C
hromatin constitutes about 40% of the nuclear volume. It can be extracted chemically (with salt, mild detergents and DNAse) or physically (electrophoresis) to leave a residual structure, the nuclear matrix (also called the nuclear scaffold), that still resembles the nucleus. Ultrastructure studies of the nuclear matrix reveal a fibrillar network of varying density divided into three components: the outer lamina–nuclear pore complex; the internal matrix; and the nucleolar matrix1. The complexity of this network strengthens the hypothesis that the nuclear matrix deter-mines nuclear organization. Although most chromatin can easily be removed from the nucleus2, a class of DNA sequences remains bound to the nuclear matrix. These sequences, called ‘matrix attachment regions’ (MARs), have been investigated both bio-chemically and genetically in order to elucidate the nature of their interactions with the nuclear matrix, and the effects of these inter-actions on DNA function. This review discusses possible roles for MARs, focusing on their performance in transgenic plant studies.
The structure of chromatin during interphase and mitosis
The nuclear matrix is defined operationally as the residual matter left after nuclear extraction. This fibrillar structure has been inter-preted to act as a skeleton that organizes the nucleus. However, there has been controversy about its structure and biochemistry3,4
. Recently, the most abundant proteins of the internal nuclear matrix have been characterized as ‘heterogeneous nuclear ribonu-cleoproteins’ (hnRNPs), a set of proteins mainly involved in RNA processing and transport; and as B23, a phosphoprotein also involved in RNA processing and transport5. One of the hnRNPs was originally characterized as the MAR-binding protein SAF-A. It follows that several previously identified matrix proteins must be minor components (each less than 1% of the total internal matrix) and probably do not participate in forming the primary structure of the matrix. These less-abundant proteins might be involved in matrix function. The structural role would belong to the hnRNPs, although these do not appear to form fibrils in vivo. Immunofluorescent staining of the major hnRNPs reveals a punc-tate, not a fibrillar, distribution. A possible determinant of the fi-brillar ultrastructure could be intermediate filaments, which are also found associated with the plant nuclear matrix. However, a residual cytoskeleton remains connected to the outer nuclear matrix and intermediate filaments could be mere contaminants of the internal matrix. Thus, the existence of a proteinaceous fibrillar network in vivo that is responsible for organizing and regulating chromatin remains controversial4.
An alternative view of the nuclear matrix is one in which hnRNAs and hnRNPs are complexed to form a granular matrix
with its major function in RNA metabolism. Thus, the matrix itself would be a result of transcription. Regulatory changes resulting in activation and repression of different gene sets might reorganize the nuclear matrix by producing new hnRNAs in dif-ferent nuclear locations and recruiting new hnRNP complexes. Consistent with this view, the major components of the nuclear matrix from different cell types are essentially invariant5
. The many reported changes in nuclear matrix protein composition between different cell types5
must involve minor, and probably non-structural, components.
A dramatic rearrangement of the nuclear structure takes place at mitosis and meiosis, when the nuclear matrix is dispersed con-currently with nuclear disintegration and chromosome conden-sation. In condensing chromosomes, the chromatin becomes associated with an insoluble scaffold that is visible as a dense chromosomal axis from which chromatin loops emanate. The major proteins of the mitotic chromosomal scaffold are not hnRNPs but topoisomerase II, a MAR-binding protein6, and ‘structural maintenance of chromosome’ (SMC) proteins7
. How-ever, these, in turn, are not predominant components of the inter-phase nuclear matrix. Therefore, the nuclear matrix and the chromosomal scaffolds have mainly different compositions.
Early studies on nuclear and chromosomal structures proposed that chromatin is segregated in domains formed by supercoiled loops8,9
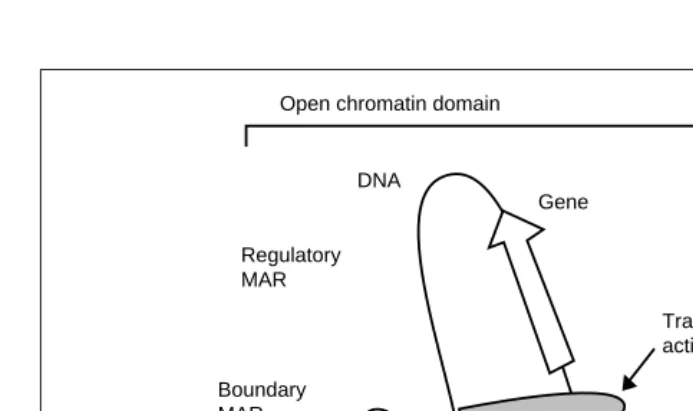
. The loop model helps explain both structural and regulat-ory features of genome organization. However, it is still unclear how the loops are formed and whether they are fixed or variable. It has been proposed that DNA is threaded through immobile replication and transcription foci in the nuclear matrix. These foci would constitute transient points of association between DNA and the matrix. Other DNA sites could be associated in a stable fash-ion, at least through part of the cell cycle. Thus, a loop of chroma-tin might be attached to the matrix at two fixed boundaries as well as a third moving point – the site of transcription or replication (Fig. 1). Fixed attachment points might segregate loop domains whose chromatin condensation and superhelical density are deter-mined by regulatory elements specific for each loop. Open chromatin domains favor gene activity; closed (compacted) chro-matin complexes suppress gene expression. The bases of the loop could thus act as boundaries preventing the regulatory factors act-ing on a loop from influencact-ing neighboract-ing loops.
