Summary The rate of ethylene production, monoterpene concentration and size of induced lesions were determined following inoculation of stems of slash pine (Pinus elliottii Englm. var. elliottii) and loblolly pine (Pinus taeda L.) with Ophiostoma minus (Hedgc.) H.P. Sydow, O. ips (Rumb.) Nannf or sterile water. In a second experiment, the rate of ethylene production and monoterpene concentration were determined following inoculation of slash pine stems with O. minus, O. ni-grocarpa (Davids.) DeHoog (a nonpathogen) or sterile water. Fungal inoculation significantly increased the rate of ethylene production compared with that of stems inoculated with sterile water. Greater rates of ethylene production were associated with greater fungal virulence. Changes in monoterpene con-centration generally mimicked changes in the rate of ethylene production, i.e., high monoterpene concentrations were asso-ciated with high rates of ethylene production.
Keywords: Ophiostoma, Pinus elliottii, Pinus taeda.
Introduction
The events that determine the success of host resistance to a pathogenic challenge occur soon after the attack (Graham and Graham 1991). Enhanced production of ethylene is an early biochemical event that is often associated with plant--micro-bial interactions (Boller 1991). However, despite many stud-ies, the extent to which ethylene is involved in host resistance remains unclear. In some studies, treatment of host plants with exogenous ethylene, or its direct precursor, prior to infection increases host resistance by either increasing the activity of enzymes involved in the synthesis of phenylpropanoids or increasing the content of hydroxyproline-rich glycoproteins in the cell wall (Stahmann et al. 1966, van Loon 1977, Esquerre-Tugaye et al. 1979, De Laat and van Loon 1982, Geballe and Galston 1982, 1983, Roby et al. 1985). However, in other studies, host defenses are induced even when ethylene biosyn-thesis is inhibited, suggesting that ethylene is a symptom of disease rather than an inducer of defensive responses (Paradies et al. 1980, Boller et al. 1983, Geballe and Galston 1983, Chappell et al. 1984, Mauch et al. 1984, Ke and Saltviet 1989).
In conifers, the development of a monoterpene-soaked le-sion is thought to be a defensive response that inhibits the colonization and spread of Ophiostoma fungi vectored by bark beetles (Scolytidae) (Berryman 1972). Artificial inoculation with different species of Ophiostoma fungi has indicated that the size and monoterpene concentration of lesions increases with increasing fungal virulence (Paine 1984, Cook and Hain 1985, 1986, Paine and Stephen 1987, Owen et al. 1987, Har-rington 1993, Popp et al. 1995), suggesting the presence of a regulatory mechanism. Because 2-chloroethylphosphonic acid, an ethylene releasing compound, is known to increase the concentration of terpenoid compounds in pines (Peters and Roberts 1977, Peters et al. 1978), we postulated that ethylene regulates the production of monoterpenes in pine stem lesions following invasion by bark beetle vectored fungi. Therefore, we designed an experiment to determine if the rate of ethylene production of slash pine (Pinus elliottii Englm. var. elliottii) and loblolly pine (Pinus taeda L.) seedlings infected with Ophiostoma fungi regulates the monoterpene concentration and size of induced lesions.
Materials and methods
Plant material
One-year-old slash pine and loblolly pine seedlings, each from a local, open-pollinated source, were planted in 12-liter pots (two or three seedlings per pot) containing a 3/1 (v/v) mix of sand and peat. Seedlings were placed in a greenhouse and watered 2--3 times weekly, and treated monthly with a com-plete fertilizer containing micronutrients. Seedlings of similar size (0.5 and 0.8 m) and age were used in the two studies.
Inoculation technique
Two consecutive 7-cm sections, starting 4 cm above the root collar, were marked on each seedling. Stem sections were surface sterilized with 70% ethanol, and two wounds to the phloem--xylem interface were made per stem section with a sterile dissecting probe. Each wound was inoculated with a 0.1-ml aliquot of inoculum.
