Permanent or transient chronic ischemic stroke in
the non-human primate: behavioral, neuroimaging,
histological, and immunohistochemical
investigations
Ebeline Bihel
1, Palma Pro-Sistiaga
2, Annelise Letourneur
1, Jerome Toutain
1,
Romaric Saulnier
1, Ricardo Insausti
2, Myriam Bernaudin
1, Simon Roussel
1and Omar Touzani
11CERVOxy team, Hypoxia and cerebrovascular pathophysiology, CI-NAPS UMR-6232, CNRS, CEA,
Universite´ de Caen, Universite´ Paris Descartes, Caen, France;2Human Neuroanatomy Laboratory, Department of Health Sciences and CRIB, School of Medicine, University of Castilla-La Mancha, Albacete, Spain
Using multimodal magnetic resonance imaging (MRI), behavioral, and immunohistochemical analyses, we examined pathological changes at the acute, sub-acute, and chronic stages, induced by permanent or temporary ischemia in the common marmoset. Animals underwent either permanent (pMCAO) or 3-h transient (tMCAO) occlusion of the middle cerebral artery (MCAO) by the intraluminal thread approach. MRI scans were performed at 1 h, 8, and 45 days after MCAO. Sensorimotor deficits were assessed weekly up to 45 days after MCAO. Immunohistological studies were performed to examine neuronal loss, astrogliosis, and neurogenesis. Remote lesions were analyzed using retrograde neuronal tracers. At day 8 (D8), the lesion defined on diffusion tensor imaging (DTI)–MRI and T2-MRI was significantly larger in pMCAO as compared with that in the tMCAO group. At D45, the former still displayed abnormal signals in T2-MRI. Post-mortem analyses revealed widespread neuronal loss and associated astrogliosis to a greater extent in the pMCAO group. Neurogenesis was increased in both groups in the vicinity of the lesion. Disconnections between the caudate and the temporal cortex, and between the parietal cortex and the thalamus, were observed. Sensorimotor impairments were more severe and long-lasting in pMCAO relative to tMCAO. The profile of brain damage and functional deficits seen in the marmoset suggests that this model could be suitable to test therapies against stroke.
Journal of Cerebral Blood Flow & Metabolism (2010) 30, 273–285; doi:10.1038/jcbfm.2009.209; published online 30 September 2009
Keywords: cerebral ischemia; chronic; functional recovery; model; MRI; non-human primate
Introduction
Many recent recommendations for preclinical inves-tigations designed to develop stroke therapies call for the use of higher order species such as non-human primates in addition to rodent models (Fisher et al, 2009). These reviews also advocate the use of multiple-outcome measures (functional deficits, neu-roimaging along with post-mortem neuropathology) both at the acute and the chronic stages of stroke to assess the efficacy of an administered treatment. In this context, we have recently described an original
stroke model in the common marmoset (Freret et al, 2008), a New World monkey, in which middle cere-bral artery (MCA) is occluded temporarily or per-manently through use of an intravascular approach. The common marmoset (Callithrix jacchus) may be considered as the species of choice to study the pathophysiology and the treatment of cerebral ische-mia. Indeed, compared with rodents this primate is closer to human in term of cerebrovascular system, brain metabolism, gray-to-white matter ratio, and rich behavioral repertoire. Weighing 250 to 400 g in the adult age, the marmosets have a brain size app-roximately four times that of the rat, breed readily in standard animal facilities, and are relatively easy to handle, which is advantageous for behavioral testing and postoperative care management (for review, see Mansfield, 2003). The recent success in generating transgenic marmosets has reinforced the value of Received 23 July 2009; revised 2 September 2009; accepted 8
September 2009; published online 30 September 2009
Correspondence: Dr O Touzani, Centre Cyceron, UMR CI-NAPS 6232, Boulevard H Becquerel, BP 5229, Caen F-14074, France. E-mail: [email protected]
using this species in modeling human diseases (Sasakiet al, 2009).
In our recently published model of stroke in the marmoset, brain damage as well as functional impairments was lesser in marmosets subjected to 3-h temporary occlusion relative to those with permanent MCA occlusion (MCAO). Although this study proved the feasibility to induce a focal cerebral ischemia in the non-human primate using the intraluminal approach, the evaluation of damage and behavioral deficits was restricted to the subacute phase (i.e., 8 days after occlusion). Therefore, using sequential magnetic resonance imaging (MRI), beha-vioral tests, histology, and immunohistochemistry, we extended here the characterization of the model and analyzed the evolution of cerebral damage and behavioral impairments in acute, subacute, and chronic stages of ischemia after permanent or temp-orary MCAO. We also examined neuronal loss, astrogliosis, and neurogenesis associated to ische-mia. Through use of neuronal retrograde tracers, we extended the characterization of the model and examined the degree of disconnections of remote brain structures not affected by initial ischemia, namely disconnection between the temporal cortex and the striatum, and between the thalamus and the parietal cortex. Both dorsal striatum and ventral parietal cortex are known to have strong connections with areas related to motor skills learning and of sensory memory consolidation (Knowlton et al, 1996; Wiseet al, 1996).
Materials and methods
All procedures were performed according to European Directives (86/609/EEC) and approved by the Regional Ethics Committee (agreement number 06-003).
Induction of Cerebral Ischemia
Six males and seven female laboratory-bred marmosets (Callithrix jacchus,285 to 370 g; aged 18 to 36 months) were used. The animals were intubated and mechanically venti-lated. Rectal temperature was kept stable with a heating pad. The tail artery and vein were cannulated for arterial pressure monitoring (Stoelting,Wooddale, IL, USA), blood sampling for gases and pH analyses, and for atracurium (0.5 mg/kg; and 0.75 mg/kg/h; Tracrium, Faulding, Royal Leamington, UK) and saline administration, respectively.
The details of the procedure of induction of ischemia have been described previously (Freretet al, 2008). Briefly, a nylon thread was inserted into the external carotid artery and gently advanced up to the origin of the MCA. The embolus was left in place in permanently occluded marmosets (pMCAO,n= 5), removed after 3 h in transiently occluded marmosets (tMCAO n= 6) or immediately in Sham-operated animals (n= 2).
At the end of the experiment, animals received an analgesic (tolfenamic acid, 4 mg/kg, intramuscularly; Tol-fedine 4%; France) and the first dose of an antibiotic
(cefamandole 15 mg/kg, intramuscularly; Kefandol, France; two other doses were administered daily during the 2 next days). After recovery from anesthesia, animals were returned to their cages and provided access to water and soft food, and observed for 1 h and daily.
