n engl j med 351;23 www.nejm.org december 2, 2004 The new england journal of medicine
2417
review article
medical progress
Contagious Acute Gastrointestinal
Infections
Daniel M. Musher, M.D., and Benjamin L. Musher, M.D.
From the Medical Service, Infectious Dis-ease Section, Michael E. DeBakey Veterans Affairs Medical Center, and the Depart-ments of Medicine and Molecular Virology and Microbiology, Baylor College of Medi-cine — both in Houston (D.M.M.); and the Department of Medicine, University of Pennsylvania School of Medicine, Philadel-phia (B.L.M.). Address reprint requests to Dr. Daniel Musher at the Infectious Disease Section, Veterans Affairs Medical Center, Houston, TX 77030, or at daniel.musher@ med.va.gov.
N Engl J Med 2004;351:2417-27.
Copyright © 2004 Massachusetts Medical Society. n our ever-shrinking world, widespread media coverage of
in-fections, ranging from the severe acute respiratory syndrome (also known as SARS) and influenza in Asia to acute gastroenteritis on cruise ships and outbreaks in day-care centers in the United States, has raised public interest in contagious diseas-es to new heights. Our purpose in this article is to examine contagion (from the Latin, tangere, to touch) — direct human-to-human spread — of acute gastrointestinal illness, defined as a syndrome of vomiting, diarrhea, or both, that begins abruptly in otherwise healthy persons and is most often self-limited.
Unlike agents that cause contagious respiratory infections,1 which are largely or
ex-clusively indigenous to humans, agents that cause acute gastrointestinal illness (Table 1) may spread from person to person or may be acquired from a common food or envi-ronmental source, often water; they may also result from exposure to animals. Food or water may serve as a primary source of contagion or may, in turn, have been contami-nated by contact with an infected person or animal. Thus, the epidemiology of acute gastrointestinal illness is complex.
Different ways of gathering, analyzing, and presenting data have generated very dif-ferent estimates of the frequency of acute gastrointestinal illness, leading to seemingly contradictory results. Estimates based on extrapolation from isolation of known diar-rheal pathogens and the numbers of stool samples submitted for study suggest that there might be 38 million cases of acute gastrointestinal illness each year.2 In contrast,
a carefully conducted questionnaire survey asking about acute, self-limited illness characterized by vomiting, diarrhea, or both found that about 1.05 cases occur per per-son per year in the United States.2,3 When this number was reduced by 25 percent on
the basis of estimates that a respiratory infection is the responsible agent in about one quarter of persons with symptoms of acute gastrointestinal illness, the resulting 0.79 case per person per year translated to 211 million cases of acute gastrointestinal illness nationally in 1997, the year for which data were available.
Earlier data from the United States and questionnaire-based studies in the Nether-lands and the United Kingdom yielded similar results.3 On the basis of reports to
pub-lic health authorities and an exchange of information between the Centers for Disease Control and Prevention and a network of participating laboratories (FoodNet),2,3 there
are thought to be about 76 million cases per year of foodborne infection. If this number and an additional 13 million cases of waterborne illness are subtracted,3 there may well
be 122 million cases of acute gastrointestinal illness each year in the United States for which human-to-human transmission is responsible. As noted above, a varying pro-portion of foodborne and waterborne outbreaks are also ultimately attributable to hu-man contamination.
i
The new england journal of medicine
salmonella
Because many principles of contagion with respect to enteric organisms were elucidated in studies of typhoid fever, it seems appropriate to begin our dis-cussion of causes of acute gastrointestinal illness with Salmonella typhosa (S. enterica serotype typhi). Al-though physicians do not always associate this or-ganism with a typical syndrome of acute gastroin-testinal illness,4 some studies suggest that diarrhea
predominates in the majority of cases.5,6S. typhi is
highly adapted to humans. Infection is virtually al-ways acquired by transmission from one person to another; an inviolable rule of epidemiology is that the occurrence of a case of typhoid fever implies an epidemiologic link to another person who either is actively infected or is chronically carrying the organ-ism and shedding it in feces. When cases result from food ingestion, individual food handlers, such as the infamous cook known as Typhoid Mary,7 are
usually found to be responsible. An infection from drinking contaminated water can also usually be traced to one or more infected persons whose ex-creta have entered the water supply.8-10
The current rarity of typhoid fever in the United States reflects good hygiene, lack of crowding, and high public health standards for home and indus-trial sewage. During the late 1990s, a breakdown of the public health infrastructure in the former Sovi-et Union led to a cessation of chlorination, the pi-rating of water lines with the use of substandard pipe fittings, and the crossing of these fittings by sewage lines, which culminated in an outbreak of 10,000 cases of typhoid fever.11
The likelihood of direct contagion depends on the number of organisms in feces or contaminated foods, their ability to survive, replicate, or both, and the infectivity of the species and the specific strain. Chronic carriers of S. typhi have 106 to 109
colony-forming units (CFU) per gram12 or more13 in their
feces. In experimental studies, ingestion of 103 CFU
of the Quailes strain of S. typhi was not infectious in volunteers, whereas nearly 50 percent of volunteers were infected by ingesting 105 or 107 CFU, and 96
percent were infected by ingesting 108 or more
CFU.14,15 The results of these experimental studies
indicate that a large inoculum is infective. However, infection in the real world will depend on the infec-tivity of the strain studied. In nature, such strains are almost certainly heterogeneous, as has been shown for other enteric16 and for respiratory1 pathogens.
The early implications of the watchwords “fingers, food, and flies,” and the frequent spread from pa-tients to nurses and physicians in the era before an-tibiotics,8 are consistent, at least in some instances
of natural infection, with low-inoculum contagion, under the assumption that large numbers of organ-isms would not be transmitted in these situations.
Infections with most other types of salmonella, except for S. paratyphi, derive from environmental sources, principally poultry and livestock. Despite the frequency with which these organisms cause acute gastrointestinal illness, there are remarkably few documented examples of person-to-person spread.17-19 An outbreak in a day-care facility was
associated with an uncertain number of secondary cases,19 and long-term surveillance of 54
perma-nent carriers of nontyphoidal salmonella identified 10 instances of transmitted infection.20 On the
ba-sis of epidemiologic studies, the infective dose of nontyphoidal salmonella is thought to be small, not exceeding 100 CFU.21,22 The paucity of
docu-mented instances of contagion may reflect the dif-ficulty of distinguishing person-to-person spread from that due to a common food source, rather than the true absence of human transmission.
shigella
Like S. typhi, shigella has no reservoir in nature and spreads from person to person (usually child to b a c t e r i a l c a u s e s
* These organisms commonly cause acute gastrointesti-nal infection in otherwise healthy children and adults in developed countries. The frequency of infection is simi-lar among such countries — for example, the United States, United Kingdom, France, and Argentina. † Symptomatic disease usually occurs only in infants or
very young children.