Changes in ethylene production and monoterpene concentration in
slash pine and loblolly pine following inoculation with bark beetle
vectored fungi
MICHAEL P. POPP,
1,2JON D. JOHNSON
1and MARK S. LESNEY
11 Department of Forestry, University of Florida, Gainesville, FL 32611, USA
2
Current address: Department of Ophthalmology, Box 100284 JHMHC, University of Florida, Gainesville, FL 32610, USA
Received September 20, 1993
Inoculation with O. minus and O. ips
Isolates of O. minus (Hedgc.) H.P. Sydow (American Type Culture Collection 15271) and O. ips (Rumb.) Nannf were obtained from the Forest Service Laboratory, Pineville, LA. Cultures were maintained and spores harvested and quantified according to the methods described by Popp et al. (1995).
Each stem wound on the loblolly pine seedlings was inocu-lated with a 0.1-ml aliquot of sterile water, O. minus spores (6.0 × 105 spores ml−1) or O. ips spores (6.0 × 105 spores ml−1). One month later, slash pine seedlings were inoculated with either sterile water, O. minus spores (3.7 × 106 spores ml−1) or O. ips spores (3.7 × 106 spores ml−1).
Inoculation with O. minus and O. nigrocarpa
Isolates of O. minus (C220) and O. nigrocarpa (Davids.) DeHoog (C283) were obtained from Dr. Tom Harrington (De-partment of Plant Pathology, Iowa State University) and sub-cultured in malt broth for 14 days. Cultures were centrifuged using aseptic techniques, and a ≈ 200-mg pellet of each fungal culture was resuspended in 3 ml of sterile water and homoge-nized for 30 s. Each stem wound on the slash pine seedlings was inoculated with a 0.1-ml aliquot of sterile water, O. minus homogenate or O. nigrocarpa homogenate.
Ethylene analysis
On each sample day, inoculated stem sections were excised from three seedlings per treatment, and the sections from each seedling were placed in a 40-ml septum-top vial. After a 30--60 min incubation period, a 1.0-ml sample of the head space was drawn from each vial with a tuberculin syringe and injected into a Hewlett-Packard 5890 gas chromatograph equipped with a packed, l.0-m, activated aluminum column and a flame ionization detector. Chromatography was carried out at 145 °C at a flow rate of 30 cm min−1. Ethylene concentration was determined by comparing the area of the sample peak to the area of a 100 pl ethylene standard. Ethylene production (pl min−1 gDW−1) was determined according to the methods of Stumpff and Johnson (1987).
Monoterpene analysis
Following ethylene analysis, stem sections were cut into smaller pieces and extracted with pentane that had been spiked with a known amount of p-cymene that served as an internal standard (Raffa and Steffeck 1988). Monoterpenes were ana-lyzed as described by Popp et al. (1995). The six major monoterpenes were summed and expressed as mg gDW−1.
Statistical analysis
Natural log transformation of the ethylene and monoterpene data were performed because the variances among treatments within days and within treatments among days were often unequal. The transformed ethylene and monoterpene values within each day were subjected to an analysis of variance.
Studies involving O. minus and O. ips inoculation, in which two seedlings were planted per pot, were treated as a one-way analysis of variance, because the pot-to-pot variation did not differ significantly. All subsequent studies were treated as a
two-way analysis of variance where pots (three seedlings per pot) served as blocks. Mean separations were performed by Duncan’s new multiple range test. All statistical analyses were performed by SAS software systems (SAS Institute Inc., Cary, NC).
Results
Inoculation of slash pine stems with O. minus and O. ips
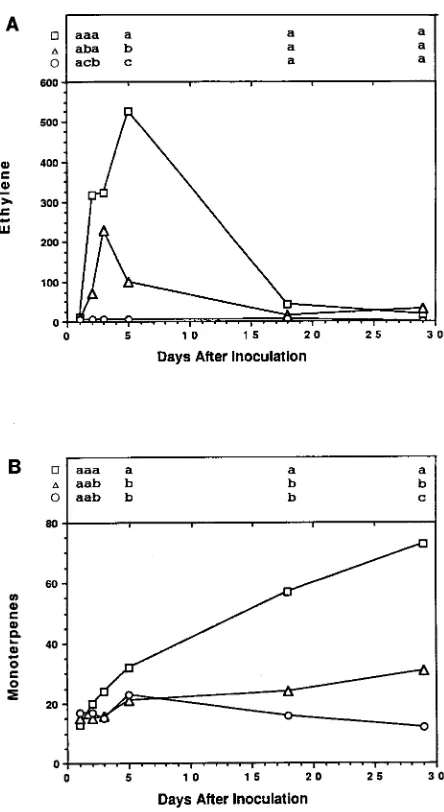
On Day 1, the rates of ethylene production in slash pine stems inoculated with O. minus or O. ips were similar to the rate in stems inoculated with sterile water (Figure 1). Subsequently, the rate of ethylene production in stems inoculated with O.