Magnetic Resonance Imaging
The animals underwent three sequential MRI examinations (7 T; Pharmascan, Bruker, France) at 1 h, 8, and 45 days after MCAO. Each MRI session comprised echo-planar dif-fusion tensor imaging (DTI, 30 difdif-fusion directions;
b= 1,000 and b= 0, echo time (TE)/repetition time (TR) = 41.1/5,000 ms, Matrix M = 128128, field of view (FOV) = 5050 mm, 10 slices, slice thickness = 1.5 mm), time of flight angiography (TE/TR = 1.8/10 ms, M = 256 19296, FOV = 404016.8 mm), T2*-weighted imaging (TE/TR = 11/400 ms, M = 256256, FOV = 5050 mm, 20 slices, thickness = 1.5 mm), and fast T2-weighted imaging (acceleration factor = 8, number of experiments = 4, TE/ TR = 60/5000 ms, M = 256192, FOV = 5050 mm, 20 slices, thickness = 1.5 mm). At day 8 (D8), a magnetiza-tion-prepared rapid acquisition gradient echo (MPRAGE) sequence (TE/TR = 15/6000 ms, M = 256256 FOV = 40 40 mm, 16 slices, thickness = 0.7 mm) was performed to calculate stereotaxic coordinates for neuronal tracer injections.
Behavioral Assessment
The behavioral tests were performed in home cages according to the procedure of Freret et al (2008), with some modifications. The animals were trained on beha-vioral tasks during 1 week (W1) preceding MCAO, evaluated on all tasks daily during the first week, and weekly during 6 weeks by the same experimenter in a blind manner.
Neurological Score
Absence (score = 2), moderate occurrence (score = 1), or presence (score = 0) of the following abnormal movements and postures were noted: forelimbs and hindlimb slipping or dangling under the perch, at rest, or during movement; hand crossing the chest; hindlimbs falling from the perch; head tilting (before and after stimulation); and reaction to a visual stimulus. A maximal score of 20 for each body side was assigned.
Tactile Stimulation
Hill-and-Valley Staircase
Marmosets must find and catch food rewards placed on five steps of two staircases located behind a Plexiglas screen attached to the front of the cage. In the hill version, there are two laterally positioned vertical slots so that the animal uses its right forelimb to retrieve the food reward situated on the right stair, and vice versa. This test, designed to study the motor impairment of each forelimb, does not allow, however, the discrimination between a motor deficit and an ipsilateral spatial deficit, which was observed for some stroke models (Marshall and Ridley, 1996). As compared with the hill version, the valley staircase dissociates the motor performances and the visual field of the marmoset and, thus, prompts to study the spatial exploration. Indeed, in the valley version there is only one centrally positioned slot and the animal uses its left forelimb to reach the right stair, and vice versa. The maximal time to catch all rewards was 300 secs for each version (Annett et al, 1994). A score was assigned for each step: 1 for the lowest step to 5 for the upper step to yield a maximal score of 15 for each forelimb.
Six-Tube Choice
Six black plastic tubes were fixed to a Plexiglas strip, which was placed horizontally at the front of the cage. Marmosets could only reach the tubes if they stood on a small central perch. The animal was required to search for the reward located in one of the six tubes. The time to reach the reward was recorded. Once the reward had been retrieved or 30 secs had passed, the array of tubes was removed and another tube was baited (Marshall et al, 2003b).
Adhesive Removal
An adhesive label was placed around each hindlimb (Annett et al, 1994). The order and latency to contact, and the latency to remove the label, were measured (600 secs maximum).
Neuronal Tracer Injections
At 30 days after the occlusion, one animal of each group was placed in a stereotaxic frame and burr holes were drilled at the intended injection sites. Pressure injections of retrograde neuronal tracers Diamidine Yellow (DY; 2%; Sigma-Aldrich, Madrid, Spain) and Fast Blue (FB; 3%, Polysciences, Eppelheim, Germany) were performed in the head of the caudate (antero-posterior = 11.5 mm; latero-medial = 3.4 mm; dorso-ventral = 5.1 mm from the inter-auricular stereotaxic plan) and the ventral parietal cortex (antero-posterior = 11.5 mm; latero-medial = 10.3 mm; dor-so-ventral = 1 mm), respectively (defined by MRI exam at 8 days with ImageJ software coordinate analysis). Analyses of the retrograde tracers transport were realized by counting positively labeled cells using a charting system (MD3-Digitizer; AccuStage, Shoreview, MN, USA) adapted
to an Olympus BX50 epifluorescence microscope (as described by Mohedano-Morianoet al, 2005).
Histology and Immunohistochemistry
Forty-five days after MCAO, the marmosets were deeply anaesthetized and transcardially perfused with a hepar-inized solution of saline followed by a solution of 4% paraformaldehyde in phosphate buffer. The brains were removed and cut in the coronal plane at 50mm with a freezing microtome (Microm, Heidelberg, Germany). One section out of five was immediately mounted for thionin staining. For immunohistochemistry, adjacent sections were incubated with glial fibrillary acidic protein (GFAP) (rabbit anti-GFAP 1:5,000; Dako, Trappes, France), NeuN (mouse anti-NeuN 1:2,000; Millipore, Chandlers Ford, Hampshire, UK), or tyrosine hydroxylase (TH) antibodies (mouse anti-tyrosine hydroxylase 1:500; Millipore, Chand-lers Ford, Hampshire, UK). Secondary biotinylated rabbit 1:500 (Sigma-Aldrich, France) and biotinylated anti-mouse antibody 1:500 (Sigma-Aldrich, Saint-Quentin-Fallavier, France) were used.
To examine neurogenesis, the marmosets received a daily injection of 5-bromo-2-deoxyuridine (BrdU, intraper-itoneal (50 mg/kg); Sigma-Aldrich, France) from D1 to D18 after MCAO. For double BrdU + NeuN immunostaining, adjacent sections were incubated with BrdU primary antibody (rat anti-BrdU, 1:100; Abcam, Paris, France) and NeuN primary antibody (mouse anti-NeuN 1:2,000; Milli-pore, Chandlers Ford, Hampshire, UK). Secondary anti-bodies were anti-rat Alexa 488 and anti-mouse Alexa 555 (1:200; Invitrogen, Cergy-Pontoise, France). For quantifica-tion of neurogenesis, the zones of interest were photo-graphed and double labeled cells were counted and expressed as a number of double labeled cells per square millimeter.