Table 1. Agents That Commonly Cause Acute Gastrointestinal Illness.*
Bacteria Salmonella Shigella Campylobacter Escherichia coli O157:H7 Clostridium difficile Viruses
Caliciviruses (Norwalk-like and related viruses) Rotavirus†
Adenovirus types 40 and 41 Astrovirus
n engl j med 351;23 www.nejm.org december 2, 2004 medical progress
2419 child) after direct contact or the ingestion of
con-taminated food. Shigellosis is highly contagious; as few as 200 CFU can cause infection,23 and the role
of a small inoculum is supported by early observa-tions, which emphasized spread by casual contact and insect vectors.24 The high level of
contagious-ness of shigellosis may be inferred from the large number of secondary cases that follow a document-ed outbreak; persons who have varying degrees of contact with infected patients are likely, themselves, to become infected.25 A very young child is the
usu-al source.26
Not surprisingly, shigella readily spreads within families,24 in custodial institutions,27 and within
and among children’s day-care centers.28,29
Day-care centers provide remarkable natural settings in which contagion in acute gastrointestinal illness can be studied28 (Table 2). In these settings, shigellosis
(Tables 1and2) may affect from one third to two thirds of children,30 with severe diarrhea increasing
the likelihood of contagion, reflecting high fecal counts of bacteria and increased chances of soil-ing.26 At least one additional case of shigellosis is
recognized in the families of about 25 percent of infected children.28 The current widespread use of
medications that reduce gastric acidity (which nor-mally eradicates salmonella and shigella) probably increases the risk of spread14 to parents of infected
children or to adults who work in day-care centers.
campylobacter
The epidemiology of infection due to campylobac-ter, perhaps now the most common bacterial cause of acute gastrointestinal illness,31,32 is similar to
that of nontyphoidal salmonella. Most infections are traced to poultry, meat, dairy products, or con-taminated water.33 Although fewer than 1000 CFU
may cause infection,16 massive foodborne
out-breaks are not often recognized, in part because this organism does not replicate in food34 and in part
because ingestion even of large numbers of organ-isms may cause symptoms in only a small propor-tion of subjects.16 Contagion within the home has
been described occasionally,35-37 and in one
house-hold, an infant was infected with the same strain that caused diarrhea in a newly acquired puppy.38 As
with nontyphoidal salmonella, the paucity of de-scriptions of human-to-human spread may reflect the difficulty of studying organisms that are present in so many food sources. Infection by campylobac-ter,39 as well as by S. typhi40 and shigella,41 has been
shown to be contagious among homosexual men.
escherichia coli o157:h7
Transmission of Escherichia coli O157:H7 occurs pri-marily through the consumption of contaminated meat, but secondary infection does occur, and a small bacterial inoculum may lead to clinical illness. For example, of 501 cases of E. coli–related diarrhea linked to hamburger consumption in an epidemic that occurred during 1992 and 1993, 48 infections (about 10 percent) were secondary.42
Person-to-person transmission occurs in day-care centers,43
among families,44 and in mental institutions45; an
attack rate — the rate of appearance of symptoms in exposed persons — of around 20 percent has been reported. It is worth noting that this form of acute gastrointestinal infection is associated with a substantial incidence of the hemolytic–uremic syn-drome, affecting up to 13 percent of young chil-dren with the infection.46
clostridium difficile
Clostridium difficile is a major cause of nosocomial colitis, generally occurring after antibiotic-induced alterations of bowel flora.47 Although the disease in
some persons results from the proliferation of an endogenous strain, infection is clearly contagious; in hospitals, both human vectors and environmen-tal contamination are implicated in the spread.48
In day-care settings, an infection in one child may be followed by the spread of C. difficile to 50 percent of the classmates, in nearly all of whom diarrhea then develops49; contagion is greatly facilitated by
the ingestion of antibiotics. Caregivers may acquire C. difficile colitis while caring for patients who have this disease. We treated an elderly woman for acute C. difficile colitis; she had been caring for her hus-band during his bout of C. difficile colitis, and she had not been taking antibiotics. Her stools contained * These factors are discussed in Pickering et al.30
Table 2. Factors That May Contribute to Contagion of Acute Gastrointestinal Illnesses within Day-Care Centers.*
Presence of one or more cases of acute gastrointestinal illness
Lack of gloving or handwashing during or after diaper changes or helping at toilet
Lack of policy to isolate or send home children with acute gastrointestinal illness
Common area for diaper changing Larger groups of children Carpeted flooring
Shared toys and classroom objects
The new england journal of medicine
C. difficile toxin, and she responded to treatment with metronidazole (unpublished data).
At least since the end of the Second World War, in developed countries, viruses have been thought to cause the vast majority of cases of acute gastroin-testinal illness, whether sporadic or part of an out-break. In the 1950s, a definitive family study by Dingle et al.50 found no isolates of salmonella or
shigella in 77 cases of acute gastrointestinal illness; these investigators concluded that most cases were due to viruses, although, at the time, they were un-able to isolate them. At that time, techniques were not available to identify campylobacter or E. coli O157:H7. The relative infrequency of bacterial acute gastrointestinal illness in developed coun-tries was confirmed by prospective studies that iden-tified salmonella, shigella, campylobacter, and E. coli O157:H7 each in 2 percent or less of fecal samples32;
these numbers have steadily declined in the past several years.51 In contrast, in underdeveloped
na-tions, one of the aforementioned bacteria, vibrio, enteropathogenic E. coli, protozoa, or intestinal par-asites cause the majority of cases of acute gastroin-testinal illness.
In the United States, the United Kingdom, north-ern Europe, and Japan, caliciviruses such as the Norwalk and Sapporo viruses are the most common cause of sporadic acute gastrointestinal illness in patients of all age groups except infants and tod-dlers, in whom rotaviruses predominate.52-54
Ade-novirus types 40 and 4155,56 and astroviruses57-59
have also been implicated. Caliciviruses and astrovi-ruses are more prevalent among outpatients, where-as rotavirus is a common cause of hospitalization.60
Features of contagion by these agents are summa-rized in Table 3.
