M
etabolism is the molecular process carried out pri-marily by protein gene products, which builds and runs plants. While genes are indifferent to time, the ancient equal to the new, metabolism deals with the bustle of events here and now, where reactions must all work together. We are enter-ing particularly excitenter-ing times in metabolic research. The ability to manipulate gene expression and affect metabolism1–4has emphasized our need to obtain much more fundamental infor-mation about the metabolic networks in plants, to revise the ways in which we think about what metabolic regulation is, and to learn how to exploit regulatory elements in plants usefully. The techniques of NMR spectroscopy offer some unique ways to help build a foundation of metabolic information about plants.
NMR and the layers of complexity in metabolism
In the simplest representations of metabolism, such as in meta-bolic maps, transforming arrows overseen by enzymes link vast numbers of metabolites (mostly small molecules). High-resolu-tion NMR spectroscopy is used to observe metabolites directly and these observations can often be used to observe enzyme activity indirectly. Unique among analytical methods, NMR allows the identification and quantitation of metabolite levels in complex mixtures, such as crude cell extracts and, in many instances, living cells (reviewed in Ref 5–9). This will not be considered in detail here because the measurement of metabolite pools was just an important beginning. More recently, NMR has been used effectively to address the complexity resulting from the abundance of points in metabolism. These branch-points offer alternative fates for many metabolites, involving both chemical transformations and distribution in different sub-cellular compartments and cells. Here, I review how NMR has been used to obtain quantitative information about fluxes in metabolic networks. Complementing the complexity of meta-bolic networks in plant cells are the wonderful non-linearities between the quantity of an enzyme, its activity in vivo, and the effect of this in vivo activity. These non-linearities are a major obstacle that impede the production of predictable alter-ations to plant metabolism using molecular genetic tools. Progress in NMR measurements of individual and of net-works of enzymatic activities in cells should be particularly valuable for understanding how pathways are regulated in plants, and for defining the key parameters that contribute to biological efficiency.
Measuring fluxes along competing metabolic routes
A recurrent feature of metabolism is that important metabolites can be synthesized by more than one pathway, therefore
meta-NMR adventures in the metabolic
labyrinth within plants
Justin K.M. Roberts
The era of metabolic engineering has begun, but there is only limited knowledge about metabolic fluxes and how they are regulated in plants. Particular challenges are the non-linearities between enzyme abundances, metabolite concentrations and metabolic fluxes, and the existence of metabolic networks that provide multiple routes between many important metabolites. NMR offers the means to distinguish and quantitate the fluxes along different routes to key metabolites. NMR can therefore help us understand and resolve the apparent paradox of, on the one hand, great metabolic flexibility evident in the natural responses of plants and, on the other hand, the unpredictable changes in metabolism reported in genetically engineered plants.
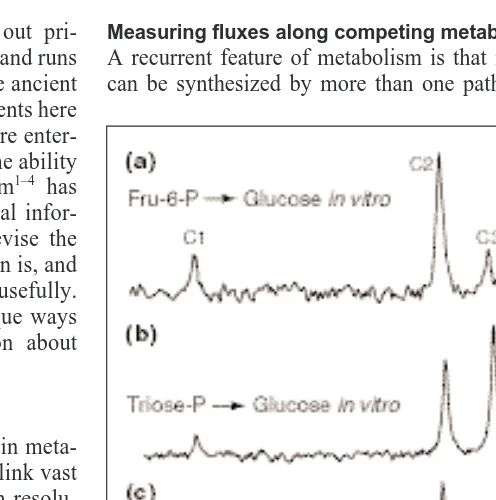
Fig. 1. The path of sucrose synthesis from starch, determined by
2
H-NMR. 2
H-NMR spectra of 2
H-labeled samples of the glucose derivative 1,2-O-isopropylidene-D-glucofuranose. Glucose derivitiz-ation is necessary to obtain simple, interpretable spectra. Peaks are labeled to show which carbon of glucose each 2
H is attached to. Signal intensity is proportional to the amount of 2
H at each position. (a) Glucose was synthesized in vitro from fructose-6-phosphate (Fru-6-P) via phosphoglucose isomerase and glucose-6-phosphatase in 2
H-labeled water, showing preferential incorporation of 2
H at C2. (b) Glucose was synthesized in vitro from dihydroxyacetone phos-phate via triose phosphos-phate isomerase, aldolase, fructose bisphos-phatase, phosphoglucose isomerase and glucose-6-phosbisphos-phatase, showing incorporation of 2