Data Analysis
To identify areas with an abnormal signal, MR images were thresholded at the mean±twice the standard deviation (s.d.) of the contralateral values (ImageJ).
The data are presented as mean±s.e.m. Scores are
expressed as median±quartile. Mann–Whitney, Wilcoxon,
and Spearman tests were used where appropriate (Statview software). AP< 0.05 was considered significant.
Results
Physiological Parameters
During the entire period of experiment, mean
arterial pressure (pMCAO: 68±3 mm Hg, tMCAO:
70±5 mm Hg, Sham: 75±2 mm Hg), heart rate
(pMCAO: 237±32 min 1; tMCAO: 230±30 min 1,
Sham: 239±79 min 1), and rectal temperature
(pMCAO: 37. 5±0.21C; tMCAO: 37.5±0.21C, Sham:
37.3±0.31C) remained stable within physiological
limits. Values for blood gases and pH were not
different between the groups studied and remained stable (for pMACO, tMACO, and Sham groups,
respectively, arterial CO2 tension was 42.5±
21.1 mm Hg, 42.3±14.2 mm Hg, and 42.4±12.3
mm Hg; arterial O2tension was 176.3±21.4 mm Hg,
174.99±21.4 mm Hg, and 178.2±12.6 mm Hg; and
blood pH was 7.384±0.1, 7.427±0.1, and 7.431±0.1). All the animals terminated the whole experimen-tal protocol and no postoperative morexperimen-tality was observed in our experiments.
Magnetic Resonance Imaging
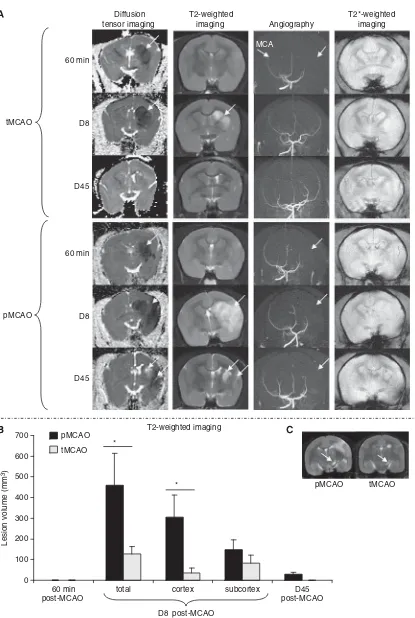
MR angiography showed disappearance of the MCA in pMCAO at all the time points analyzed. Reperfu-sion was also confirmed by this approach in tMCAO (Figure 1A).
At 60 mins after MCAO, a marked decrease in apparent diffusion coefficient (ADC) was observed mainly in the subcortical structures (Figure 1A). The volume of tissue displaying these changes was not different between pMCAO and tMCAO
(208.0±58.2 mm3 and 298.5±67.5 mm3for pMCAO
and tMCAO, respectively; Mann–Whitney test
P= 0.23). At this time, no abnormal signal was
observed on T2-MRI (Figure 1A). At D8, the total volume of lesion and the cortical lesion, measured on either DTI or T2-MRI, were significantly higher in the pMCAO as compared with that in the tMCAO
group (Mann–Whitney test P< 0.05; Figure 1B).
In addition, an abnormal signal was observed in
the substantia nigra at D8 (6.5±2.2 mm3 and
3.3±0.6 mm3for pMCAO and tMCAO, respectively;
Figure 1C). The volume of this lesion was correlated to the volume of abnormal signal in the striatum
(Spearman test, P= 0.0037; R= 0.918).
At D45, the area with an abnormal signal, on either DTI or T2-MRI, decreased remarkably in the pMCAO
group (21.5±16.7 mm3) and completely resolved in
the tMCAO group. However, both groups of animals showed atrophy of the ipsilateral hemisphere
rela-tive to the contralateral one (pMCAO: 4.8±1.7% and
tMCAO: 3.1±1.5%, Wilcoxon test P< 0.05). In all
animals, T2*-MRI did not reveal any abnormal signal during the entire period of examination (Figure 1A), suggesting absence of obvious hemorrhage. For Sham-operated animals, no abnormal signal was observed on all the imaging modalities at any of the time points analyzed.
Behavioral Assessments
Before MCAO, all the animals showed no deficit on behavioral tasks. Sham-operated animals also showed no deficit during the entire period of investigation.
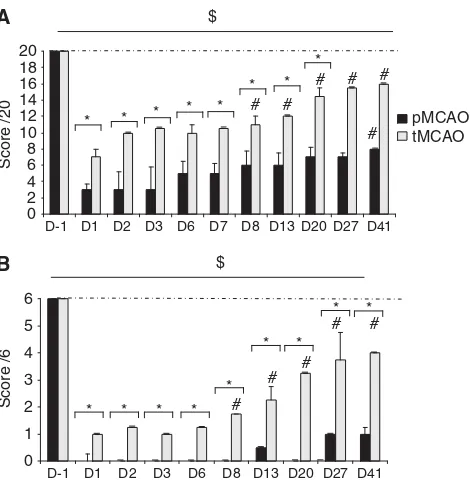
Neurological Score
After induction of ischemia, no animal showed
significant ipsilateral deficit (pMCAO: 18±1;
tMCAO: 19±1 at D1). However, all marmosets
exhibited long-lasting neurological impairment
(pMCAO: score = 2±1 at D1 and 8±0 at D41,
Wilcoxon test P= 0.027 and P= 0.025 and tMCAO:
score = 6±1 at D1 and 16±0 at D41, Wilcoxon test
P= 0.042 and 0.039). This deficit was more
promi-nent and more durable for pMCAO as compared with that for tMCAO (Figure 2A).
Tactile Stimulation
At 1 day after ischemia, a decrease of contralateral tactile perception was observed. Although this deficit was long lasting for both groups (pMCAO
score = 0±1 at D1 and 1±1 at D41, and tMCAO
score = 1±1 at D1 and 4±4 at D41; Wilcoxon test
P< 0.05 as compared with D1), the pMCAO group
presented a stronger deficit as compared with the tMCAO group at all the time points analyzed
(Mann–Whitney test P< 0.05 for comparison
bet-ween pMCAO and tMCAO; Figure 2B). The animals subjected to transient ischemia presented with
partial recovery from D8 (Wilcoxon test P= 0.026 as
compared with D1).