Within families, acute gastrointestinal illnesses are spread chiefly by young children, whose hygiene is not as consistently good as that of adults and who are dependent on, and therefore in intimate contact with, their parents and caregivers.50 As shown by
Dingle et al.,50 20 percent of persons have
sympto-matic infection after exposure to a family member with acute gastrointestinal illness. The likelihood of secondary infection increases from 10 percent when symptoms are mild to 30 percent if severe vomiting and diarrhea are present, reflecting increased vol-umes of infective excreta that presumably contain higher concentrations of infective particles.
caliciviruses
Calciviruses, of which Norwalk-like viruses are the prototype, cause more than 90 percent of outbreaks of acute gastrointestinal illness in the United States and account for about 23 million cases of diarrheal disease per year, according to the pathogen-associ-ated method of calculation.2,61 As already noted, if
the same percentages are applied to cases of acute gastrointestinal illness identified by questionnaire, the incidence of calicivirus-induced infection is far greater; there may be a total of 74 million cases each year in the United States. Outbreaks have been reported in nursing homes and on military bases and school campuses, but Norwalk-like viruses on cruise ships have made national headlines in the past few years.62,63 Attack rates have been as high
as 41 percent, reflecting the propensity of infection with Norwalk-like viruses to cause emesis and vo-luminous stools, the large number of organisms in stools and vomitus, and the low inoculum (fewer than 100 viral particles) required to produce infec-tion. The extent of spread in such closed environ-ments may involve nearly 100 percent of exposed persons, since experimental ingestion of infectious material causes symptoms in only 50 to 80 percent of subjects.64,65
Although consumption of contaminated food or water causes large outbreaks of infection with Norwalk-like virus, the importance of person-to-person transmission has been recognized since the initial identification of this organism in an outbreak that affected one third of family members and 50 percent of school contacts.66 Well-documented
sec-ondary outbreaks62 indicate person-to-person,
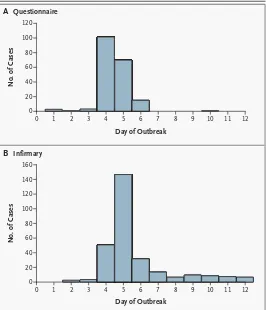
rath-er than foodborne, transmission. For example, in a hyperacute outbreak67 traced to a food handler in a
college dining hall, about 20 percent of all cases occurred after the dining hall was closed and were therefore thought to reflect secondary person-to-person spread (Fig. 1). In a large community out-break in Sweden, secondary cases appeared in one third of the households in which a case occurred.68
Contagion by Norwalk-like viruses has been docu-mented in other circumstances as well. When Brit-ish soldiers with acute gastrointestinal illness were airlifted out of a combat zone, two flight medics and one hospital staff member subsequently became ill; fecal samples from both the patients and the medi-cal personnel contained Norwalk-like viruses.69 In
n
en
gl
j
m
ed
351;23
www.nejm.org
december
2, 2004
medical progress
2421
* This table is subject to the limitations of the medical literature (for some organisms, clinical studies are more detailed, whereas for other organisms the documentation may not exist, al-though the clinical syndromes may be very similar). In the entries in the columns “Incubation Period,” “Duration of Symptoms,” and “Duration of Shedding,” the numbers in parentheses indicate the range. “Shedding” is the time during which the infectious agent can be recovered from feces after the end of illness. CFU denotes colony-forming units, RT-PCR reverse-tran-scriptase–polymerase-chain-reaction assay, and EIA enzyme immunoassay.
† This column reflects the authors’ assessment of the likelihood of human-to-human spread, based on all the available sources of information as presented in the text. ‡ Experimental studies show a high inoculum, but some clinical observations suggest a low inoculum.
Table 3. Relevant Features of Selected Acute Gastrointestinal Infections.*
Agent
Quantity of Inoculum
to Cause Disease
Usual Mode of Transmission
Incubation
Period Usual Symptoms Diagnostic Methods
Duration
of Symptoms Duration of Shedding
Probability of Human-to-Human Spread†
Salmonella typhi High
(105 CFU)‡
Human contact, prepared food, contaminated water
5–14 days Fever, abdominal pain, diarrhea
Blood culture, fecal culture
3–4 wk 2–6 wk, rarely lifetime High
Salmonella (nontyphoidal)
Low (102–103 CFU)
Poultry, eggs, meat 24 hr (8–24 hr)
Diarrhea, fever Fecal culture 2–4 days 5 wk, rarely lifetime Very low
Shigella Low (≤102 CFU)
Human contact, prepared food, contaminated water
3 days (1–7 days)
Diarrhea, fever Fecal culture 3 days (2–6)
Days to weeks Very high
Campylobacter Low Poultry, milk, tap water 3 days (1–7 days)
Diarrhea, fever Fecal culture 3 days (1–7)
50% negative after 3 wk Very low
Calicivirus Low Human contact (feces, vomitus), prepared food
1 day (1–2 days)
Diarrhea, vomiting, fever
RT-PCR 2 days (1–3)
3 days (1 day to weeks) Very high
Rotavirus Very low Human contact 2 days Fever, vomiting, diarrhea (in infants)
EIA, latex agglutination
4 days (3–9)
4 days (2–7 days) Very high
Astrovirus Unknown Human contact 1–2 days Diarrhea EIA (not commer-cially available)
2–5 days (1–14)
4 days (1 day to weeks) High
Adenovirus types 40 and 41
Unknown Human contact (feces, possibly vomitus)
2–3 days Diarrhea, vomiting, fever
EIA (not commer-cially available)
2–4 days (1–7)
5 days (3–11 days) Low
Giardia Low (≤102 organisms)
Tap water, human contact 9 days (1–2 wk)
Abdominal discomfort, diarrhea
Microscopical exam-ination of feces
1–8 wk 3 wk–6 mo High
Cryptosporidium Very low (1–2 cysts)
Tap water, human contact 7 days (1–14 days)
Diarrhea, abdominal pain, headache, fever
Microscopical exam-ination of feces
10–12 days (3–60)
7 days Very low
The new england journal of medicine
team (17 percent) later had acute gastrointestinal illness due to a Norwalk-like virus with an identical genogroup.70
Whereas bacteria causing diarrheal disease are presumably shed exclusively in feces, caliciviruses are detected in vomitus and feces,67 and contact
with either source may result in infection. Airborne transmission may have caused an outbreak in a ger-iatric facility in which 9 of 14 employees who con-tracted acute gastrointestinal illness had no direct contact with the feces of residents.71 Similar
in-stances have been cited in other locales, such as cruise ships,72 hospitals,73 and restaurants,74 which
suggests that a small inoculum can spread disease by aerosol. Caliciviruses persist in an infective form in the environment75 and are resistant to
deactiva-tion by ordinary cleansing agents,76 although they
are inactivated by exposure to household bleach di-luted 1:10.77 This explains why, once they are in the
environment, for example in a day-care setting or a cruise ship, they are so difficult to eradicate.