Are the same loops maintained throughout the cell cycle? The different composition of the interphase nuclear matrix and the mitotic chromosomal scaffold is consistent with the possibility of different loop-determination mechanisms, perhaps dependent on the different roles for interphase and mitotic chromatin. The
Nuclear matrix attachment regions
and plant gene expression
Rachel Holmes-Davis and Luca Comai
major role of interphase chromatin is transcription; mitotic chroma-tin must achieve extreme compaction and, during meiosis, chromatin must engage in pairing, crossing-over and chromosome segregation.
Matrix attachment regions
Division of interphase chromatin in loops averaging 85 kb (Ref. 8) implies that a diploid cell with a genome of 109nucleotide
pairs should have 23 000 loop-attachment sites. If these sites are fixed, and specific sequences are responsible for the attachment, the responsible sequences should be dispersed throughout the genome. They should bind the nuclear matrix (assuming that the nuclear matrix organizes the loops). Furthermore, they might define regulatory domains and have assayable genetic properties. Matrix attachment regions fit all of these criteria. Matrix attach-ment regions are identified by an in vitro binding assay that measures the affinity of a cloned DNA fragment for the nuclear matrix. Binding to the matrix is reversible, resulting in an equilib-rium between bound and soluble DNA. An excess of competitor DNA is used to demonstrate binding specificity. An alternative strategy for identifying MARs involves assaying the continued association of an endogenous DNA fragment with the matrix after nuclear extraction and removal of unbound DNA. Drawbacks to these assays have been described3. Primarily, it is difficult to
ascertain whether a given DNA fragment is actually bound to the nuclear matrix in vivo, because DNA fragments that are not bound initially could bind after the extraction.
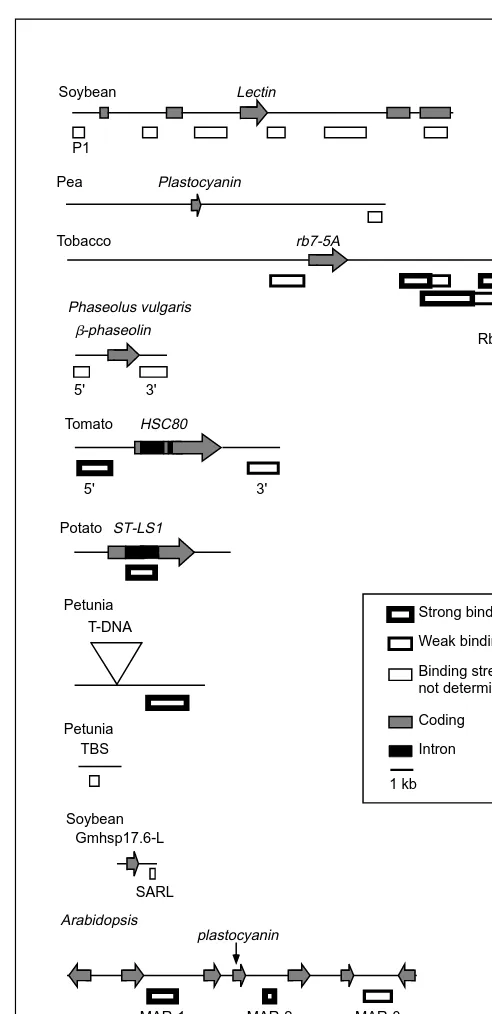
Most MARs have been isolated from DNA close to genes, although this observed distribution is probably biased because transcribed sequences are the most frequently studied. However, a high density of MARs has been observed in animal centromeric chromatin10. The position of known plant MARs in their genomic
regions is shown in Figs 2 and 311–23
. Highly expressed genes, such as adh1, hsc80 and b-phaseolin, are closely flanked by
MARs (Fig. 2), but other genes can be more distant from associated MARs. Putative loops have different sizes and contain different numbers of genes. Matrix attachment regions in the extended genomic region surrounding adh1 of maize have been mapped24 (Fig. 3). Chromatin loops of 7 to 70 kb could be formed from the binding of these sequences to the nuclear matrix. Interestingly, the MAR in the 5'-direction from the adh1 gene corre-sponds to a region of chromatin that is hypersensitive to nuclease digestion and is necessary for adh1 regulation15,25
. Matrix attachment regions share cer-tain characteristics: they are AT-rich and often incorporate conserved sequence motifs; they have DNA-unwinding properties as a result of regions with base-unpairing potential; and they derive their matrix-binding activity from the synergistic action of multiple units that bind the matrix at low efficiency26
. A minimum length of about 300 bp is needed for activity. Matrix attachment regions bind nuclear matrices of widely divergent origin. Proteins of the nuclear matrix recognize conserved features of these sequences: base-unpairing regions; A-tracts; and the minor groove of oligo-dA–oligo dT-regions. These binding properties suggest that all MARs are similar and interchangeable. However, considerable bio-chemical and genetic evidence implies that MARs contain specific binding modules in addition to general ones. For example, the thy-mocyte (thymus cell)-specific MAR-binding protein SATB-1 rec-ognizes the base-unpairing region of all MARs, but has a higher affinity for MARs containing a 5'-[C/A]TAATA-3' motif, which specifically interacts with a homeodomain in SATB-1 (Ref. 27). The 5'-MAR from the HSC80 gene of tomato has binding properties distinct from the 3'-HSC80 MAR (Ref. 20). The 5'-MAR of b -phaseolin has distinct regulatory properties from the 3'-MAR (Ref. 13). Thus, some MARs have both generic and specific properties. This heterogeneity can complicate functional analysis, because results obtained with different MARs might not be comparable.