H into C2–C5. (c) Glucose isolated from sucrose synthesized in intact bean leaves in vivo after being sealed with2
bolic outcome is the consequence of competition among many possible reactions and regulatory mechanisms. Generally, metab-olite pool size measurements cannot be used to unambiguously distinguish the contributions of multiple pathways, and estimates based on in vitro enzyme activities are not easily extrapolated to conditions in vivo. The NMR approach to this problem relies on the discrimination of alternative pathways using isotopes. The iso-topic enrichment at specific atoms in a metabolite can reflect the route by which it was synthesized. NMR spectra show the sum of isotopomers (isotope isomers) in a mixture, and quantitation of the isotopic enrichment within individual metabolites allows the relative fluxes through the distinct pathways to be determined quantitatively. The many examples described in this section all rely on this principle. In general, labeling patterns are observed more easily in plant extracts than in vivo because the resolution is greater, and the quantitation of individual peaks and the un-ambiguous assignment of peaks are much easier. In vitro, deuterium from heavy water (2
H2O) is incorporated into glucose differently when made from fructose-6-phosphate compared with synthesis from triose-phosphate9
(Fig. 1). Labeling patterns of the glucose moiety of sucrose, obtained from leaves during darkness, match the action of phosphoglucose isomerase but do not match syn-thesis via triose-phosphate in vivo. From these results, it was in-fered9
that during the night, carbon from starch breakdown in the chloroplasts is exported primarily as hexose – in contrast with the
situation during the day, when reduced carbon is exported pri-marily as triose-phosphate. This method has been extended re-cently to aid the understanding of some of the origins of deuterium abundances in plants, which is important in the field of climate– plant interactions. It appears that chloroplast phosphoglucose iso-merase is displaced from equilibrium in contrast with the cytosolic isomerase10.
Most of the studies using NMR have focused on specific pathways of [1-13C]glucose metabolism. In nature, the level of 13C is 1.1%, the balance being primarily 12
C. Hence, ‘natural abundance’ 13 C-NMR spectra of plant metabolites show signals from molecules that are present at high concentrations: .~1 mmol/g fresh weight for in vivo spectra11, depending on the acquisition time and the spectrometer. Sensitivity is highest in cell extracts because signal peaks are sharper. Selective enrichment of [1-13C]glucose at carbon 1 to 99% 13
C means that plant metabolites derived from the C1 of glucose can be seen at concentrations .~10 nmol/g fresh weight, together with signals from abundant metabolites that are not enriched with 13
C. The relative 13C-enrichment at different positions in a given molecule can be determined from the relative peak intensities of 13
C-NMR spectra, analogous to Fig. 1. Absolute 13C-enrichment at a specific
Fig. 2. Determination of 13
C isotopic enrichment in C1 of glucose-6-phosphate by 1
H-NMR. 1
H-NMR spectrum of 1
H attached to C1 of glucose-6-phosphate extracted from [1-13
C]glucose-labeled maize root tips. Doublet 1, 1
H attached to C1 of [1-12
C]glucose-6-phosphate. Doublet 2, 1
H attached to C1 of [1-13
C]glucose-6-phos-phate (this signal is shifted because of 1
H-13
C spin–spin coupling, which splits the signal to positions on either side of Doublet 1; the
13
C-coupled 1
H-signal having a higher chemical shift than peak 1 overlapped with the water signal, and is not shown). The ratio
12
C:13
C at C1 of glucose-6-phosphate is given by the intensity of doublet 1, divided by twice the intensity of doublet 2. Figure reproduced, with permission, from Ref. 29.
Fig. 3. Competing metabolic reactions in tissues fed [1-13
C]glu-cose and studied by 13
carbon in a metabolite can be measured by 1
H-NMR (Fig. 2). Alter-natively, absolute 13C-enrichments can be determined by combining the values of the overall 13
C:12
C ratios in each metabolite, deter-mined by gas-chromatography-mass spectroscopy (GC-MS), with 13
C-NMR measurements of relative 13
C-enrichment12 .