rotaviruses
Rotaviruses are a prominent cause of severe diar-rheal disease in children under the age of two years. Infection is highly contagious, indicating that a very small inoculum is infectious, since the feces of infected children usually contain no more than 100 CFU per gram. When a rotavirus is introduced into a family, about 50 percent of exposed children and 15 to 30 percent of exposed adults become infected, although some proportion of infected children and most infected adults remain asymptomatic.28,78-80
Most adults who are infected become so within the family, whereas most infections in very young chil-dren are acquired outside the family — for exam-ple, in day-care settings.28,81 Like caliciviruses,
ro-taviruses survive well on environmental surfaces82
and are difficult to inactivate,83 although diluted
household bleach seems to be effective.84 The
con-gruence of the small size of the inoculum required for infection,85,86 the survival of the pathogen in
the environment, and its resistance to most com-mon cleansing agents renders this virus very diffi-cult to control in closed populations; the same is true of the Norwalk-like viruses.
adenovirus types 40 and 41
Enteric adenoviruses, types 40 and 41, which have been identified only recently by application of novel techniques, are found in the feces of about 3 per-cent of all young children with acute gastrointesti-nal illness.55,87,88 These viruses are readily
trans-mitted from child to child, with disease developing in about half of infected children; most infected adults remain asymptomatic.89-91 In a prospective,
five-year investigation,91 adenovirus type 40 or 41
was found in all 10 outbreaks in which other or-ganisms had not been identified; 38 percent of all fecal samples studied were positive. Nevertheless, one family study suggested that this organism is much less contagious than rotavirus.55 In one
pro-longed outbreak of acute gastrointestinal illness in Figure 1. Contagion (Primary and Secondary Infection) in a Foodborne
Out-break of Infection.
An outbreak of a presumed calicivirus infection was traced to a single food handler who prepared salads in a college dining hall.67 Panel A shows the number of cases of acute gastrointestinal infection that developed each day, as reported on a questionnaire by persons who ate or worked in the dining hall. Panel B shows the numbers of persons who presented to the college in-firmary each day with symptoms of acute gastrointestinal disease. The dining hall was closed at the end of day 4. Patients who presented to the college in-firmary on days 5 through 7 were presumed to have acquired the infection in the dining hall. The long “tail” on the right side in Panel B is thought to reflect transmission of infection from persons initially infected by ingestion of taminated food (primary cases) to other students who did not ingest the con-taminated food (secondary cases). The graphs are adapted from Kilgore et al.67
No. of Cases
80 100
60
40
20
0
0 1 2 3 4 5 6 7 8 9 10 11 12
Day of Outbreak
120
No. of Cases
80 100
60
40
20
0
0 1 2 3 4 5 6 7 8 9 10 11 12
Day of Outbreak
120 140 160
A
B
Questionnaire
n engl j med 351;23 www.nejm.org december 2, 2004 medical progress
2423 persons hospitalized for long periods, rotavirus and
adenovirus type 40 or 41 were isolated in nearly equal proportions.92
astrovirus
Astroviruses, which are perhaps less well studied than the viruses already described, cause outbreaks of acute gastrointestinal illness — generally, but not always,93 by person-to-person spread. Day-care94
and kindergarten57 attendees, military recruits,95
and mothers and children in maternal-care facili-ties96 have been implicated, and pediatric97,98 and
geriatric92,99,100 hospital wards have been involved.
During outbreaks in day-care centers, 50 to 90 per-cent of children and up to 25 perper-cent of adults may have disease57,94,96; secondary cases occur in the
families of one third of affected children.57 This
ap-parently high rate of contagion belies results show-ing disease in only a very small proportion of hu-man volunteers after experimental ingestion of astrovirus101; the lower rate in the study is perhaps
attributable to differences between naturally ac-quired strains and those used experimentally.
Cryptosporidium, Giardia lamblia, and Entamoeba his-tolytica cause acute diarrheal disease, with transmis-sion via a small inoculum (fewer than 100 organ-isms).102-104 Once regarded as waterborne,105 these
organisms are now known to spread through day-care centers by way of the fecal–oral route with a substantial likelihood of secondary infection among family members, especially women of childbear-ing age.
cryptosporidium
Because it can be difficult to eradicate cryptospo-ridium from drinking water, large outbreaks of infection have occurred.106 Nevertheless,
person-to-person spread of cryptosporidium107 is well
doc-umented in homes, schools, and day-care centers. Cryptosporidium may infect 40 percent of house-hold members who have contact with young chil-dren with diarrhea, but fewer than 10 percent of household members whose contact is with asymp-tomatic carriers become infected107 — again
illus-trating the importance of diarrhea in contagion. When adults are infected, the risk for secondary in-fection in families is less than 5 percent108; in part,
this low rate of risk is consistent with the better hy-giene of adults, as compared with children, and, in
part, it may be due to other, uncertain causes. Food handlers may also spread this organism.109
giardia
Outbreaks of infection with giardia in child-care settings are associated with overall attack rates (in-cluding clinical and subclinical cases) of 17 to 47 percent among attendees and 10 to 35 percent among adult workers.110 When a young child
be-comes infected, there is a 5 to 25 percent chance that one or more family members will contract the disease.28,110 Severe giardiasis occurs most
com-monly in young children and women of childbear-ing age,111 probably reflecting host susceptibility
together with the effect of the size of the inoculum. Giardia also spreads among participants in swim-ming classes112 and among homosexual men.113
e. histolytica
Outbreaks of E. histolytica infection in schools are generally traced to contaminated water sources. Person-to-person spread has, however, been docu-mented in homes, schools, and day-care centers, as well as among homosexual men.30,114,115
Never-theless, somewhat surprisingly, documented spread within families is unusual.116,117
In nearly all instances, transmission of acute gastro-intestinal illness is due to organisms that are present transiently on the hands.118 The distinction between
transient and resident flora is important in under-standing apparent discrepancies relating to trans-mission of acute gastrointestinal illness. Washing the hands for 30 seconds with soap or detergent and water may not substantially reduce the total num-ber of bacteria that are present on relatively clean hands119; in contrast, handwashing reduces by
about 95 percent the numbers of bacteria or viruses that are applied to the hands experimentally120,121
or that are acquired exogenously under natural con-ditions122; and handwashing clearly reduces the
spread of acute gastrointestinal illness in day-care and family settings.123-125 The explanation is that
exogenously acquired organisms or transient flora (the ones that are likely to transmit infection) are removable by washing, whereas resident flora (the ones that are normally present) are not.