Matrix attachment regions as boundary, regulatory and chromosomal organization elements
When data from all experimental systems are considered, four possible roles emerge for MARs (Fig. 1):
• A boundary-element role28
. Boundary elements define domains of independent gene regulation. They resist the spreading of differing chromatin conformation states and block the actions of regulatory elements from crossing the boundary.
• A chromatin-regulation role29,30
. Chromatin-regulatory el-ements modify chromatin conformation. The term regulatory element is used here in a broad sense, defining a cis-acting el-ement that stimulates or represses gene expression. Regulatory MARs would facilitate the binding and action of regulatory proteins, perhaps providing a critical discontinuity in com-pacted chromatin. Such perturbation may serve as a ‘beach-head’, where the initial landing of chromatin-opening proteins takes place and from where the switch in conformation can occur. In addition, because of their DNA unwinding properties,
Fig. 1. Possible roles of matrix attachment regions (MARs) illustrated in the context of
com-pacted and open chromatin domains of a eukaryotic chromosome. Three MARs are present in the DNA segment shown, corresponding to the domain boundary and regulatory sites. Domain boundaries prevent the spreading of alternative chromatin states along the chromosome, thus insulating the domain. The regulatory MAR is shown facilitating the access of a transcription activator to the gene promoter via the formation of a ternary complex consisting of a MAR, matrix and activator. By stabilizing an environment favorable to transcription, a regulatory MAR may mimic the action of boundary elements in a transgenic assay.
Nuclear matrix Gene
Transcription activator
Closed chromatin
Closed chromatin
Regulatory MAR
Boundary MAR
Boundary MAR Open chromatin domain
MARs function as topological switches storing and releasing supercoil-generated energy26
.
• A role as components of origins of DNA replication31. This
function is mechanistically related to the chromatin-regulation role, in that both transcription and replication require access to DNA by proteins. In yeast origins, for example, MARs serve an auxiliary role, contributing to an origin’s efficiency32.
• A chromosome-organization role. Matrix attachment regions may tether chromatin to the mitotic chromosomal scaffold during mitosis and meiosis. Matrix attachment regions are common in centromere-associated DNA10, and appear to be
important in mitotic chromosome assembly and maintenance of chromosome shape during metaphase33.
Transgenic studies with plant matrix attachment regions
Chromatin effects on gene expression are elucidated by compar-ing expression between transiently transformed and stable trans-genic cells. In a transient assay, the expression of the gene is determined before integration into the genome and presumably before a higher order of chromatin organization can be estab-lished. With transgenes, by contrast, expression is assayed after genomic integration and establishment of normal chromatin organization. Enhancers function equally well in both assays, whereas elements that affect chromatin organization function only after integration into the genome22,34–37.