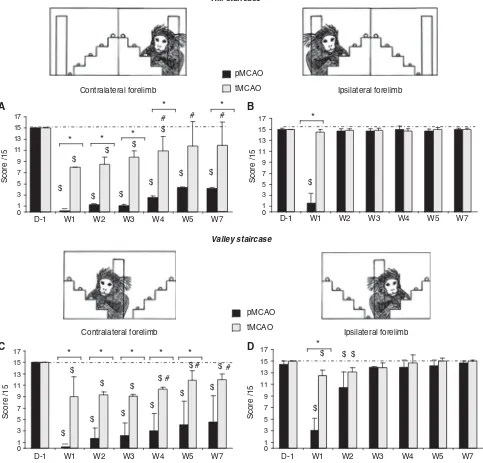
Hill-and-Valley Staircase
Before ischemia, all the marmosets completed the two forms of the staircase test without significant mistake and no difference in performances between the right and left forelimbs was observed. None-theless, 1 week (W1) after ischemia, the animals showed difficulties to catch the reward with the contralateral forelimb (Figure 3B). This deficit was bilateral at W1 for pMCAO group (Figure 3A). Ipsi-lateral deficit totally recovered at W2 and persisted
for the contralateral forelimb (score = 0±0 at W1,
P= 0.001 and score = 4.2±0.8 P= 0.042 at W7
(Wilcoxon test) as compared with D-1).
For the tMCAO group, the deficit was observed
until W5 (score = 7±1 at W1P= 0.02, 11.9±1 at W4
P= 0.04, Wilcoxon test, compared with D1). Indeed,
partial recovery was observed for tMCAO since
W4 (P= 0.04 Wilcoxon test, compared with W1).
This deficit was more severe in the pMCAO group during the time of observation (Mann–Whitney test
P< 0.05 for comparison between pMCAO and
tMCAO; Figure 3B).
As in the hill staircase test, the valley version revealed a contralateral motor deficit, which was more pronounced and durable for the pMCAO group as compared with the tMCAO group (Figure 3C). In addition, the valley staircase revealed a contralateral perception deficit (Figure 3D). Indeed, we observed significant decrease in the ipsilateral forelimb
score (i.e., contralateral space) (score = 10.5±2.6
and 13.1±0.2 at W2, and Wilcoxon test P= 0.04
and P= 0.04 as compared with D1 for pMCAO and
tMCAO pMCAO
0 100 200 300 400 500 600 700
60 min post-MCAO
total
D8 post-MCAO *
Lesion volume (mm
3)
T2-weighted imaging 60 min
D8
D45
60 min
D8
D45 pMCAO
tMCAO
* Diffusion tensor imaging
T2-weighted
imaging Angiography
T2*-weighted imaging
MCA
tMCAO pMCAO
D45 post-MCAO subcortex
cortex
Figure 1 (A) Representative images of ADC maps obtained by DTI, T2-weighted imaging, MR angiography, and T2*-weighted imaging at 60 mins, D8, and D45 after permanent MCAO (pMCAO) and 3-h temporary MCAO (tMCAO) the abnormal signals are indicated by arrows. (B) Evolution of brain damage volume (mean±s.e.m.) measured on T2-MRI images.*P< 0.05 between tMCAO and pMCAO groups. (C) Example of abnormal signal observed in the substantia nigra on T2-weighted imaging at 8 days after MCAO for one animal in each group (arrows indicate the abnormal signal in the substantia nigra).
this deficit recovered at W3 after the induction of ischemia.
Adhesive Removal
After MCAO, a contralateral tactile deficit was measured in both groups (time = 600±0 and 268.6± 92.7 secs, Wilcoxon testP= 0.04 andP= 0.02, respec-tively, for pMCAO and tMCAO), with partial recov-ery from the fourth week (W4) (time = 404.4±220.9 s at W6 and time = 67.3±22.7 s at W4, Wilcoxon test P= 0.04 and P= 0.02 as compared with D1, respec-tively, for pMCAO and tMCAO). This deficit was more severe for pMCAO (Mann–Whitney test, P< 0.05 at W2 until W5).
A bilateral motor coordination deficit was also evidenced. The time spent to remove adhesive labels was increased as compared with pre-operative measurements (W1: time = 600±0 and 466.7± 107.9 secs, respectively, for pMCAO and tMCAO for the contralateral side, and time = 573.8±238.1 and 457.9±111.8 secs, respectively, for pMCAO and tMCAO for the ipsilateral side).
In the ipsilateral side, we observed a partial recovery from W5 for the two groups (pMCAO time = 298.2±149.1 secs and tMCAO time = 194.9± 78.2 secs, Wilcoxon test P< 0.05 as compared with D1) and in the contralateral side from W4 for tMCAO and W5 for pMCAO (pMCAO time = 298.2±149.1
secs and tMCAO time = 194.9±78.2 secs; Wilcoxon testP< 0.05 as compared with W1).
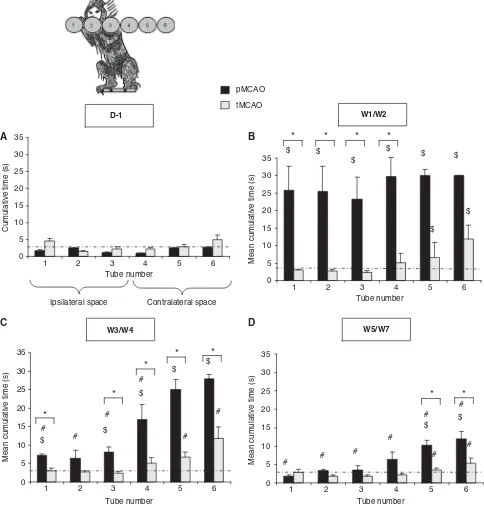
Six Tubes Choice
Ischemia induced a deficit of spatial exploration as the animals spent more time to find the rewards located in the contralateral space (i.e., tubes 4 to 6). As shown in Figure 5B, at 1 week after MCAO, the marmosets subjected to temporary MCAO showed contralateral alteration of visual perception (W1/W2: time = 11.9±3.9 secs for tube 6; Wilcoxon test P= 0.02 as compared with D1), which was transient so as at W3; times to retrieve the rewards were not different as compared with performances before ischemia (Figure 4C and 4D, Wilcoxon test, P= 0.61 as compared with D-1 for tube 6). For animals subjected to permanent MCAO, bilateral visual perception deficit was observed during the first 2 weeks after the ischemia (Figure 4B). However, these animals displayed only a contralateral hemineg-lect, which was long lasting (W5/W7: time = 11.92± 2.08 secs for tube 6; Wilcoxon test P= 0.04 as compared with D-1). When the two groups of animals were compared, the hemineglect, revealed by this test, was more severe for the pMCAO group during W4/W5 and W5/W7 (Mann–Whitney test P< 0.05 for comparison between pMCAO and tMCAO for tubes 5 and 6).