Whereas the antibacterial substances in house-hold soaps do not prevent acute gastrointestinal ill-ness,126 additional field studies with alcohol-based
p r o t o z o a l c a u s e s
p r e v e n t i o n
The new england journal of medicine
gels may be warranted in day-care centers and other sites where the risk of person-to-person transmis-sion is particularly high. As noted above, washing environmental surfaces with solutions containing diluted household bleach (1:10) greatly reduces the counts of bacteria and viruses that are implicated in acute gastrointestinal illness, but this type of appli-cation is not always practicable.
Acute gastrointestinal illness is exceedingly com-mon; viruses, bacteria, and protozoa are the princi-pal recognized causes. Some causative organisms, such as calicivirus, rotavirus, astrovirus, adenovirus types 40 and 41, S. typhi, and shigella, are indige-nous to humans; person-to-person spread follows direct contact or human contamination of food or water. In contrast, nontyphoidal salmonella, cam-pylobacter, and pathogenic E. coli are prevalent in meat, poultry, and dairy foods; human-to-human spread is documented infrequently relative to the total number of cases of infection with these bacte-rial agents. This lower rate of documentation may reflect the difficulty, in an individual case, of deter-mining whether some common food source is re-sponsible or in distinguishing an environmental source from a human source.
As a general matter, the failure to identify a common source for most sporadic, presumably vi-ral, acute gastrointestinal illnesses does not exclude the possible link to an unrecognized foodborne out-break. The essential point remains, however, that — even though the visibility of an outbreak tends to focus attention on foodborne infection — the great majority of cases are sporadic and spread from per-son to perper-son. Although free-living protozoa, such as cryptosporidia or giardia, are widespread in na-ture, contagion is also well documented.
The likelihood of contagion depends on the age and self-reliance of an infected person, the nature of the social interaction within the potentially in-volved group, the intensity of the symptoms, the
concentration of organisms in the potentially in-fective material, the likelihood that the organism will survive direct transmission or survive in the en-vironment, and other, less well understood factors. The immune status of the host undoubtedly plays a role in determining whether symptomatic disease or subclinical infection results, but the nature of such immune factors is poorly understood.127
Within families, young children are the usual source for contagion because of their exposure to other children, their imperfect personal hygiene, and their dependence on adults. Severely affect-ed persons are more contagious because they dis-charge greater volumes of infective material that contain large numbers of infectious particles. The likelihood of contagion varies with the concentra-tion of organisms in excreta, the capacity of the or-ganisms to survive and replicate in food or persist in the environment, and the number required to in-fect. Spread of acute gastrointestinal illness is com-mon and problematic in all closed environments such as day-care centers, schools, and cruise ships. Person-to-person transmission is best prevented by the practice of excellent personal hygiene both by infected persons and by those exposed to them. Fecal–oral transmission is the usual route of spread of acute gastrointestinal illness, but caliciviruses and probably adenoviruses are present in vomitus, so kissing or sharing utensils should also be avoid-ed. Dilution by handwashing reduces the inoculum of causative organisms, greatly diminishing the risk of contagion. There is no apparent benefit from the antibacterial agents in soaps, although the regular use of alcohol-based gels will probably reduce trans-mission. The use of diluted household bleach on environmental surfaces may be necessary to inter-rupt transmission of viral or protozoal agents.
Dr. Daniel Musher reports having received Merit Review Funding from the Department of Veterans Affairs, grant support from Ro-mark Laboratories, and consulting fees from Aventis.
We are indebted to Marsha Sullivan and the staff of the Medical Library at the Michael E. DeBakey Veterans Affairs Medical Center, Houston, for their help.
s u m m a r y a n d c o n c l u s i o n s
r e f e r e n c e s
1. Musher DM. How contagious are
com-mon respiratory infections? N Engl J Med 2003;348:1256-66.
2. Mead PS, Slutsker L, Dietz V, et al.
Food-related illness and death in the United States. Emerg Infect Dis 1999;5:607-25.
3. Imhoff B, Morse D, Shiferaw B, et al.
Burden of self-reported acute diarrheal
ill-ness in FoodNet surveillance areas, 1998-1999. Clin Infect Dis 2004;38:Suppl 3:S219-S226.
4. Lesser CF, Miller SI. Salmonellosis. In:
Braunweld E, Fauci AS, Kasper DL, Jameson JL, eds. Harrison’s principles of internal med-icine. 15th ed. New York: McGraw-Hill, 2001: 970-5.
5. Roy SK, Speelman P, Butler T, Nath S,
Rahman H, Stoll BJ. Diarrhea associated with typhoid fever. J Infect Dis 1985;151: 1138-43.
6. Mathieu JJ, Henning KJ, Bell E, Frieden
n engl j med 351;23 www.nejm.org december 2, 2004 medical progress
2425
7. Leavitt JW. Typhoid Mary: captive to the
public’s health. Boston: Beacon Press, 1996.
8. Osler W. The principles and practice of
medicine. New York: D. Appleton, 1914.
9. Smith DT, Conant NF, eds. Zinsser
mi-crobiology. New York: Appleton-Century-Crofts, 1960.
10. Typhoid fever — Washington. MMWR
Morb Mortal Wkly Rep 1972;21:290.
11.Mermin JH, Villar R, Carpenter J, et al.
A massive epidemic of multidrug-resistant typhoid fever in Tajikistan associated with consumption of municipal water. J Infect Dis 1999;179:1416-22.
12.Davis BD, Dulbecco R, Eisen HN,
Gins-berg HS, Wood WBJ. Microbiology. New York: Harper & Row, 1967.