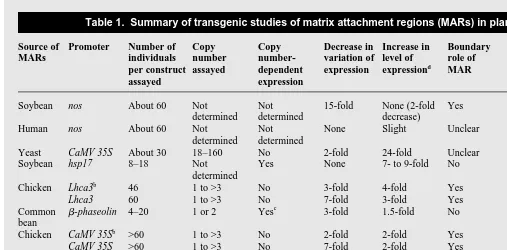
Studies of MARs in plants are summarized in Table 1. To assay MAR function, appropriate constructs (Fig. 4) and selectable markers are introduced by either direct DNA transfer or
Agrobac-terium-mediated transformation. These methods have common
features, but also important differences. A drawback with both methods is that when the selectable marker is linked to the MAR–reporter construct, selection for the marker may prevent the retrieval of low-level-expressing transgenes. Furthermore, silenc-ing interactions can occur if DNA sequences homologous to the transgene (either transgenic or endogenous) are present in the genome38
. Differences between the two methods involve the pat-tern of DNA insertion into the target genome. Direct DNA trans-fer often generates multicopy insertions consisting of intact and rearranged copies of the transforming gene34,35. In contrast,
Fig. 2. Map position of the known plant matrix attachment regions (MARs) with respect to genes or other relevant features of the source region. (The adh1 locus from maize is shown separately in Fig. 3.) The DNA regions of different species are shown at the same scale. Genes are represented by arrows indicating the direc-tion of transcripdirec-tion and are marked with the gene name. Matrix attachment regions are represented by open boxes below the source DNA and name, if available. There are no characterized genes in the proximity of the two petunia MARs: TBS is a trans-formation booster sequence and T-DNA is a vector carrying a transgene. References for the different species, starting from the top of the diagram: soybean, 14; pea, 12; tobacco, 13; Phaseolus
vulgaris, 18; tomato, 20; potato, 11; petunia, 17; petunia, 19;
soy-bean, 16; Arabidopsis, 22; rice, 23; and tomato, 21.
P1
Lectin Soybean
SARL Gmhsp17.6-L Soybean
rb7-5A Tobacco
Rb7 MAR Phaseolus vulgaris
5' 3'
b-phaseolin
Strong binding Weak binding
Intron Coding
1 kb
Binding strength not determined Tomato
5' 3'
HSC80 Pea Plastocyanin
Petunia T-DNA
TBS Petunia
ST-LS1 Potato
MAR-1 MAR-2 MAR-3 plastocyanin
Arabidopsis
Rice
NB-1 NB-2
Tomato
RBCS1 RBSC2 RBCS3A
Fig. 3. Putative chromatin loops defined by matrix attachment
regions (MARs) in the adh1 region of maize. This diagram is a representation of the 280 kb region surrounding the maize adh1 gene based on the work of Avramova and colleagues24
Agrobacterium T-DNA insertion produces fewer copies and often
only a single copy. Insertion of the T-DNA is usually within or near active genes39, thus pre-selecting an open chromatin domain
that may provide endogenous boundaries.
The boundary role
Because of the influence of work in animal systems6, the focus of
plant studies has been to investigate the boundary-role hypothesis. Boundary elements placed in a gene-flanking arrangement should insulate the gene from regulatory elements residing outside the MAR-defined domain. These endogenous regulatory elements may otherwise enhance or suppress the expression of a transgene. In independent transformation events having the same transgene copy number, boundary elements should therefore reduce the variability of expression. Furthermore, within a given transform-ant, each transgene copy should add to the level of expression, resulting in copy number-dependent expression.
The first MAR study using plants14
showed that soybean P1 MARs flanking a transgene reduced variability in gene expression among independent transformants. Because expression of the MAR-flanked transgene was half that of the control, it was inferred that in certain transgenic events the expression of the con-trol gene was increased by chromosomal position effects and that the flanking MARs conferred position-independent expression. In contrast, a human MAR had little effect. A subsequent study showed that the chicken lysozyme A-element MAR reduced the variability of transgene expression and increased the average level of gene expression37,40
. In tobacco plants, the A-element achieved these two effects by reducing or eliminating low-level expressors, without affecting the maximum expression level. These results resemble those seen in animal studies. However, copy number-dependent expression was observed exclusively in the one to two copy range with a light-dependent plant promoter, and not at all
Table 1. Summary of transgenic studies of matrix attachment regions (MARs) in plantsa
Source of Promoter Number of Copy Copy Decrease in Increase in Boundary Regulatory Ref. MARs individuals number number- variation of level of role of role of
per construct assayed dependent expression expressiond
MAR MAR assayed expression
Soybean nos About 60 Not Not 15-fold None (2-fold Yes Unclear 14 determined determined decrease)
Human nos About 60 Not Not None Slight Unclear Unclear 14 determined determined
Yeast CaMV 35S About 30 18–160 No 2-fold 24-fold Unclear Yes 34 Soybean hsp17 8–18 Not Yes None 7- to 9-fold No Yes 16
determined Chicken Lhca3b
46 1 to >3 No 3-fold 4-fold Yes No 37
Lhca3 60 1 to >3 No 7-fold 3-fold Yes No 37
Common b-phaseolin 4–20 1 or 2 Yesc
3-fold 1.5-fold No Yes 18 bean
Chicken CaMV 35Sb
>60 1 to >3 No 2-fold 2-fold Yes Unclear 40
CaMV 35S >60 1 to >3 No 7-fold 2-fold Yes Unclear 40
Tobacco CaMV 35S 16–17 1–31 No None 140-fold Unclear Yes 35 Tomato HSC80 11–32 1–5 No None Yes Unclear Maybe 20 Common CaMV 35S/ 2–10 Not Not Not Yes No Yes 41 bean b-phaseolin determined relevant relevant
a
The transgenic material was the whole plant, except in three reports14,34,35
in which callus was used.
b
This result was also seen in the control.
c
Both of these studies were done with MARs flanking either the GUS gene alone, or the GUS and NPT genes.
d
An increase in expression is only seen when both MARs and introns are present.