Correlation of the Functional Impairment with the Extent of the Lesion
To determine whether the brain damage observed at 8 days after MCAO was predictive for behavioral deficits, we performed correlation analyses between the behavioral data obtained at the same period and the lesion volume measured by DTI-MRI and T2-MRI. The volume of subcortical damage mea-sured with T2-MRI was correlated with the score of the contralateral forelimb at the valley-and-hill staircase tests 2 weeks after the occlusion (P= 0.049, R = 0.6 and P= 0.02, R = 0.7, respec-tively, for the valley and hill staircase). Similarly, there was a correlation with the deficit observed in the tactile stimulation test at D8 (P= 0.04, R = 0.6) and in the adhesive removal test at W2 (time to contact the contralateral label, P= 0.017, R = 0.68). The volume of cortical damage was correlated with the degree of deficit as measured in the valley staircase (ipsilateral score = contralateral hemispace: P< 0.001, R = 0.95 and contralateral forelimb score P= 0.02, R = 0.67) and the hill staircase tests (contralateral forelimbP= 0.02, R = 0.66) at 2 weeks after occlusion.
The volumes of the lesion determined with DTI-MRI at D8 also correlated with the behavioral data, although the statistical significance was weaker as compared with that obtained with T2-MRI results. 0
Figure 2 Evolution of contralateral neurological score (A) and
tactile stimulation score (B) in the pMCAO (n= 5 black bars) and tMCAO (n= 6, gray bars) groups (median±quartile). *P< 0.05 for difference between groups (Mann–Whitney test); $P< 0.05 in comparison with D1 indicating presence of deficit (Wilcoxon test); and#P< 0.05 in comparison with D1 indicat-ing recovery (Wilcoxon test). Dotted line represents the performances of Sham-operated animals.
Remote Injury
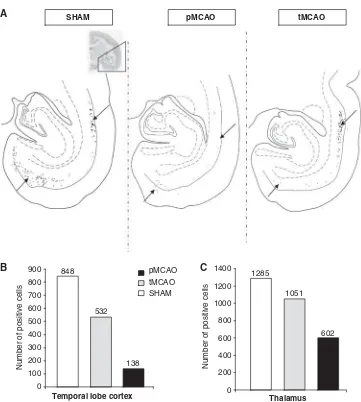
The sites of tracer’s injection were similar in each group. The injection of DY into the caudate nucleus revealed a reduction of labeled neurons in the temporal cortex of occluded animals in comparison with Sham-operated animal (Figure 5A and 5B), the decrease being more pronounced in the pMCAO animal. Similarly, the injection of FB into ventral parietal cortex showed a decrease of labeled neurons in the ipsilateral thalamus of animals subjected to
ischemia as compared with that in Sham-operated animal, without major difference between pMCAO and tMCAO (Figure 5C).
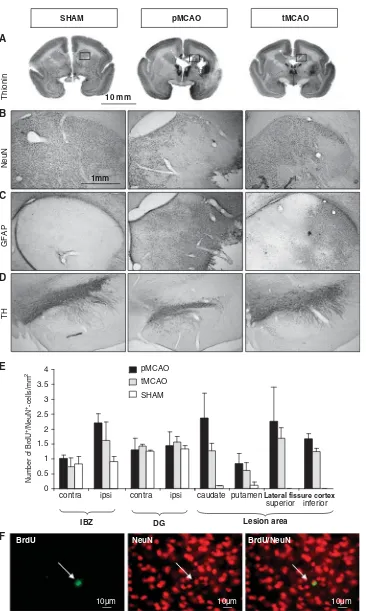
Histology and Immunochemistry
At 45 days after MCAO, thionin staining showed both an increase in staining located in the caudate nucleus and the putamen, and a lack of staining in the cortex at the lateral fissure cortex (Figure 6A).
* *
Figure 3 Evolution of performances in the hill (AandB) and valley (CandD) staircase tasks for the pMCAO (n= 5, black bars) and tMCAO (n= 6, gray bars) groups (median±quartile). (AandC) Scores to retrieve rewards with the contralateral forelimb. (BandD) Scores to retrieve rewards with the ipsilateral forelimb.*P< 0.05 for difference between groups (Mann–Whitney test);$P< 0.05 in comparison with D1 indicating presence of deficit (Wilcoxon test); and #P< 0.05 in comparison with W1 indicating recovery (Wilcoxon test). Dotted line represents the performances of Sham-operated animals.
The increase in thionin coloration corresponded to reactive gliosis as shown by GFAP immunostaining (Figure 6C). Moreover, using a specific neuronal marker, NeuN, widespread neuronal loss in caudate, putamen, parietal, and temporal cortices (Figure 6B) was observed. The use of tyrosine hydroxylase (TH)
immunostaining showed a decrease of labeling in the substantia nigra, confirming the changes seen on MRI at D8 (Figure 6D). All of these changes were more pronounced in permanently occluded marmosets relative to those subjected to transient ischemia.
tMCAO pMCAO
0 5 10 15 20 25 30 35
1 2 3 4 5 6
1 2 3 4 5 6 1 2 3 4 5 6
1 2 3 4 5 6
Cumulative time (s)
Tube number
0 5 10 15 20 25 30
35 $ $ $
$
$
$ $
$
Mean cumulative time (s)
* * * *
Tube number
*
0 5 10 15 20 25 30 35
$ #
# #
$
$ #
$
# $
# * *
*
*
Tube number
0 5 10 15 20 25 30 35
#
# #
#
# #
$ $
# #
* *
Tube number W1/W2
W3/W4 W5/W7
D-1
A B
C D
Mean cumulative time (s) Mean cumulative time (s)
Ipsilateral space Contralateral space
Figure 4 Six-tube search task, illustrated in panelA, with results shown as latency (±s.e.m.) to find food reward hidden in 1 of 6 tubes positioned from marmoset’s far right (tube 1) to their far left (tube 6) by pMCAO (n= 4, black bars) and tMCAO (n= 4, gray bars) marmoset before surgery; (B) mean 1 and 2 weeks after MCAO; (C) mean 3 and 4 weeks after MCAO; and (D) 5 and 7 weeks after MCAO.*P< 0.05 for difference between groups (Mann–Whitney test);$P< 0.05 in comparison with D1 indicating presence of
deficit (Wilcoxon test); and#P< 0.05 in comparison with W1/W2 indicating recovery (Wilcoxon test). Dotted line represents the
BrdU/NeuN labeling revealed in both groups the existence of an ischemia-induced increase in neuro-genesis in the ischemic boundary zone between the subventricular zone and the striatal lesion, as well as in the caudate, putamen, and the cortex, but not in the dentate gyrus (Figure 6E). Although these BrdU/ NeuN labeling were more pronounced for the marmosets subjected to ischemia (Mann–Whitney testP= 0.06 as compared with Sham-operated), there was no difference between temporarily and perma-nently occluded animals.