13.Hornick RB, Greisman SE, Woodward
TE, DuPont HL, Dawkins AT, Snyder MJ. Ty-phoid fever: pathogenesis and immunolog-ic control. 2. N Engl J Med 1970;283:739-46.
14. Idem. Typhoid fever: pathogenesis and immunologic control. N Engl J Med 1970; 283:686-91.
15.Glynn JR, Hornick RB, Levine MM,
Bradley DJ. Infecting dose and severity of ty-phoid: analysis of volunteer data and exami-nation of the influence of the definition of illness used. Epidemiol Infect 1995;115:23-30.
16.Black RE, Levine MM, Clements ML,
Hughes TP, Blaser MJ. Experimental Cam-pylobacter jejuni infection in humans. J In-fect Dis 1988;157:472-9.
17. Ager EA, Top FH Sr. Salmonellosis. In:
Top FH Sr, Wehrle PF, eds. Communicable and infectious diseases. St. Louis: C.V. Mos-by, 1972:567-80.
18.Buchwald DS, Blaser MJ. A review of
hu-man salmonellosis: II. Duration of excretion following infection with nontyphi Salmo-nella. Rev Infect Dis 1984;6:345-56.
19.Newcomb S, Broadhurst L, Kissane K.
Salmonella outbreak in an American child development center in Germany. Mil Med 1997;162:783-7.
20.Musher DM, Rubenstein AD.
Perma-nent carriers of nontyphosa salmonellae. Arch Intern Med 1973;132:869-72.
21.A waterborne epidemic of
salmonello-sis in Riverside, California, 1965: epidemio-logic aspects. Am J Epidemiol 1971;93:33-48.
22.Kapperud G, Gustavsen S, Hellesnes I,
et al. Outbreak of Salmonella typhimurium infection traced to contaminated chocolate and caused by a strain lacking the 60-mega-dalton virulence plasmid. J Clin Microbiol 1990;28:2597-601.
23.DuPont HL, Hornick RB, Snyder MJ,
Li-bonati JP, Formal SB, Gangarosa EJ. Immu-nity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis 1972;125:12-6.
24.Davison WC. A bacteriological and
clin-ical consideration of bacillary dysentery in
adults and children. Medicine (Baltimore) 1922;1:389-580.
25.Wharton M, Spiegel RA, Horan JM, et al.
A large outbreak of antibiotic-resistant shigellosis at a mass gathering. J Infect Dis 1990;162:1324-8.
26. Lewis JN, Gangarosa EJ. Shigellosis. In:
Top FH Sr, Wehrle PF, eds. Communicable and infectious diseases. St. Louis: C.V. Mos-by, 1972:585-91.
27.Levine MM, Dupont HL, Gangarosa EJ,
et al. Shigellosis in custodial institutions. II. Clinical, immunologic and bacteriologic re-sponse of institutionalized children to oral attenuated shigella vaccines. Am J Epidemi-ol 1972;96:40-9.
28.Pickering LK, Evans DG, DuPont HL,
Vollet JJ III, Evans DJ Jr. Diarrhea caused by shigella, rotavirus, and giardia in day-care centers: prospective study. J Pediatr 1981; 99:51-6.
29.Litwin CM, Leonard RB, Carroll KC,
Drummond WK, Pavia AT. Characterization of endemic strains of Shigella sonnei by use of plasmid DNA analysis and pulsed-field gel electrophoresis to detect patterns of transmission. J Infect Dis 1997;175:864-70.
30.Pickering LK, Bartlett AV, Woodward
WE. Acute infectious diarrhea among chil-dren in day care: epidemiology and control. Rev Infect Dis 1986;8:539-47.
31.Blaser MJ, Wells JG, Feldman RA,
Pol-lard RA, Allen JR. Campylobacter enteritis in the United States: a multicenter study. Ann Intern Med 1983;98:360-5.
32.Slutsker L, Ries AA, Greene KD, Wells
JG, Hutwagner L, Griffin PM. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann In-tern Med 1997;126:505-13.
33.Kapperud G, Espeland G, Wahl E, et
al. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am J Epidemiol 2003;158:234-42.
34.Skirrow MB. Campylobacter. Lancet
1990;336:921-3.
35.Blaser MJ, Waldman RJ, Barrett T,
Er-landson AL. Outbreaks of Campylobacter enteritis in two extended families: evidence for person-to-person transmission. J Pediatr 1981;98:254-7.
36.Gillespie IA, O’Brien SJ, Adak GK, et al.
Point source outbreaks of Campylobacter jejuni infection — are they more common than we think and what might cause them? Epidemiol Infect 2003;130:367-75.
37.Oosterom J, den Uyl CH, Banffer JR,
Huisman J. Epidemiological investigations on Campylobacter jejuni in households with a primary infection. J Hyg (Lond) 1984;93: 325-32.
38.Wolfs TF, Duim B, Geelen SP, et al.
Neonatal sepsis by Campylobacter jejuni: genetically proven transmission from a household puppy. Clin Infect Dis 2001;32: E97-E99.
39.Gaudreau C, Michaud S. Cluster of
erythromycin- and ciprofloxacin-resistant Campylobacter jejuni subsp. jejuni from 1999 to 2001 in men who have sex with men, Quebec, Canada. Clin Infect Dis 2003; 37:131-6.
40.Reller ME, Olsen SJ, Kressel AB, et al.
Sexual transmission of typhoid fever: a mul-tistate outbreak among men who have sex with men. Clin Infect Dis 2003;37:141-4.
41.Shigella sonnei outbreak among men
who have sex with men — San Francisco, California, 2000–2001. MMWR Morb Mor-tal Wkly Rep 2001;50:922-6.
42.Bell BP, Goldoft M, Griffin PM, et al.
A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburg-ers: the Washington experience. JAMA 1994;272:1349-53.
43.Belongia EA, Osterholm MT, Soler JT,
Ammend DA, Braun JE, MacDonald KL. Transmission of Escherichia coli O157:H7 infection in Minnesota child day-care facili-ties. JAMA 1993;269:883-8.
44.Spika JS, Parsons JE, Nordenberg D,
Wells JG, Gunn RA, Blake PA. Hemolytic uremic syndrome and diarrhea associated with Escherichia coli O157:H7 in a day care center. J Pediatr 1986;109:287-91.
45.Pavia AT, Nichols CR, Green DP, et al.