Fig. 4. A genetic assay of matrix attachment region (MAR) func-tion. Matrix attachment regions are placed in different positions with respect to a reporter gene (from top to bottom): control con-struct; control construct with enhancer; flanking arrangement to test boundary function; an enhancer-blocking placement to test boundary function; 3'-placement to test chromatin-regulatory function. The different genes are compared during transient trans-formation and in stably transformed lines. Chromatin-organizing elements are expected to have no effects during transient trans-formation. Upon integration into the host genome, boundary el-ements should confer chromosomal position-independent and copy-dependent expression of a flanked transgene. A boundary element placed between a promoter and an enhancer should block the enhancing action on the promoter. Chromatin-regulatory el-ements should increase expression of close genes whether they flank them or not. They may also appear to have a boundary effect by establishing an open chromatin domain that is less susceptible to position effects.
Promoter Gene
with a constitutive viral promoter.
There is ambiguity in the results obtained from the transgene-flanking test for boundary elements used in the experiments described. Elements that establish an open chromatin environ-ment favorable to gene expression, but do not function as bound-ary elements, can also decrease variability among different transformants by ensuring that the transgene is expressed opti-mally regardless of the neighboring chromatin context. Such a possibility is tested by making a construct that contains a single MAR instead of two flanking ones. If a single MAR is insufficient for the effect, a boundary role is further supported. Yet it is poss-ible that the doubling of the MAR and not its flanking arrange-ment is responsible for the observed effect. A double MAR should be tested in a non-flanking arrangement to rule out the hypothesis that simple doubling of the element augments its activity.
A more direct assay for boundary-element function is the enhancer-blocking assay28
. In this assay, a putative boundary el-ement is placed between an enhancer and the promoter of a reporter gene. A boundary element will prevent the enhancer from increasing the expression of the reporter. This assay was used to study the b-phaseolin MARs from common bean (Phaseolus vul-garis)41and led to the unexpected observation that the CaMV 35S
enhancer did not boost expression of the reporter when the two were separated by a control 1 kb DNA fragment. This indicated that the CaMV 35S enhancer can act only at close range. When the 5'-MAR replaced the control fragment, the CaMV 35S enhancer activated the reporter. The 3'-MAR acted like the control fragment, and thus a boundary function is unlikely for the b-phaseolin MARs. The 5'-MAR acted as an ‘enhancer
facilita-tor’, an action that may result from the opening of chromatin30, or
from the bending of DNA42
to bring the enhancer and promoter in close contact. In order to use the enhancer-blocking assay in plants successfully, an enhancer that is capable of activating pro-moters at a considerable distance must first be identified.
The chromatin-regulation role
A MAR can affect the expression level of a neighboring gene, whether flanking it or not. This action may result in enhancement of gene expression or may confer specialized gene regulation that acts in a similar way to a locus control-like region43. A study in
tobacco callus cells resulted in strong enhancement of transgene expression34. The cells were transformed with a yeast ‘ARS1’
MAR-flanked GUS reporter gene using direct DNA transfer. The
GUS gene was expressed at a level 24-fold higher than a control
gene. In addition, the hypothesis that a stronger-binding MAR would have a stronger effect on gene expression was tested. The
Rb7 tobacco MAR, which binds more strongly to the matrix than
the ARS1 MAR, exhibited a 140-fold increase in reporter gene expression over the control35
.
The enhancing effect exhibited in these studies required flank-ing MARs, suggestflank-ing a possible boundary function for the ARS1 MAR. The authors hypothesized that MARs might act by pre-venting homologous gene silencing35
: control genes would be silenced by the presence of multiple copies, while MAR-flanked transgenes would be protected from homologous interactions, perhaps by a close association with the nuclear matrix. These studies using cultured calli transformed by direct DNA transfer provided the strongest enhancement of expression by MARs. The more modest results obtained with Agrobacterium-mediated transformation suggest that differences between the two systems are crucial for the action of these elements.
Another explanation that does not involve chromatin organiz-ation may also apply to these observorganiz-ations. The large number of linked intact and truncated transgenes generated in direct DNA
transfer suggests the possibility of spurious antisense effects in silencing. Because AT-rich elements can function as efficient polyadenylation sites44
, it is possible that an AT-rich region such as a MAR, when positioned in the 3'-direction from the reporter, may cause termination of an unexpected converging transcript. If the transcript is not terminated, it could silence a reporter gene with an antisense RNA. Consequently, by unexpectedly function-ing as a transcription terminator, a MAR may appear to increase expression.