Discussion
The major finding in this study is that the intralum-inal approach to induce cerebral ischemia in
common marmosets consistently results in cortical and subcortical brain damage, evidenced by MRI, histology, and immunohistochemistry, as well as in long-lasting functional deficits, which are reduced by reperfusion instituted as late as 3 h after the occlusion. This makes this model suitable for testing therapeutic interventions for stroke. The importance of employing non-human primates in the study of the pathophysiology and the treatment of brain ischemia has been highlighted in many recent reviews (Fisher et al, 2009, Gladstone et al, 2002, STAIR, 1999). In this context, although marmosets are not so close phylogenetically to human as compared with old word monkeys (i.e., Cerco-pithecidae), they present several advantages over both rodents and large primates (e.g., macaques and baboon), making them potentially valuable in
Number of positive cells
tMCAO pMCAO
SHAM
Temporal lobe cortex SHAM
A pMCAO tMCAO
0 200 400 600 800 1000 1200 1400
1285
1051
602
Thalamus 0
100 200 300 400 500 600 700 800
900 848
532
138
Number of positive cells
B C
Figure 5 (A) Illustration of cell labeling in the temporal cortex after DY injection in the dorsal caudate nucleus (arrows) in three
marmosets (Sham, pMCAO, and tMCAO). Quantification of retrograde tracer transport from the caudate nucleus to the temporal lobe cortex (B), and from the parietal cortex to the thalamus (C), after DY and FB injections in dorsal caudate nucleus and ventral cortex, respectively. The data are shown as mean number of positive cells per slice labeled.
pMCAO tMCAO SHAM
A
B
C
D
E
F
1mm 10 mm
Number of BrdU
+/NeuN +-cells/mm
2
IBZ
tMCAO pMCAO
SHAM
superior
DG 0
0.5 1 1.5 2 2.5 3 3.5 4
Lesion area
NeuN
GFAP
TH
Thionin
10µm
10µm 10µm
BrdU NeuN BrdU/NeuN
inferior Lateral fissure cortex putamen
contra ipsi contra ipsi caudate
Figure 6 Representative photomicrographs illustrating thionin staining (A) and immunostaining in the head of caudate with NeuN (B) and GFAP (C), and with tyrosine hydroxylase (TH) in the substantia nigra (D) in one animal per group. (E) Quantification of double labeling (NeuN/BrdU-positive cells) is represented as mean (±s.e.m.) of double labeled cells per square millimeter per structure. IBZ, ischemic boundary zone; DG, dentate gyrus (pMCAO (n= 4), tMCAO (n= 4), and Sham (n= 2)). (F) Illustration of BrdU-, NeuN-, and BrdU/NeuN-positive cells in the IBZ (arrows indicate a BrdU/NeuN-positive cell).
modeling stroke and for testing stroke therapies (Freret et al, 2008; Marshall and Ridley (1996);
Marshall et al, 2003a; Virley et al, 2004). We have
recently shown the feasibility to induce focal cerebral ischemia by the intraluminal approach in the common marmoset. This method has several advantages over the previously described models in this species: (1) the strict control of ischemia durations; (2) the absence of craniotomy with subsequent preservation of intracranial pressure and brain temperature; (3) the simplicity of the surgical procedure, which resembles the procedure widely performed in rodents in many laboratories; and (4) the comparative non-invasiveness as severe disability and mortality was not observed in the postoperative period. Overall, this approach allowed us to perform behavioral and neuropathological analyses in the subacute stage of infarction.
The use of multiple MRI modalities is important to understand the evolution of the ischemic lesion. At 1 h after MCAO, DTI-MRI showed reduction of ADC values especially in the subcortical structures. Tissue volumes affected by these changes were similar for both groups of marmosets, reflecting a similar degree of the initial blood flow reduction in those animals. At this time, and in accordance with the literature, T2-MRI did not show any abnormal change since vasogenic edema is not yet established. However, 8 days after MCAO, both T2-MRI and DTI-MRI revealed the presence of abnormal signals that were volumetrically reduced in the tMCAO group, indi-cating that restoration of blood flow as late as 3 h after MCAO could reduce the brain lesion, especially in the cortex. These findings imply that in this model of stroke, the lesion evolves at least during 3 h after the insult, an evolution closer to what is observed for human relative to rodents (Touzani and Baron, 2007). Although the absolute ADC values are comparable between marmosets (455±36 and 756±2510 6mm2/s for the lesion area and the
contralateral hemisphere, respectively), rodents (486±62 and 716±5810 6mm2/s in lesioned and contralateral striatum; Wang et al, 2003), and
humans (436±72 and 742±14410 6mm2/s;
East-wood et al, 2003), the profile of evolution of ADC
abnormalities observed in our study concords more with that reported for humans for which the ADC values were diminished during 10 days after the ictus and reversed thereafter (Eastwood et al, 2003;
Munoz Maniega et al, 2004). In contrast, the acute
reduction of ADC values for rodents normalizes in general at 4 days after the occlusion (Neumann-Haefelinet al, 2000). The diminished ADC observed
at 8 days after MCAO proved that the time course of cerebral damage in the marmoset was closer to human pathology than rodent pathology. This rela-tive slow evolution of the lesion, as compared with that for rodents, could be explained by the existing differences in neuronal and glial densities and white-to-gray matter ratio, and, thus, basal brain metabolism between rats and primates (Kennedy
et al, 1978). In accordance with our previous study
(Freret et al, 2008), T2*-MRI showed absence of
hemorrhage confirming that introduction of the filament did not damage the vessel, and absence of hemorrhagic transformation in the sub-acute and chronic stage. The absence of micro-hemorrhage in this model was also confirmed by histological analyses (Perls coloration) at 8 days after stroke (Freretet al, 2008).