mi-Figure 1. (A) Mean rate of ethylene production (pl gDW−1 min−1) and (B) mean total monoterpene concentration (mg gDW−1) in stem sec-tions of 2-year-old slash pine seedlings inoculated with sterile water (s), O. minus spores (n) or O. ips spores (h). Each point represents
nus increased significantly to reach a maximum rate of 228.6 pl gDW−1 min−1 on Day 3, which represented a 36-fold increase above the rate of ethylene production in control stems. The rate of ethylene production in stems inoculated with O. ips in-creased more rapidly than in stems inoculated with O. minus and reached a maximum rate of 526.6 pl gDW−1 min−1 on Day 5, which represented a 103-fold increase above the rate in control stems and a 2-fold increase above the maximum rate in stems inoculated with O. minus. After reaching maximum values, rates of ethylene production in the fungal-inoculated stems gradually decreased to the rate in control stems (Figure 1).
The monoterpene concentration in stems inoculated with O. minus gradually increased from 15.2 mg gDW−1 on Day 1 to 30.9 mg gDW−1 on Day 29 (Figure 1). However, there was no significant increase in the monoterpene concentration of stems inoculated with O. minus until Day 29 despite a significant increase in ethylene production on Days 2, 3 and 5 (Figure 1). The monoterpene concentration in stems inoculated with O. ips gradually increased from 13.2 mg gDW−1 on Day 1 to 73.1 mg gDW−1 on Day 29, which represented a 6-fold increase in concentration above that of control stems and a 2-fold increase above that of stems inoculated with O. minus. The stems inoculated with O. ips exhibited a significant increase in monoterpene concentration 1 day after a significant increase in ethylene production was observed. Three days after the rate of ethylene production had become significantly higher in stems inoculated with O. ips than in stems inoculated with O. minus, there was a significant increase in monoterpene concentration in stems inoculated with O. ips compared with stems inocu-lated with O. minus (Figure 1).
Both O. minus and O. ips induced disease symptoms as indicated by their ability to inhibit callus formation (Table 1). However, only O. ips induced lesions that were significantly larger than the lesions induced by sterile water (Table 1).
Inoculation of loblolly pine stems with O. minus and O. ips
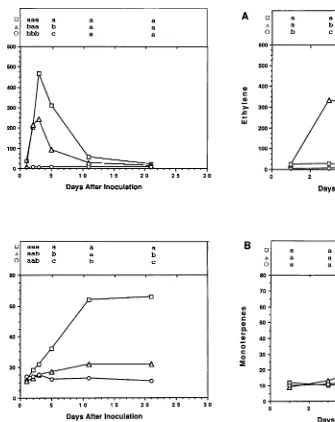
Responses of loblolly pine to bark beetle vectored fungi were similar to those of slash pine. On Day 1, the rates of ethylene production in loblolly pine stems inoculated with O. minus or O. ips were similar to the rate in stems inoculated with sterile
water (Figure 2). Subsequently, the rate of ethylene production in stems inoculated with O. minus increased significantly and reached a maximum of 243.0 pl gDW−1 min−1 on Day 3, which represented an approximate 39-fold increase above the rate in control stems. The rate of ethylene production in stems lated with O. ips increased more rapidly than in stems inocu-lated with O. minus and reached a maximum on Day 3, which represented a 76-fold increase above the maximum rate in control stems and a 2-fold increase above the maximum rate observed in stems inoculated with O. minus. After reaching maximum values, rates of ethylene production in the fungal-inoculated stems gradually decreased to control rates (Fig-ure 2).