Introduction of the soybean MAR ‘SARL’ in the 5'-direction
from the reporter gene resulted in an eightfold increase in trans-gene expression16. In addition, this resulted in copy
number-dependent expression in the one to five copy range. However, adding the SARL in the 3'-direction from the transgene, either
alone or in constructs with a 5'-SARL MAR, did not enhance
expression. Thus, copy number-dependent expression, often inter-preted to result from a boundary function, can be conferred by a single MAR element.
Another study that supports a regulatory role for MARs used the b-phaseolin MAR from common bean18. This MAR
func-tioned in a similar way to the SARLtype: a single MAR placed in
the 5'-direction from the reporter doubled its expression. In addi-tion, a single 5'-MAR reduced the variation in gene expression. A regulatory role was further indicated by the ability of the 5'-MAR to facilitate promoter activation by the CaMV 35S enhancer in a separate study41.
Finally, studies on the effects of the Heat Shock Cognate 80 (HSC80) MARs on transgene expression driven by the HSC80 promoter showed that both the 5'- and 3'-MARs and the gene’s introns were necessary for expression20,36. The absence of any of
these elements severely decreased or eliminated gene expression. Thus, the HSC80 MARs and the introns appear to act synergisti-cally to facilitate the activity of the HSC80 promoter.
Roles in DNA replication and chromosome organization
The possibility that MARs participate in DNA replication or chro-mosome organization has not been investigated in plants. Evi-dence supporting an auxiliary role for MARs in origins of DNA replication has been obtained in yeast32
and mammals31
. The involvement of MARs in chromosome structure has been probed by treating mitotic Xenopus extracts with synthetic proteins in-corporating multiple copies of the AT-tract binding domain (multi-AT hook: MATH). This treatment disrupted chromosome structure and assembly, but not condensation, suggesting that MARs are important for the maintenance of chromosome shape during mitosis33. The physical properties of MARs make them
possible participants in other chromosomal phenomena, such as sister-chromatid adhesion, homolog pairing, recombination and centromeric function.
Challenges for research on matrix attachment regions
Several problems preclude assigning a single function for MARs in plants. Two of these problems are intrinsic to the system and cannot easily be addressed. First, a single role might not exist: MARs may be a heterogeneous group of elements that share only the ability to bind to the nuclear matrix, but also interact with dif-ferent matrix components. Consequently, MARs may fall into different functional classes. Second, because MARs are relatively large, elements independent of the MARs could be ‘hidden’ inside and produce spurious results.
Additional considerations for studies of MARs involve experi-mental design. To eliminate unnecessary variables, the size and spacing26,37
that preserve the distance between the reporter gene and other elements. Such stuffer elements should also be AT-rich so as to mimic the composition of the MARs. In choosing the transfor-mation system, selection for a linked transfortransfor-mation marker, and the tendency of Agrobacterium T-DNA to insert as a single copy (or a few copies) preferentially within or near active genes39,
might compromise the analysis of the effects of MARs on trans-genes. A possible approach to circumvent this problem is to use transposable elements engineered to move the MAR–transgene construct to new sites after the original transformation, assuring the availability of single-copy, unselected events.
In reviewing these transgenic studies in plants, it appears that there are inconsistencies similar to those observed in animal sys-tems6,26. Although differences in approach can make comparisons
difficult, the information obtained does provide an understanding of how MARs affect transgenes. Progress in this field, however, would be greatly facilitated by studies in which side-by-side com-parisons of MARs are performed in the absence of selection, and with standardized vector and reporter-gene components.
Conclusions
Recent research has shown that MARs are common plant genomic elements that can contribute to gene regulation, but the mecha-nism for this is unclear. When the experimental data are consid-ered with respect to the boundary-element role and the chromatin-regulation role, evidence for the boundary-element role is weak. Rather, MARs might act in a regulatory role to fa-cilitate the transition from closed to open chromatin, so stabilizing and augmenting gene expression. These data and other recent dis-coveries on nuclear matrix structure caution against the canonical interpretation of the role of MARs as solely loop anchors that insulate domains of independent gene function. Alternative roles involving chromosome function deserve consideration, as well as the possibility that different functional classes of MARs might exist. A comparative survey of plant MARs may uncover elements useful in transgenic applications.
Acknowledgements
We would like to thank Drs Arnold Bendich, Delene Oldenburg and Kyle Serikawa for their critical comments. We acknowledge support by the National Science Foundation grant DMB 9205573 to L.C. and PHS NRSA T32 GM07270 from the National Institute of General Medical Sciences (National Institutes of Health) and the University of Washington P.M.I.F. Fellowship to R.H-D.