Hemolytic-uremic syndrome during an out-break of Escherichia coli O157:H7 infec-tions in instituinfec-tions for mentally retarded persons: clinical and epidemiologic obser-vations. J Pediatr 1990;116:544-51.
46.Rowe PC, Orrbine E, Lior H, et al. Risk
of hemolytic uremic syndrome after sporad-ic Eschersporad-ichia coli O157:H7 infection: re-sults of a Canadian collaborative study. J Pe-diatr 1998;132:777-82.
47. Bartlett JG. Antibiotic-associated
diar-rhea. N Engl J Med 2002;346:334-9.
48.Johnson S, Adelmann A, Clabots CR,
Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis 1989;159:340-3.
49.Kim K, DuPont HL, Pickering LK.
Out-breaks of diarrhea associated with Clostridi-um difficile and its toxin in day-care centers: evidence of person-to-person spread. J Pedi-atr 1983;102:376-82.
50.Dingle JH, Badger GF, Jordan WS Jr.
Ill-ness in the home: a study of 25,000 illIll-nesses in a group of Cleveland families. Cleveland: Press of Western Reserve University, 1964.
51.Allos BM, Moore MR, Griffin PM, Tauxe
RV. Surveillance for sporadic foodborne dis-ease in the 21st century: the FoodNet per-spective. Clin Infect Dis 2004;38:Suppl 3: S115-S120.
52.Glass RI, Noel J, Ando T, et al. The
epi-demiology of enteric caliciviruses from humans: a reassessment using new diag-nostics. J Infect Dis 2000;181:Suppl 2:S254-S261.
53.Hedlund KO, Rubilar-Abreu E,
Svens-son L. Epidemiology of calicivirus infections
The new england journal of medicine
in Sweden, 1994-1998. J Infect Dis 2000; 181:Suppl 2:S275-S280.
54.de Wit MA, Koopmans MP, Kortbeek
LM, et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol 2001;154:666-74.
55.Rodriguez WJ, Kim HW, Brandt CD, et
al. Fecal adenoviruses from a longitudinal study of families in metropolitan Washing-ton, D.C.: laboratory, clinical, and epidemio-logic observations. J Pediatr 1985;107:514-20.
56.Mistchenko AS, Huberman KH, Gomez
JA, Grinstein S. Epidemiology of enteric ad-enovirus infection in prospectively moni-tored Argentine families. Epidemiol Infect 1992;109:539-46.
57.Konno T, Suzuki H, Ishida N, Chiba R,
Mochizuki K, Tsunoda A. Astrovirus-associ-ated epidemic gastroenteritis in Japan. J Med Virol 1982;9:11-7.
58.Herrmann JE, Taylor DN, Echeverria P,
Blacklow NR. Astroviruses as a cause of gas-troenteritis in children. N Engl J Med 1991; 324:1757-60.
59.Naficy AB, Rao MR, Holmes JL, et al.
As-trovirus diarrhea in Egyptian children. J In-fect Dis 2000;182:685-90.
60.Waters V, Ford-Jones EL, Petric M, Fearon
M, Corey P, Moineddein R. Etiology of com-munity-acquired pediatric viral diarrhea: a prospective longitudinal study in hospitals, emergency departments, pediatric practices and child care centers during the winter ro-tavirus outbreak, 1997 to 1998. Pediatr In-fect Dis J 2000;19:843-8.
61.Fankhauser RL, Monroe SS, Noel JS, et
al. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with out-breaks of gastroenteritis in the United States. J Infect Dis 2002;186:1-7.
62.Norovirus activity — United States,
2002. MMWR Morb Mortal Wkly Rep 2003; 52:41-5. [Erratum, MMWR Morb Mortal Wkly Rep 2003;52:113.]
63.Outbreaks of gastroenteritis associated
with norovirus on cruise ships — United States, 2002. MMWR Morb Mortal Wkly Rep 2002;51:1112-5.
64.Schreiber DS, Blacklow NR, Trier JS.
The mucosal lesion of the proximal small in-testine in acute infectious nonbacterial gas-troenteritis. N Engl J Med 1973;288:1318-23.
65.Parrino TA, Schreiber DS, Trier JS,
Kapikian AZ, Blacklow NR. Clinical immu-nity in acute gastroenteritis caused by Nor-walk agent. N Engl J Med 1977;297:86-9.
66.Adler JL, Zickl R. Winter vomiting
dis-ease. J Infect Dis 1969;119:668-73.
67.Kilgore PE, Belay ED, Hamlin DM, et al.
A university outbreak of gastroenteritis due to a small round-structured virus: applica-tion of molecular diagnostics to identify the etiologic agent and patterns of transmis-sion. J Infect Dis 1996;173:787-93.
68.Gotz H, Ekdahl K, Lindback J, de Jong
B, Hedlund KO, Giesecke J. Clinical
spec-trum and transmission characteristics of in-fection with Norwalk-like virus: findings from a large community outbreak in Swe-den. Clin Infect Dis 2001;33:622-8.
69.Outbreak of acute gastroenteritis
asso-ciated with Norwalk-like viruses among British military personnel — Afghanistan, May 2002. MMWR Morb Mortal Wkly Rep 2002;51:477-9.
70.Becker KM, Moe CL, Southwick KL,
MacCormack JN. Transmission of Norwalk virus during a football game. N Engl J Med 2000;343:1223-7.
71.Gellert GA, Waterman SH, Ewert D, et
al. An outbreak of acute gastroenteritis caused by a small round structured virus in a geriatric convalescent facility. Infect Control Hosp Epidemiol 1990;11:459-64.
72.Ho MS, Glass RI, Monroe SS, et al. Viral
gastroenteritis aboard a cruise ship. Lancet 1989;2:961-5.
73.Sawyer LA, Murphy JJ, Kaplan JE, et al.
25- to 30-nm virus particle associated with a hospital outbreak of acute gastroenteritis with evidence for airborne transmission. Am J Epidemiol 1988;127:1261-71.
74.Marks PJ, Vipond IB, Carlisle D, Deakin
D, Fey RE, Caul EO. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol Infect 2000; 124:481-7.
75.Kuusi M, Nuorti JP, Maunula L, et al.
A prolonged outbreak of Norwalk-like cali-civirus (NLV) gastroenteritis in a rehabilita-tion centre due to environmental contami-nation. Epidemiol Infect 2002;129:133-8.