In a study of isoprenoid biosynthesis, [1-13C]glucose was injected inside the receptacles bearing compound flowers of chamomile (Chamaemelum nobile), and the C15terpenoids subse-quently extracted and analyzed by NMR and GC-MS (Ref. 13). The labeling pattern of these sequiterpenes indicates that the con-tribution of the classical mevalonate pathway of terpenoid synthe-sis14 is small relative to the contribution from the pathway originating at triose-phosphate and pyruvate15
(Fig. 3; reviewed in Ref. 16). Moreover, the labeling of different 5C units in the
ter-penoids is not uniform, suggesting that the sequential addition of 5C units occurs in distinct compartments13
. Comparisons of labeling patterns also reveal compartmenta-tion between different terpenoids in liver-worts, where, in contrast with chamomile, sequiterpenoids appear to be synthesized via the mevalonate pathway; other ter-penoids have labeling patterns that indicate exclusive synthesis from triose-phosphate and pyruvate17
.
Other pairs of pathways in [1-13 C]glucose-labeled maize root tips analyzed using NMR include sucrose hydrolysis and glu-cose uptake, with the result that sucrose turnover appears to be a major futile cycle in plants18
. Perhaps even more complex is the balance between carbon flow for bio-synthesis and catabolism (energy produc-tion), which is regulated at multiple interfaces between the citric acid cycle and the rest of primary metabolism. Several of these interfaces have been studied: phos-phoenol pyruvate (PEP) carboxylase and citrate synthase18
; malic enzyme and pyru-vate kinase12,18; PEP carboxylase and the flux from 2-oxoglutarate to malate12
(Fig. 3). During sugar starvation, the flux of carbon via PEP carboxylase is stopped quickly, whereas the flux of endogenous carbo-hydrates to respiratory intermediates de-creases more slowly as catabolism of fatty acids increases19
. During the first few min-utes of low oxygen stress, malic enzyme is activated, serving to deplete cytoplasmic pools of malate and aspartate, which can mitigate cytoplasmic acidosis12
.
The metabolism of glucose in glycolysis and the pentose phosphate pathway (PPP) is too complicated to unravel quantitatively and unambiguously using [1-13
C]glucose alone18. The oxidative PPP releases the C1 of glucose as CO2. However, the PPP inter-mediates become labeled with 13C because the label is scrambled between C1 and C6 of hexose-phosphates (via exchange between fructose-phosphate and triose-phosphate), in addition to the mixing of glycolytic and PPP intermediates. Combin-ing results from experiments usCombin-ing [1-13
C]glucose with data using [2-14
C]glucose indicates that almost a third of the glucose entering maize root tips is metabo-lized via the PPP (Ref. 18). In studies where only [1-13
C]hexoses are used, only qualitative conclusions on the involvement of the PPP are possible20
. These results point to the need for further work with NMR and other techniques to describe this complicated net-work more fully, including an explanation of the seductively sim-ple result that maize root tips release label from [1-13C]glucose and [6-13
C]glucose at equal rates, resulting from processes such as pentane synthesis18. Experiments using additional labeled sub-strates, and analysis of isotope distribution in intermediates, will be invaluable21,22. The advantage of data from more intermediates is primarily in reducing the degrees of freedom contained within the metabolic models used to determine metabolic fluxes, which
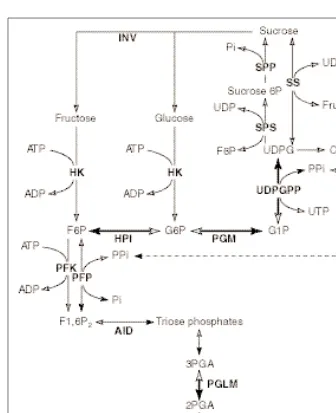
Fig. 4. Cytosolic pathways of carbohydrate metabolism in heterotrophic plant cells under
hypoxia, studied by 2-dimensional 31
reduces the number of untested assumptions associated with each measurement of relative enzyme fluxes.