References
01 Moreno Diaz de la Espina, S. (1995) Nuclear matrix isolated from plant cells,
in Structural and Functional Organization of the Nuclear Matrix (Berezney, R. and Kwang, W.J., eds), pp. 75–139, Academic Press
02 Nickerson, J.A. et al. (1997) The nuclear matrix revealed by eluting chromatin
from a cross-linked nucleus, Proc. Natl. Acad. Sci. U. S. A. 94, 4446–4450 03 Jackson, D.A. and Cook, P.R. (1995) The structural basis of nuclear function,
in Structural and Functional Organization of the Nuclear Matrix (Berezney, R. and Kwang, W.J., eds), pp. 125–149, Academic Press
04 Singer, R.H. and Green, M.R. (1997) Compartmentalization of eukaryotic
gene expression: causes and effects, Cell 91, 291–294
05 Mattern, K.A. et al. (1997) Major internal nuclear matrix proteins are common
to different human cell types, J. Cell. Biochem. 65, 42–52
06 Luderus, M.E.E. and van Driel, R. (1997) Nuclear matrix-associated regions,
in Nuclear Organization, Chromatin Structure, and Gene Expression (van Driel, R. and Otte, A.P., eds), pp. 99–115, Oxford University Press 07 Heck, M.M.S. (1997) Condensins, cohesins, and chromosome architecture:
how to make and break a mitotic chromosome, Cell 91, 5–8
08 Benyajati, C. and Worcel, A. (1976) Isolation, characterization, and structure
of the folded interphase genome of Drosophila melanogaster, Cell 9, 393–407 09 Paulson, J.R. and Laemmli, U.K. (1997) The structure of histone depleted
metaphase chromosomes, Cell 12, 817–828
10 Strissel, P.L. et al. (1996) Scaffold attachment regions in
centromere-associated DNA, Chromosoma 105, 122–133
11 Mielke, C. et al. (1990) Hierarchical binding of DNA fragments derived from
scaffold-attached regions: correlation of properties in vitro and function
in vivo, Biochemistry 29, 7475–7485
12 Slatter, R.E., Dupree, P. and Gray, J.C. (1991) A scaffold-associated DNA
region is located downstream of the pea plastocyanin gene, Plant Cell 3, 1239–1250
13 Hall, G., Jr et al. (1991) Nuclear scaffolds and scaffold-attachment regions in
higher plants, Proc. Natl. Acad. Sci. U. S. A. 88, 9320–9324
14 Breyne, P. et al. (1992) Characterization of a plant scaffold attachment region
in a DNA fragment that normalizes transgene expression in tobacco, Plant
Cell 4, 463–471
15 Avramova, Z. and Bennetzen, J.L. (1993) Isolation of matrices from maize
leaf nuclei: identification of a matrix-binding site adjacent to the Adh1 gene,
Plant Mol. Biol. 22, 1135–1143
16 Schöffl, F. et al. (1993) An SAR sequence containing 395 bp DNA fragment
mediates enhanced, gene-dosage-correlated expression of a chimaeric heat shock gene in transgenic tobacco plants, Transgenic Res. 2, 93–100
17 Dietz, A. et al. (1994) A plant scaffold attached region detected close to a
T-DNA integration site is active in mammalian cells, Nucleic Acids Res. 22, 2744–2751
18 van der Geest, A.H.M. et al. (1994) The b-phaseolin gene is flanked by matrix
attachment regions, Plant J. 6, 413–423
19 Galliano, H. et al. (1995) The transformation booster sequence from Petunia
hybrida is a retrotransposon derivative that binds to the nuclear scaffold, Mol. Gen. Genet. 247, 614–622
20 Chinn, A.M. and Comai, L. (1996) The Heat Shock Cognate 80 gene of
tomato is flanked by matrix attachment regions, Plant Mol. Biol. 32, 959–968
21 Meier, I. et al. (1997) The tomato RBCS3A promoter requires integration into
the chromatin for correct organ-specific regulation, FEBS Lett. 415, 91–95
22 van Drunen, C.M. et al. (1997) Analysis of the chromatin domain organisation
around the plastocyanin gene reveals an MAR-specific sequence element in
Arabidopsis thaliana, Nucleic Acids Res. 25, 3904–3911
23 Nomura, K. et al. (1997) Isolation and characterization of matrix associated
region DNA fragments in rice (Oryza sativa L.), Plant Cell Physiol. 38, 1060–1068
24 Avramova, Z. et al. (1995) Matrix attachment regions and transcribed sequences
within a long chromosomal continuum containing maize Adh1, Plant Cell 7, 1667–1680
25 Paul, A-L. and Ferl, R.J. (1993) Osmium tetroxide footprinting of a scaffold
attachment region in the maize Adh1 promoter, Plant Mol. Biol. 22, 1145–1151
26 Bode, J. et al. (1996) Scaffold/matrix-attached regions: topological switches
with multiple regulatory functions, Crit. Rev. Eukaryotic Gene Expr. 6, 115–138
27 Dickinson, L.A., Dickinson, C.D. and Kohwi-Shigematsu, T. (1997) An
atypical homeodomain in SATB1 promotes specific recognition of the key structural element in a matrix attachment region, J. Biol. Chem. 272, 11463–11470