In the chronic stage, both DTI and T2-MRI showed apparent reduction of the lesion, which was more pronounced in tMCAO. This profile of evolution that depends on the duration and severity of the ischemia, has been reported in many studies both of rodents and primates (Esneault et al, 2008;
Wegener et al, 2006, Hirouchi et al, 2007), and
reflects the occurrence of inflammation, glial reac-tion, and reorganization of the affected hemisphere at the chronic stage. This reorganization gives place to shrinkage of the ipsilateral hemisphere as observed in our study. Cavitation formation was observed only in some pMCAO marmosets, which were, however, smaller to that reported by Marshall et al (2003b)
in a model of permanent extraluminal occlusion of the MCA in the marmoset, a model in which almost the entire hemisphere is damaged.
In this study, based on the use of a battery of sensorimotor tests, we have assessed the evolution of functional deficits during 45 days after MCAO. We have demonstrated the presence of hemiparesis, hemianesthesia, and hemineglect in both groups. In general, the contralateral deficits were more severe and long lasting in the marmosets subjected to pMCAO relative to those subjected to tMCAO. The time course of the deficits seen in pMCAO was globally in agreement with that reported by Marshall and Ridley (1996). But the long-term evolution of deficits in tMCAO was not described previously. Here we have shown a long-lasting neurological impairment in this group, although the animals displayed better spontaneous recovery. Nonetheless, we observed a bilateralism of deficits in some tests such as the hill staircase during the first week. This can be attributed to the presence of nystagmus and spontaneous rotations at this time and prevented marmosets to focus on the task. These spontaneous rotations also explain the bilateral deficits shown in pMCAO in the six tubes task. In the adhesive removal task, we also observed an increase of time to remove the adhesive from the ipsilateral hind limb, which can be explained by the presence of contralateral hemiparesis, which makes it difficult for the animal to use its affected limbs to remove the adhesive label in the non-affected ones. In general, the functional deficits seen in this model both in the acute and the chronic stages of ischemia were in accordance with the situation observed in man (Feigin et al, 2003; Varonaet al, 2004).
Post-mortem analyses confirmed the reorganiza-tion of the ipsilateral hemisphere. Indeed, thionin staining revealed the presence of cavitation in some
animals in the pMCAO group and cellular loss especially in the parieto-temporal cortex around the lateral fissure. In both groups, and in contrast to what we observed at 8 days after MCAO (Freret et al, 2008), thionin staining also elicited an increase of signal in the striatum and the frontoparietal cortex, reflecting the high cellular density that corresponds to reactive gliosis. The latter was indeed confirmed by GFAP staining that closely matched the area with increased thionin labeling. The use of a specific neuronal marker, NeuN, revealed neuronal loss in the striatum and the cortex, which was more prominent in pMCAO relative to tMCAO.
Additionally, TH labeling revealed neuronal loss located in the substantia nigra, which confirmed the MRI observations at 8 days. This substantia nigra lesion has been described previously in rat and macaque after chronic stroke (Abe et al, 2003; Hirouchiet al, 2007), and described retrograde degeneration of nigro-striatal neurons. In our study, we found also an increase in GFAP labeling, confirming a glial reaction associated to the substantia nigra lesion, but the significance of this remote lesion in the evolution of ischemia-induced deficit is not established.
The analysis of double NeuN + BrdU immunostain-ing allowed the quantification of the neurogenesis in our model. The effect of age on neurogenesis in the marmoset’s brain was previously described by Leuner et al(2007), but this phenomenon has never been described under pathological conditions. It is widely accepted that ischemia increased neurogen-esis, and that new neurons migrate from neurogenic regions (subventricular zone) to the infarct area (for review, see Zhang et al, 2008). In this study, we observed, for the first time, increase of neurogenesis in marmosets after stroke as reported for rodents. This increase was observed in caudate and putamen nuclei, and in the lateral fissure cortex and ischemic boundary zone, but not in the hippocampus. This observation is of interest since this model of stroke could also be employed in studies aiming to test therapeutic strategies designed to increase neurogen-esis. Overall, these data emphasize the importance of using different markers of cellular loss and cellular proliferation to evaluate the amplitude and the type of tissue changes during the maturation of the ischemic lesion.
Although remote injuries should not be claimed based solely on the neuronal tracer results, we showed that neuronal disconnections between re-mote brain structures are not affected by the initial ischemia and the lesion areas. The use of FB-retrograde neuronal tracer allowed us to show an alteration of neuronal thalamic projections to the ventral parietal cortex, a zone perfused by the MCA and then affected by the ischemia as suggested for humans (Nagasawa et al, 1994). These data are in agreement with studies that described delayed atrophy of the thalamus after MCAO in rodents and humans, although in these studies no tracer injec-tions where realized to confirm this disconnection
(Freretet al, 2006; Nakaneet al, 2002; Stebbinset al, 2008). Similarly, DY-retrograde neuronal tracer revealed temporal cortex projections to caudate nucleus as previously described in another healthy non-human primate study (Selemon and Goldman-Rakic, 1988). These projections were markedly altered in animals subjected to MCAO, suggesting retrograde degeneration after the lesion of the cau-date (Nagasawa et al, 1994). Of note, this ischemia-induced alteration of these projections did not result in any changes in MRI signal (both DTI and T2-MRI). This suggests that the use of the neuronal tracer approach would be more sensitive in depicting dis-connections than MRI alone. Although these results are preliminaries analyses, they evidence the pre-sence of disconnections between structures in this marmoset stroke model. The significance of these disconnections for functional deficits is not comple-tely known. Secondary thalamic and temporal cortex atrophies could underlie, at least in part, sensor-imotor and memory disturbances seen in patients.
In conclusion, through use of multiple analyses with MRI, behavioral tests, histology, and immuno-chemistry, we show that focal stroke in the marmo-set, induced by intraluminal occlusion of the MCA, results in widespread brain damage and long-lasting functional deficits that are reduced by reperfusion. This makes this model suitable to test new therapies against stroke.
Acknowledgements
This study was supported by the French Centre National de la Recherche Scientifique (CNRS) and the french conseil re´gional de Basse-Normandie. The Ministry of Foreign Affairs (Spain) for the Picasso project is also acknowledged.
Conflict of interest
The authors declare no conflict of interest.