Another example of the analysis of dual, convergent pathways studied to date is the incorporation of formate or glycine into serine in Arabidopsis23,24
. These studies showed the largely independent operation of these two sources of C1 carbons. Similar experiments with non-photosynthetic tissues suggested a serine–glycine cycle during serine catabolism, involving reactions in both the cytosolic and mitochondrial compartments25
. The metabolic NMR approach should be increasingly useful for complementing the great advances in our knowledge of the structures of enzymes involved in one carbon metabolism26,27. This combination offers the exciting promise that the consequences on in vivo metabolism of changing structural properties of plant enzymes in defined ways will be determined. Such an approach would constitute a major advance over present metabolic engineering strategies in plants.
The experiments described above deal with metabolic reactions occurring on a timescale of minutes or longer. There are many reactions in plant cells were metabolites turnover on the timescale of seconds, and under favorable circumstances these can be quan-titated by the technique of saturation-transfer NMR. This is a labeling technique, but, rather than using isotopic labeling as described, label is created in metabolites of interest by orienting the magnetic nuclei using radiowaves. Transfer of the labeled nuclei to other metabolites can be monitored if it occurs before the label decays (usually within a few seconds). Recently, a 2-dimensional 31P-NMR approach has been used to estimate unidirectional fluxes through several enzymes of central metabolism in hypoxic maize root tips28
(Fig. 4). This method has the distinct advantage over previous 1-dimensional saturation transfer NMR experiments where only single exchange reactions were observed29
. Most interestingly, and relevant to the PPi enigma30, the flux to UDPG, catalyzed by UDP-glucose pyrophosphorylase, is higher than the reverse flux to glucose-1-phosphate28. This result suggests that the pyrophos-phorylase is generating large quantities of PPi, which could be valuable in sustaining glycolysis under these hypoxic conditions, which limit ATP synthesis via oxidative phosphorylation.
Compartmentation of metabolism
The widespread use of NMR to study compartmentation of pro-tons, Pi and organic acids between the cytoplasm and the vacuole has been reviewed previously5–8. A study of plant response to the fungal elicitor cryptogein31
illustrates how NMR measurements of cytoplasmic pH can be integrated with other measurements in a sophisticated way, in this case to identify the sequence of mem-brane and metabolic events preceding the hypersensitive reaction. In contrast with phosphates and organic acids, most amino acids give identical NMR signals whether they are in the acidic vacuole or in the more alkaline cytoplasm. Recently, this problem was overcome by rapidly and reversibly alkalinizing the cytoplasm of cultured cells, so that the natural abundance 13C-NMR signal from cytoplasmic amino acids was shifted
selectively and resolved11
(Fig. 5). The concentration of homoserine and other amino acids was much higher than in the vacuole. This technique has been extended to observe proline in leaf tissue, which also showed that proline accumulated pref-erentially in the cytoplasm32
. It will be interesting to see how this new in situ method compares to the more commonly used destructive approaches of non-aqueous tissue fractionation or protoplast fractionation (discussed in Ref. 11). It is noteworthy that the concentrations of cytoplasmic Pi determined by in vivo 31P-NMR (reviewed in Ref. 6) are approximately an order of
magnitude smaller than those found using the technique of non-aqueous fractionation9. This discrepancy has profound implications
for our understanding of energy metabolism and sub-cellular transport in plants.
In addition to compartmentation between pools of metabolites by the membrane barriers, compartmentation can also occur at the level of the physical state of metabolites. For example, plant mitochondria contain enzyme-bound nucleotides, which cannot be removed by extensive washing33. A trace of bound ATP in mitochondria is able to prime the phosphorylation of AMP by mitochondria, cata-lyzed by the combined action of adenylate kinase and ATP syn-thase, in a response that mirrors the metabolic transformation that occurs in plants emerging from dormancy and prolonged anoxia.
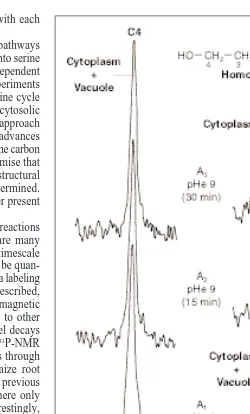
Fig. 5. Compartmentation of homoserine between the cytoplasm and
the vacuole, determined by in vivo13C-NMR following
manipu-lation of cytoplasmic pH. Acer pseudoplatanus cells were incubated in 0.1 mM homoserine (with natural 13C abundance) for 12 h. Spectra
show 13C resonances from C3 and C4 of homoserine inside the cells.