28 Geyer, P.K. (1997) The role of insulator elements in defining domains of gene
expression, Curr. Opin. Genet. Dev. 7, 242–248
29 Mirkovitch, J., Mirault, M-E. and Laemmli, U.K. (1984) Organization of the
higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold, Cell 39, 223–232
30 Bodnar, J.W. and Bradley, M.K. (1996) A chromatin switch, J. Theor. Biol.
183, 1–7
31 Dijkwel, P.A. and Hamlin, J.L. (1995) Origins of replication and the nuclear
matrix: the DHFR domain as a paradigm, in Structural and Functional
Organization of the Nuclear Matrix (Berezney, R. and Kwang, W.J., eds),
pp. 455–484, Academic Press
32 Amati, B. et al. (1990) Nuclear scaffold attachment stimulates, but is not
Drosophila ftz SAR, EMBO J. 9, 4007–4016
33 Strick, R. and Laemmli, U.K. (1995) SARs are cis DNA elements of
chromosome dynamics: synthesis of a SAR repressor protein, Cell 83, 1137–1148
34 Allen, G.C. et al. (1993) Scaffold attachment regions increase reporter gene
expression in stably transformed plant cells, Plant Cell 5, 603–613
35 Allen, G.C. et al. (1996) High-level transgene expression in plant cells: effects
of a strong scaffold attachment region from tobacco, Plant Cell 8, 899–913
36 Chinn, A.M., Payne, S.R. and Comai, L. (1996) Variegation and silencing of
the Heat Shock Cognate 80 gene are relieved by a bipartite downstream regulatory element, Plant J. 9, 325–339
37 Mlynárová, L. et al. (1994) Reduced position effect in mature transgenic
plants conferred by the chicken lysozyme matrix-associated region, Plant Cell 6, 417–426
38 Meyer, P. and Saedler, H. (1996) Homology-dependent gene silencing in
plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 23–48
39 Koncz, C. et al. (1989) High-frequency T-DNA-mediated gene tagging in
plants, Proc. Natl. Acad. Sci. U. S. A. 86, 8467–8471
40 Mlynárová, L. et al. (1995) The MAR-mediated reduction in position effect
can be uncoupled from copy number-dependent expression in transgenic
plants, Plant Cell 7, 599–609
41 van der Geest, A.H.M. and Hall, T.C. (1997) The b-phaseolin 5' matrix
attachment region acts as an enhancer facilitator, Plant Mol. Biol. 33, 553–557
42 von Kries, J.P. et al. (1990) A non-curved chicken lysozyme 5' matrix
attachment site is 3' followed by a strongly curved DNA sequence, Nucleic
Acids Res. 18, 3881–3885
43 Festenstein, R. et al. (1996) Locus control region function and
heterochromatin-induced position effect variegation, Science 271, 1123–1125
44 Luehrsen, K.R. and Walbot, V. (1994) Intron creation and polyadenylation in
maize are directed by AU-rich RNA, Genes Dev. 8, 1117–1130
Rachel Holmes-Davis and Luca Comai*are at the Dept of Botany, University of Washington, Box 355325, Seattle, WA 98195, USA.
*Author for correspondence (tel +1 206 543 4841; fax +1 206 685 1728; e-mail comai@u.washington.edu).
T
he ability to recognize ‘self’ from ‘non-self’ is a character-istic feature of the immune system. Experiments on cell fusion and related studies have provided ample evidence that such recognition occurs at the cellular level, but the possi-bility that recognition exists at the genomic level has received relatively little attention. Although a vast number of genetically engineered plants has now been produced, many examples exist in which the introduced gene is not functioning (i.e. is silenced), or is functioning aberrantly1–4. These observations suggest that anabil-ity to recognize self from non-self exists at the nucleic acid level and that some cases of gene silencing reflect this ability (Box 1).
Protection against foreign DNA
The concept that eukaryotic cells have effective defense mecha-nisms against the uptake, integration and faithful maintenance of
foreign DNA5–7or their transcribed products4evolved following
the observation that bacteria protect their DNA by selectively degrading invading bacteriophage DNA using restriction-modifi-cation systems. In these systems, cellular DNA is methylated at 4–8 bp recognition sequences, a reaction catalyzed by a methyl-transferase, and unmethylated non-self (viral) DNA containing these sequences is degraded via the action of the cognate endo-nuclease. Mouse methyltransferase contains extensive homology to the bacterial enzyme, suggesting that it arose via fusion of a prokaryotic methyltransferase gene to a second gene of unknown function5. The efficacy of methylation in the repression of genes
and transposable elements8,9
, and inactivation of duplicated and extraneous sequences7,10–12, probably led to its retention as a
prin-cipal component of genome intruder recognition and inactivation systems during the evolution of complex genomes.