References
Abe O, Nakane M, Aoki S, Hayashi N, Masumoto T, Kunimatsu A, Mori H, Tamura A, Ohtomo K (2003) MR imaging of postischemic neuronal death in the sub-stantia nigra and thalamus followingmiddle cerebral artery occlusion in rats.NMR Biomed16:152–9 Annett LE, Martel FL, Rogers DC, Ridley RM, Baker HF,
Dunnett SB (1994) Behavioral assessment of the effects of embryonic nigral grafts in marmosets with unilateral 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol125:228–46
Eastwood JD, Engelter ST, Delong DM, Provenzale JM (2003) Quantitative assessment of the time course of infarct signal intensity on diffusion-weighted images. Am J Neuroradiol24:680–7
Bernaudin M (2008) Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral
ischemia in rats.J Cereb Blood Flow Metab28:1552–63
Feigin VL, Lawes CM, Bennett DA, Anderson CS (2003) Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in
the late 20th century.Lancet Neurol2:43–53
Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH (2009) For the STAIR Group Update of the Stroke Therapy Academic Industry Roundtable
Preclinical Recommendations.Stroke40:2244–50
Freret T, Bouet V, Toutain J, Saulnier R, Pro-Sistiaga P, Bihel E, Mackenzie ET, Roussel S, Schumann-Bard P, Touzani O (2008) Intraluminal thread model of focal
stroke in the non-human primate. J Cereb Blood Flow
Metab28:786–96
Freret T, Chazalviel L, Roussel S, Bernaudin M, Schumann-Bard P, Boulouard M (2006) functional outcome follo-wingtransient middle cerebral artery occlusion in the rat: correlation between brain damage and behavioral
impairment.Behav Neurosci120:1285–98
Gladstone DJ, Black SE, Hakim AM, Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery (2002) Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic
directions.Stroke33:2123–36
Hirouchi Y, Suzuki E, Mitsuoka C, Jin H, Kitajima S, Kohjimoto Y, Enomoto M, Kugino K (2007) Neuroima-ging and histopathological evaluation of delayed neu-rological damage produced by artificial occlusion of the middle cerebral artery in Cynomolgus monkeys: estab-lishment of a monkey model for delayed cerebral
ischemia.Exp Toxicol Pathol59:9–16
Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L (1978) Local cerebral glucose utilization in the
normal-conscious macaque monkey.Ann Neurol4:293–301
Leuner B, Kozorovitskiy Y, Gross CG, Gould E (2007) Diminished adult neurogenesis in the marmoset brain
precedes old age.Proc Natl Acad Sci USA104:17169–73
Knowlton BJ, Mangels JA, Squire LR (1996) A neostriatal
habit learning system in humans.Science273:1399–402
Mansfield K (2003) Marmoset models commonly used in
biomedical research.Comp Med53:383–92
Marshall JW, Cummings RM, Bowes LJ, Ridley RM, Green AR (2003a) Functional and histological evidence for the protective effect of NXY-059 in a primate model
of stroke when given4 hours after occlusion. Stroke
34:2228–33
Marshall JW, Ridley RM (1996) Assessment of functional impairment followingpermanent middle cerebral artery
occlusion in a non-human primate species.
Neurode-generation5:275–86
Marshall JW, Ridley RM, Baker HF, Hall LD, Carpenter TA, Wood NI (2003b) Serial MRI, functional recovery, and long-terminfarct maturation in a non-human primate
model of stroke.Brain Res Bull61:577–85
Munoz Maniega S, Bastin ME, Armitage PA, Farrall AJ, Carpenter TK, Hand PJ, Cvoro V, Rivers CS, Wardlaw JM (2004) Temporal evolution of water diffusion param-eters is different in grey and white matter in human
ischae-mic stroke.J Neurol Neurosurg Psychiatry75:1714–8
Mohedano-Moriano A, Martinez-Marcos A, Mun˜oz M, Arroyo-Jimenez MM, Marcos P, Artacho-Pe´rula E, Blaizot X, Insausti R (2005) Reciprocal connections between olfactory structures and the cortex of the rostral
superior temporal sulcus in the Macaca fascicularis
monkey.Eur J Neurosci22:2503–18
Nagasawa H, Kogure K, Itoh M, Ido T (1994) Multi-focal metabolic disturbances in human brain after cerebral infarction studied with 18FDG and positron emission
tomography.Neuroreport5:961–4
Nakane M, Tamura A, Sasaki Y, Teraoka A (2002) MRI of secondary changes in the thalamus followinga cerebral
infarct.Neuroradiology44:915–20
Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Moseley ME (2000) Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood–brain barrier damage, and edema
formation.Stroke31:1965–72
Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T (2009) Generation of transgenic
non-human primates with germline transmission.
Nature459:523–7
Selemon LD, Goldman-Rakic PS (1988) Common
cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the Rhesus monkey: evidence for a distributed neural network
subserving spatially guided behavior. J Neurosci
8:4049–68
Stebbins GT, Nyenhuis DL, Wang C, Cox JL, Freels S, Bangen K, deToledo-Morrell L, Sripathirathan K, Mose-ley M, Turner DA, Gabrieli JD, Gorelick PB (2008) Gray matter atrophy in patients with ischemic stroke with
cognitive impairment.Stroke39:785–93
Stroke Therapy Academic Industry Roundtable (STAIR) (1999) Recommendations for Standards Regarding Pre-clinical Neuroprotective and Restorative Drug
Develop-ment.Stroke30:2752–8
Touzani O, Baron JC (2007) Duration and threholds of the
ischemic penumbra in different species. In: The
Ischemic Penumbra (Donnan G, Baron JC, Davis S, Sharp F, eds), New York, NY, USA, Informa Healthcare Inc. pp 37–57
Varona JF, Bermejo F, Guerra JM, Molina JA (2004) prognosis of ischemic stroke in young adults. Study of
272 cases.J Neurol251:1507–14
Virley D, Hadingham SJ, Roberts JC, Farnfield B, Elliott H, Whelan G, Golder J, David C, Parsons AA, Hunter AJ (2004) A new primate model of focal stroke: endothelin-1-induced middle cerebral artery occlusion and
reperfu-sion in the common marmoset. J Cereb Blood Flow
Metab24:24–41
Wang L, Yushmanov VE, Liachenko SM, Tang P, Hamilton RL, Xu Y (2003) Late reversal of cerebral perfusion and water diffusion after transient focal ischemia in rats.
J Cereb Blood Flow Metab22:253–61
Wegener S, Weber R, Ramos-Cabrer P, Sprenger C, Wiedermann D, Villringer A, Hoehn M (2006) Temporal profile of T2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient
focal cerebral ischemia in the rat. J Cereb Blood Flow
Metab26:38–47
Wise SP, Murray EA, Gerfen CR (1996) The frontal
cortex-basal ganglia system in primates. Crit Rev Neurobiol
10:317–56
Zhang RL, Zhang ZG, Chopp M (2008) Ischemic stroke and
neurogenesis in the subventricular zone.
Neuropharma-cology55:345–52