A1, spectrum obtained before any addition, external pH 5 6. A2and
A3, spectra obtained 15 and 30 min, respectively, after alkalinization
Metabolite transport
The transfer and metabolism of carbon from the root to the symbiont has been investigated using a split-culture system to grow carrot roots colonized with mycorrhizal fungi34. Although the fungus cannot use sugars in the external medium directly, it can take 13
C-labeled hexoses from the root effectively, and convert them to lipids, which are transported to the distant mycelium. The synthesis of fungal lipids within the colonized root is sustained by reductant from the oxidative PPP. This study shows how profoundly the metabolism of the symbiont is affected by the presence of the host plant.
Sucrose movement into and within the hypocotyl of castor bean seedlings can be followed by 13C-NMR imaging35 and spec-troscopy35,36
. The experiments involve feeding 13
C-labeled sugars to the cotyledons, and observing 13C in the distant hypocotyl. The challenge is to obtain sufficient signal, particularly for high spatial resolution imaging. This work indicates that significant technical improvements have been made, and we can anticipate further advances in the near future because all the NMR measurements described in this review will become easier and applicable to more metabolic processes in plants.
References
1 Haake, V. et al. (1998) A moderate decrease of plastid aldolase activity
inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 14, 147–157
2 Trethewey, R.N. et al. (1999) Tuber-specific expression of a yeast invertase
and a bacterial glucokinase in potato leads to an activation of sucrose phosphate synthase and the creation of a sucrose futile cycle. Planta 208, 227–238
3 Dai, N. et al. (1999) Overexpression of Arabidopsis hexokinase in tomato
plants inhibits growth, reduces photosynthesis, and induces rapid senescence.
Plant Cell 11, 1253–1266
4 Nuccio, M.L. et al. (1999) The metabolic engineering of plants for osmotic
stress resistance. Curr. Opin. Plant Biol. 2, 128–134
5 Ratcliffe, R.G. (1994) In vivo NMR studies of higher plants and algae. Adv.
Bot. Res. 20, 43–123
6 Roberts, J.K.M. (1996) Plant physiology. In Encyclopedia of Nuclear Magnetic
Resonance (Grant, D.M. and Harris, R.K., eds), pp. 3627–3633, Wiley
7 Shachar-Hill, Y. and Pfeffer, P., eds (1996) Nuclear Magnetic Resonance in
Plant Biology, American Society of Plant Physiologists, Rockville, MD, USA
8 Schneider, B. (1997) In vivo nuclear magnetic resonance spectroscopy of
low-molecular-weight compounds in plant cells. Planta 203, 1–8
9 Schleucher, J. et al. (1998) Export of carbon from chloroplasts at night. Plant
Physiol. 118, 1439–1445
10 Schleucher, J. et al. (1999) Intramolecular deuterium distributions reveal
disequilibrium of chloroplast phosphoglucose isomerase. Plant Cell Environ. 22, 525–533
11 Aubert, S. et al. (1998) Transport, compartmentation, and metabolism of
homoserine in higher plant cells: carbon-13- and phosphorus-31-nuclear magnetic resonance studies. Plant Physiol. 116, 547–557
12 Edwards, S. et al. (1998) Contribution of malic enzyme, pyruvate kinase,
phosphoenolpyruvate carboxylase, and the Krebs cycle to respiration and biosynthesis and to intracellular pH regulation during hypoxia in maize root tips observed by nuclear magnetic resonance and gas chromatography-mass spectrometry. Plant Physiol. 116, 1073–1081
13 Adam, K-P. and Zapp, J. (1998) Biosynthesis of the isoprene units of
chamomile sesquiterpenes. Phytochemistry 48, 953–959
14 Stryer, L. (1995) Biochemistry, W.H. Freeman
15 Eisenreich, W. et al. (1996) Studies on the biosynthesis of taxol: the taxane
carbon skeleton is not of mevalonoid origin. Proc. Natl. Acad. Sci. U. S. A. 93, 6431–6436
16 Lichtenthaler, H.K. (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 47–65
17 Adam, K.P. et al. (1998) Involvement of the mevalonic acid pathway and the
glyceraldehyde-pyruvate pathway in terpenoid biosynthesis of the liverworts
Ricciocarpos natans and Conocephalum conicum. Arch. Biochem. Biophys.