Between Days 1 and 21, the monoterpene concentration of stems inoculated with O. minus gradually increased from 10.6 to 21.6 mg gDW−1, which represented an approximate 2-fold increase above the concentration in control stems. The corre-sponding increase in stems inoculated with O. ips was from 10.9 to 65.8 mg gDW−1, which represented an approximate 3-and 6-fold increase in concentration above that in O. minus 3-and control stems, respectively (Figure 2).
Both O. minus and O. ips induced disease symptoms as indicated by their ability to inhibit callus formation and induce lesions that were significantly larger than the lesions induced by sterile water (Table 1). Lesions induced by O. ips were significantly larger than those induced by O. minus.
Inoculation of slash pine stems with O. minus and O. nigrocarpa
On Day 1, rates of ethylene production in stems inoculated with O. minus (22.7 pl gDW−1 min−1) or O. nigrocarpa (24.8 pl gDW−1 min−1) were 10-fold greater than the rate in stems inocu-lated with sterile water (Figure 3). The rate of ethylene produc-tion in stems inoculated with O. nigrocarpa remained more or less constant until Day 3, and then it gradually decreased to control values (Figure 3). In contrast, the rate of ethylene production in stems inoculated with O. minus increased to a maximum on Day 3, which represented an approximate 57-fold increase above that in the control stems (Figure 3), and then gradually declined to control rates (Figure 3).
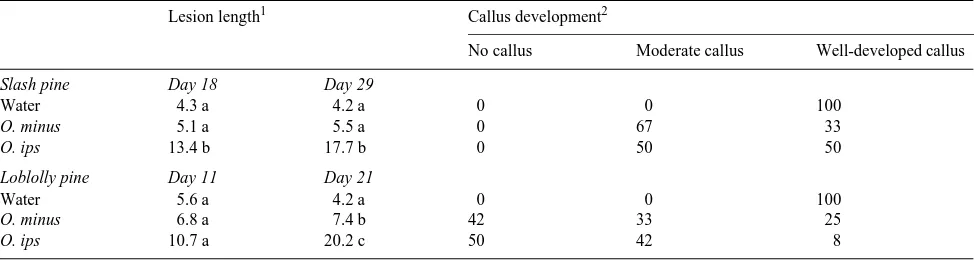
Table 1. Mean lesion length (mm) and percentage of wounds with different amounts of callus tissue around the margins of wounds after inoculating stem sections of 2-year-old slash pine and loblolly pine with sterile water, or spores of O. minus or O. ips.
Lesion length1 Callus development2
No callus Moderate callus Well-developed callus
Slash pine Day 18 Day 29
Water 4.3 a 4.2 a 0 0 100
O. minus 5.1 a 5.5 a 0 67 33
O. ips 13.4 b 17.7 b 0 50 50
Loblolly pine Day 11 Day 21
Water 5.6 a 4.2 a 0 0 100
O. minus 6.8 a 7.4 b 42 33 25
O. ips 10.7 a 20.2 c 50 42 8
The monoterpene concentrations of stems inoculated with sterile water, O. minus or O. nigrocarpa were similar on Days 1, 3 and 5. However, by Day 9, the monoterpene concentration of stems inoculated with O. minus was 2-fold greater than that of stems inoculated with O. nigrocarpa and 3-fold greater than that of stems inoculated with sterile water (Figure 3).
Discussion
Both O. minus and O. ips are mildly pathogenic in slash pine and loblolly pine as indicated by their ability to produce lesions and delay wound healing. A comparison of the O. ips and O. minus pathogens indicated that O. ips was the more
virulent: it induced larger lesions and caused greater inhibition of callus formation than O. minus. Although O. minus induced disease symptoms, e.g., it delayed callus formation in slash pine, this pathogen did not induce larger lesions than those induced by sterile water indicating that lesion size alone may not always be a reliable estimator of host resistance or fungal virulence.
