant Anal. 34 (15–16) (2003) 2259–2275.
[15] W.T. Frankenberger Jr., J.B. Johanson, Factors affecting invertase activity in soils, Plant Soil 74 (1983) 313–323. [16] A.M.V. Garzillo, L. Badalucco, F. De Cesare, S. Grego, V.
Buonocore, Synthesis and characterization of an acid phosphatase-polyresorcinol complex, Soil Biol. Biochem. 28 (1996) 1155–1161.
[17] M.H. Graham, R.J. Haynes, Organic matter accumulation and fertilizer-induced acidification interact to affect soil microbial and enzyme activity on a long-term sugarcane management experiment, Biol. Fertil. Soils 41 (2005) 249–256. [18] R.J. Haynes, R.S. Swift, Effects of lime and phosphate
additions on changes in enzyme activities, microbial biomass and levels of extractable nitrogen, sulphur, and phosphorus in an acid soil, Biol. Fertil. Soils 6 (1988) 153–158. [19] M.L. Jackson, Soil Chemical Analysis, Prentice Hall of India
pvt. Ltd., New Delhi, 1962.
[20] D.S. Jenkinson, J.N. Ladd, Microbial biomass in soil: measurement and turnover, in: E.A. Paul, J.N. Ladd (Eds.), Soil Biochemistry, Dekker, New York, 1981, pp. 415–471. [21] E. Kandeler, J. Luxhoi, D. Tscherko, J. Magid, Xylanase,
invertase and protease at the soil-litter interface of a loamy sand, Soil Biol. Biochem. 31 (1999) 1171–1179.
[22] E.P. Kandeler, M. Stemmer, M.H. Gerzabek, Tillage changes microbial biomass and enzyme activities in particle-size fractions of a Hapic Chernozem, Soil Biol. Biochem. 31 (1999) 1253–1264.
[23] S. Kundu, R. Bhattacharaya, V. Prakash, B.N. Ghosh, H.S. Gupta, Carbon sequestration and relationship between carbon addition and storage under rainfed soybean-wheat rotation in a sandy loam soil of the Indian Himalayas, Soil Till. Res. 92 (1–2) (2007) 87–95.
[24] J. Kucharski, T. Niklewska, The effect of manuring with straw on microbial properties of light soil, Pol. J. Soil Sci. 24 (2) (1991) 171–177.
[25] J.N. Ladd, J.H.A. Butler, Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates, Soil Biol. Biochem. 4 (1972) 19–30.
[26] A.E. Linkins, R.L. Sinsabaugh, C.A. McClaugherty, J.M. Mellilo, Cellulose activity on decomposition of leaf litters in microcosms, Plant Soil 123 (1990) 17–25.
[27] S.M. Marianari, B. Ceccanti, A. Grego, The influence of organic and mineral fertilizers on biological and physical properties, Bioresour. Technol. 72 (2000) 9–17.
[28] F. Magdof, L. Lanyon, B. Liebhardt, Nutrient cycling, transformations and flows: implications for a more sustainable agriculture, Adv. Agron. 60 (1997) 1–73. [29] D.A. Martens, K.L. Loeffelmann, Improved accounting of
carbohydrate carbon from plants and soils, Soil Biol. Biochem. 34 (2002) 1393–1399.
[30] G.L. Miller, Use of dinitrosalicylic acid reagent for determination of reducing sugar, Anal. Chem. 31 (1969) 426–428.
[31] P. Nannipieri, R.L. Johnson, A.E. Paul, Criteria for
measurement of microbial growth and activity in soil, Soil Biol. Biochem. 10 (1978) 223–229.
[32] P. Nannipieri, E. Kandeler, P. Ruggiero, Enzyme activities and microbiological and biochemical processess in soil, in: R.G.
Burns, R.P. Dick (Eds.), Enzymes in Environment, Marcel Dekker, Inc, New York, pp. 1–33.
[33] S.R. Olsen, C.V. Cole, F.S. Watanabe, L.A. Dean, Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate Circular 939, US Department of Agriculture, 1954. [34] J.A. Parham, S.P. Deng, W.R. Raun, G.V. Johnson, Long term
cattle manure application in soil, I. Effect on soil phosphorous levels, microbial biomass C, and
dehydrogenase and phosphatase activities, Biol. Fertil. Soils 35 (2002) 328–337.
[35] A.E. Richarson, Soil microorganisms and phosphorous availability, in: C.E. Pankhurst, B.M. Doube (Eds.), Soil Biota, CSIRO, East Melbourne, 1994.
[36] H. Rodriguez, R. Fraga, Phosphate solubilising bacteria and their role in plant growth promotion, Biotechnol. Adv. 17 (1999) 319–339.
[37] D.J. Ross, Invertase and amylase activities as influenced by clay minerals, soil-clay fractions and topsoils under grassland, Soil Biol. Biochem. 15 (1983) 287–293.
[38] P.A. Sanchez, R.H. Miller, Organic matter and soil fertility management in acid soils of the tropics, in: , Transactions of the 13th International Congress on Soil Science, vol. 6, Hamburg, 1986, pp. 609–625.
[39] F. Schinner, W. Von Mersi, Xylanase, CM-cellulase and invertase activity in soil, an improved method, Soil Biol. Biochem. 22 (1996) 511–515.
[40] T.W. Speir, D.J. Ross, Soil phosphatase and sulfatase, in: R.G. Burns (Ed.), Soil Enzyme, Academic Press, New York, 1978, pp. 197–250.
[41] T.W. Speir, J.C. Cowling, Phosphatase activities of pasture plants and soils: Relationship with plant productivity and soil P fertility indices, Biol. Fertil. Soils 12 (1991) 189–194. [42] M.J. Swift, P. Woomer, Organic matter and the sustainability
of agricultural systems: definition and measurement, in: K. Mulongoy, R. Merckx (Eds.), Soil Organic Matter Dynamics and Sustainability of Tropical Agriculture, John Wiley and Sons, Chichester, UK, 1993, pp. 3–18.
[43] M.A. Tabatabai, J.M. Bremner, Use of
p-nitrophenylphosphate for assay of soil phosphatase activity, Soil Biol. Biochem. 1 (1969) 301–307.
[44] M.A. Tabatabai, J.M. Bremner, Assay of urease activity in soils, Soil Biol. Biochem. 4 (1972) 479–487.
[45] M.A. Tabatabai, Soil enzymes, in: R.W. Weaver, J.S. Angle, P.S. Bottomley (Eds.), Methods of Soil Analysis, Part 2. Microbiological and Biochemical Properties, SSSA, Madison, WI, 1994, pp. 775–833.
[46] K.R. Tate, The biological transformations of P in soil, Plant Soil 76 (1984) 245–256.
[47] T. Teklay, A. Nordgren, A. Malmer, Soil respiration
characteristics of tropical soils from agricultural and forestry land-uses at Wondo Genet (Ethiopia) in response to C, N and P amendments, Soil Biol. Biochem. 38 (2006) 125–133. [48] M.B. Turrion, B. Glaser, D. Solomon, A. Ni, W. Zech, Effects of
deforestation on phosphorus pools in mountain soils of the Alay Range, Khyrgyzia, Biol. Fertil. Soils 31 (2) (2000) 134–142. [49] A. Walkley, J.A. Black, Estimation of soil organic carbon by
chromic acid titration method, Soil Sci. 17 (1934) 29–38. [50] T. Wlodarczyk, Nitrogen transformations and their
circumstances, Acta Agrophys. 63 (2002) 123–158. [51] A.L. Wright, K.R. Reddy, Phosphorus loading effects on