354, 181–187
18 Dieuaide-Noubhani, M. et al. (1995) Quantification of compartmented
metabolic fluxes in maize root tips using isotope distribution from 13C- or 14
C-labeled glucose. J. Biol. Chem. 270, 13147–13159
19 Dieuaide-Noubhani, M. et al. (1997) Sugar-starvation-induced changes in
carbon metabolism in excised maize root tips. Plant Physiol. 115, 1505–1513
20 Krook, J. et al. (1998) Sucrose and starch metabolism in carrot (Daucus
carota L.) cell suspensions analysed by 13
C-labelling: indications for a cytosol and a plastid-localized oxidative pentose phosphate pathway. J. Exp. Bot. 49, 1917–1924
21 ap Rees, T. (1980) Contributions of metabolic pathways to respiration. In The
Biochemistry of Plants (Vol. 2) (Davies, D.D., ed.), pp. 1–29, Academic Press
22 ap Rees, T. (1988) Hexose phosphate metabolism by nonphotosynthetic
tissues of higher plants. In The Biochemistry of Plants (Vol. 14) (Preiss, J., ed.), pp. 1–33, Academic Press
23 Prabhu, V. et al. (1996) 13
C nuclear magnetic resonance detection of interactions of serine hydroxymethyltransferase with C1-tetrahydrofolate synthase and glycine decarboxylase complex activities in Arabidopsis. Plant
Physiol. 112, 207–216
24 Prabhu, V. et al. (1998) Effects of sulfanilamide and methotrexate on 13
C fluxes through the glycine decarboxylase/serine hydroxymethyltransferase enzyme system in Arabidopsis. Plant Physiol. 116, 137–144
25 Mouillon, J.M. et al. Glycine and serine catabolism in non-photosynthetic
higher plant cells. Their role in C1 metabolism. Plant J. (in press)
26 Cohen-Addad, C. et al. (1997) Structural studies of the glycine decarboxylase
complex from pea leaf mitochondria. Biochimie 79, 637–643
27 Guilhaudis, L. et al. (1999) Investigation of the local structure and dynamics
of the H subunit of the mitochondrial glycine decarboxylase using heteronuclear NMR spectroscopy. Biochemistry 38, 8334–8346
28 Roscher, A. et al. (1998) Unidirectional steady state rates of central
metabolism enzymes measured simultaneously in a living plant tissue. J. Biol.
Chem. 273, 25053–25061
29 Chang, K. and Roberts, J.K.M. (1991) Quantitation of rates of transport,
metabolic fluxes, and cytoplasmic levels of inorganic carbon in maize root tips during potassium ion uptake. Plant Physiol. 99, 291–297
30 Stitt, M. (1998) Pyrophosphate as an energy donor in the cytosol of plant cells:
an enigmatic alternative to ATP. Bot. Acta, 111, 167–175
31 Pugin, A. et al. (1997) Early events induced by the elicitor cryptogein in
tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell 9, 2077–2091
32 Aubert, S. et al. (1999). Subcellular compartmentation of proline in the leaves
of the subantarctic Kerguelen cabbage Pringlea antiscorbutica R. Br. In vivo
13C-NMR study. Plant Cell Environ. 22, 255–259
33 Roberts, J.K.M. et al. (1997) Cooperation and competition between adenylate
kinase, nucleoside diphosphokinase, electron transport, and ATP synthase in plant mitochondria studied by 31
P-nuclear magnetic resonance. Plant Physiol. 113, 191–199
34 Pfeffer, P.E. et al. (1999) Carbon uptake and the metabolism and transport of
lipids in an arbuscular mycorrhiza. Plant Physiol. 120, 587–598
35 Heidenreich, M. et al. (1998) Investigation of carbohydrate metabolism and
transport in castor bean seedlings by cyclic J cross polarization imaging and spectroscopy. J. Magn. Reson. 132, 109–124
36 Kalusche, B. et al. (1999) Sucrose unloading in the hypocotyl of the Ricinus
communis L. seedling measured by 13
C-nuclear magnetic resonance spectroscopy in vivo. Planta 208, 358–364
Justin K.M. Roberts is at the Dept of Biochemistry, University of California, Riverside, CA 92521, USA.