Inoculation of stems with O. ips or O. minus significantly increased the rate of ethylene production compared with that of stems inoculated with sterile water. In both tree species, greater rates of ethylene production were associated with larger lesions, reduced callus formation and higher monoter-pene concentrations.
Figure 2. (A) Mean rate of ethylene production (pl gDW−1 min−1) and (B) mean total monoterpene concentration (mg gDW−1) in stem sec-tions of 2-year-old loblolly pine seedlings inoculated with sterile water (s), O. minus spores (n) or O. ips spores (h). Each point represents the mean of 3--4 seedlings. The same letters within a day indicate no significant difference among means based on Duncan’s new multiple range test (P = 0.05).
Figure 3. (A) Mean rate of ethylene production (pl gDW−1 min−1) and (B) mean total monoterpene concentration (m gDW−1) in stem sections of 18-month-old slash pine seedlings inoculated with sterile water (s), O. minus homogenate (n) or O. nigrocarpa homogenate (h).
Although inoculation of slash pine stems with O. nigro-carpa induced significant changes in ethylene production and monoterpene concentration, the magnitude of the responses was small compared to the responses observed following in-oculation with O. minus. Thus our study confirmed that O. ni-grocarpa is either a nonpathogen or an avirulent pathogen (cf. Paine 1984, Owen et al. 1987, Raffa and Smally 1988, Har-rington 1993, Popp et al. 1995).
In all cases, the rate of ethylene production, monoterpene concentration, lesion size and callus formation were highly correlated, and the increase in monoterpene concentration oc-curred after the rate of ethylene production increased. One interpretation of these results is that recognition of the fungus by the host induces ethylene synthesis, which in turn, induces monoterpene synthesis. Ethylene would, therefore, be acting as a second messenger inducing monoterpene synthesis in advance of the fungus, thus conferring host resistance. An alternative hypothesis is that fungal wall fragments or endo-genous elicitors liberated by the action of fungal-derived pec-tolytic enzymes induce the synthesis of both ethylene and monoterpenes. These responses would then reflect tissue dam-age and differential fungal success rather than host resistance.
Acknowledgments
We thank Drs. Bob Bridges and Tom Harrington for providing fungal isolates, Drs. Dudley Huber, Gary Hodge and Maria Mattiacci for help with statistical analyses, and Janice Osmond for technical support. We also thank Drs. Ken Raffa, Tom Harrington and John Davis for review-ing earlier versions of this manuscript. This is journal series R-04316 of the Institute of Food and Agricultural Sciences, University of Florida, Gainesville. The work was partially supported by U.S. De-partment of Agriculture grant 89-37250-4522 to J.D.J.
References
Berryman, A.A. 1972. Resistance of conifers to invasion by bark beetle fungus associations. BioScience 22:598--601.
Boller, T. 1991. Ethylene in pathogenesis and disease resistance. In The Plant Hormone Ethylene. Eds. A.K. Mattoo and J.C. Suttle. CRC Press, Boca Raton, FL, pp 293--314.
Boller, T., A. Gehri, F. Mauch and U. Vogeli. 1983. Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157:22--31.
Chappell, J., K. Hahlbrock and T. Boller. 1984. Rapid induction of ethylene biosynthesis in cultured parsley cells by fungal elicitor and its relationship to the induction of phenylalanine ammonia-lyase. Planta 161:475--480.
Cook, S.P. and F.P. Hain. 1985. Qualitative examination of the hyper-sensitive response of loblolly pine, Pinus taeda L., inoculated with two fungal associates of the southern pine beetle, Dendroctonus
frontalis Zimmermann (Coleoptera: Scolytidae). Environ. Entomol.
14:396--400.
Cook, S.P. and F.P. Hain. 1986. Defensive mechanisms of loblolly and shortleaf pine against attack by southern pine beetle, Dendroctonus
frontalis Zimmermann, and its fungal associate, Ceratocystis minor
(Hedgecock) Hunt. J. Chem. Ecol. 12:1397--1406.
De Latt, M.M. and L.C. van Loon. 1982. Regulation of ethylene biosynthesis in virus-infected tobacco leaves. II. Time course of levels of intermediates and in vivo conversion rates. Plant Physiol. 69:240--245.
Esquerre-Tugaye, M., C. Lafitte, D. Mazau, A. Toppan and A. Touze. 1979. Cell surfaces in plant--microorganism interactions. II. Evi-dence for the accumulation of hydroxyproline-rich glycoproteins in the cell wall of diseased plants as a defense mechanism. Plant Physiol. 64:320--326.
Geballe, G.T. and A.W. Galston. 1982. Ethylene as an effector of wound-induced resistance to cellulase in oat leaves. Plant Physiol. 70:788--790.
Geballe, G.T. and A.W. Galston. 1983. Wound-induced lignin forma-tion and resistance to cellulase in oat leaves. Phytopathology 73:619--623.
Graham, T.L. and M.Y. Graham. 1991. Cellular coordination of mo-lecular responses in plant disease. MPMI 4:415--422.
Harrington, T.C. 1993. Plant diseases caused by Ophiostoma and
Leptographium. In Ceratocystis and Ophiostoma: Taxonomy,
Ecol-ogy, and Pathology. Eds. M.J. Wingfield, K.A. Seifert and J.F. Webber. APS Press, St. Paul, MN, pp 120--132.
Ke, D. and M.E. Saltviet Jr. 1989. Wound-induced ethylene produc-tion, phenolic metabolism and susceptibility to russet spotting in iceberg lettuce. Physiol. Plant. 76:412--418.
Mauch. F., L.E. Hadwiger and T. Boller. 1984. Ethylene: symptom, not signal for the induction of chitinase and β-1,3-glucanase in pea pods by pathogens and elicitors. Plant Physiol. 76:607--611.
Owen, D.R., K.Q. Lindhal Jr., D.L. Wood and J.R. Parmeter Jr. 1987. Pathogenicity of fungi isolated from Dendroctonus valens, D.
bre-vicomis, and D. ponderosae to pine seedlings. Phytopathology
77:631--636.
Paine, T.D. 1984. Seasonal response of ponderosa pine to inoculation of the mycangial fungi from the western pine beetle. Can. J. Bot. 62:551--555.
Paine, T.D. and F.M. Stephen. 1987. Fungi associated with the south-ern pine beetle: avoidance of the induced response in loblolly pine. Oecologia 74:377--379.
Paradies, I., J.R. Konze, E.F. Elstner and J. Paxton. 1980. Ethylene: indicator but not inducer of phytoalexin synthesis in soybean. Plant Physiol. 66:1106--1109.
Peters, W.J. and D.R. Roberts. 1977. Ethrel bipyridilium synergism in slash pine. In Proc. of the Lightwood Res. Coordinating Counc. Atlantic Beach, FL, pp 78--83.
Peters, W.J., D.R. Roberts and J.W. Munson. 1978. Etherl-diquat-paraquat interaction in lightwood formation. In Proc. of the Light-wood Res. Coordinating Counc. Atlantic Beach, FL, pp 31--39. Popp, M.P., J.D. Johnson and M.S. Lesney. 1995. Response of slash
pine to inoculation with bark beetle vectored fungi. Tree Physiol. 15:619--623.
Raffa, K.F. and E.B. Smally. 1988. Response of red and jack pine to inoculation with microbial associates of the pine engraver, Ips pini (Coleoptera: Scolytidae). Can. J. For. Res. 18:581--586.
Raffa, K.F. and R.J. Steffeck. 1988. Computation of response factors for quantitative analysis of monoterpenes by gas-liquid chromatog-raphy. J. Chem. Ecol. 14:1385--1390.
Roby, D., A. Toppan and M. Esquerre-Tugaye. 1985. Cell surfaces in plant--microorganism interactions. V. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hy-droxyproline-rich glycoproteins in plants. Plant Physiol. 77:700--704.
Stahmann, M.A., B.G. Clare and W. Woodbury. 1966. Increased resis-tance and enzyme activity induced by ethylene and ethylene pro-duction by black rot infected sweet potato tissue. Plant. Physiol. 41:1505--1512.