76.Gulati BR, Allwood PB, Hedberg CW,
Goyal SM. Efficacy of commonly used disin-fectants for the inactivation of calicivirus on strawberry, lettuce, and a food-contact sur-face. J Food Prot 2001;64:1430-4.
77.Thurston-Enriquez JA, Haas CN,
Jacan-gelo J, Gerba CP. Chlorine inactivation of ad-enovirus type 40 and feline calicivirus. Appl Environ Microbiol 2003;69:3979-85.
78.Wenman WM, Hinde D, Feltham S,
Gurwith M. Rotavirus infection in adults: re-sults of a prospective family study. N Engl J Med 1979;301:303-6.
79.Rodriguez WJ, Kim HW, Brandt CD, et
al. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epi-demiologic observations. Pediatr Infect Dis J 1987;6:170-6.
80.Koopman JS, Monto AS, Longini IM Jr.
The Tecumseh Study. XVI: Family and com-munity sources of rotavirus infection. Am J Epidemiol 1989;130:760-8.
81.Bartlett AV III, Reves RR, Pickering LK.
Rotavirus in infant-toddler day care centers: epidemiology relevant to disease control strategies. J Pediatr 1988;113:435-41.
82.Keswick BH, Pickering LK, DuPont HL,
Woodward WE. Survival and detection of ro-taviruses on environmental surfaces in day care centers. Appl Environ Microbiol 1983; 46:813-6.
83.Abad FX, Pinto RM, Bosch A.
Disinfec-tion of human enteric viruses on fomites. FEMS Microbiol Lett 1997;156:107-11.
84.Ojeh CK, Cusack TM, Yolken RH.
Evalu-ation of the effects of disinfectants on rota-virus RNA and infectivity by the polymerase chain reaction and cell-culture methods. Mol Cell Probes 1995;9:341-6.
85.Vesikari T, Ruuska T, Bogaerts H, Delem
A, Andre F. Dose-response study of RIT 4237 oral rotavirus vaccine in breast-fed and formula-fed infants. Pediatr Infect Dis 1985; 4:622-5.
86.Graham DY, Dufour GR, Estes MK.
Minimal infective dose of rotavirus. Arch Vi-rol 1987;92:261-71.
87.Brandt CD, Kim HW, Rodriguez WJ, et
al. Adenoviruses and pediatric gastroenteri-tis. J Infect Dis 1985;151:437-43.
88.Vizzi E, Ferraro D, Cascio A, Di Stefano
R, Arista S. Detection of enteric adenovirus-es 40 and 41 in stool specimens by mono-clonal antibody-based enzyme immunoas-says. Res Virol 1996;147:333-9.
89.Richmond SJ, Caul EO, Dunn SM,
Ash-ley CR, Clarke SK, Seymour NR. An out-break of gastroenteritis in young children caused by adenoviruses. Lancet 1979;1: 1178-81.
90.Chiba S, Nakata S, Nakamura I, et al.
Outbreak of infantile gastroenteritis due to type 40 adenovirus. Lancet 1983;2:954-7.
91.Van R, Wun CC, O’Ryan ML, Matson
DO, Jackson L, Pickering LK. Outbreaks of human enteric adenovirus types 40 and 41 in Houston day care centers. J Pediatr 1992; 120:516-21.
92.Dupuis P, Beby A, Bourgoin A,
Lussier-Bonneau MD, Agius G. Epidemic of viral gastroenteritis in an elderly community. Presse Med 1995;24:356-8. (In French.)
93.Oishi I, Yamazaki K, Kimoto T, et al.
A large outbreak of acute gastroenteritis as-sociated with astrovirus among students and teachers in Osaka, Japan. J Infect Dis 1994;170:439-43.
94.Mitchell DK, Monroe SS, Jiang X,
Mat-son DO, Glass RI, Pickering LK. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse tran-scriptase-polymerase chain reaction. J In-fect Dis 1995;172:1437-44.
95.Belliot G, Laveran H, Monroe SS.
Out-break of gastroenteritis in military recruits associated with serotype 3 astrovirus infec-tion. J Med Virol 1997;51:101-6.
96.Oppermann H, Mueller B, Takkinen J,
Klauditz W, Schreier E, Ammon A. An out-break of viral gastroenteritis in a mother-and-child health clinic. Int J Hyg Environ Health 2001;203:369-73.
97.Kurtz JB, Lee TW, Pickering D.
Astrovi-rus associated gastroenteritis in a children’s ward. J Clin Pathol 1977;30:948-52.
98.Ashley CR, Caul EO, Paver WK.
Astrovi-rus-associated gastroenteritis in children. J Clin Pathol 1978;31:939-43.
n engl j med 351;23 www.nejm.org december 2, 2004 medical progress
2427
Wilson SA. Outbreaks of astrovirus type 1 and rotavirus gastroenteritis in a geriatric in-patient population. J Hosp Infect 1989; 14:9-14.
100. Gray JJ, Wreghitt TG, Cubitt WD, Elliot
PR. An outbreak of gastroenteritis in a home for the elderly associated with astrovirus type 1 and human calicivirus. J Med Virol 1987;23:377-81.
101. Kurtz JB, Lee TW, Craig JW, Reed SE.
Astrovirus infection in volunteers. J Med Vi-rol 1979;3:221-30.
102. DuPont HL, Chappell CL, Sterling CR,
Okhuysen PC, Rose JB, Jakubowski W. The
infectivity of Cryptosporidium parvum in
healthy volunteers. N Engl J Med 1995;332: 855-9.
103. Overturf GD. Endemic giardiasis in the
United States — role of the daycare center. Clin Infect Dis 1994;18:764-5.
104. Okhuysen PC. Traveler’s diarrhea due
to intestinal protozoa. Clin Infect Dis 2001; 33:110-4.
105. Marshall MM, Naumovitz D, Ortega Y,
Sterling CR. Waterborne protozoan patho-gens. Clin Microbiol Rev 1997;10:67-85. [Erratum, Clin Microbiol Rev 1998;11:404.]
106. Mac Kenzie WR, Hoxie NJ, Proctor
ME, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med 1994;331:161-7. [Erratum, N Engl J Med 1994;331:1035.]
107. Heijbel H, Slaine K, Seigel B, et al.
Out-break of diarrhea in a day care center with spread to household members: the role of cryptosporidium. Pediatr Infect Dis J 1987; 6:532-5.
108. MacKenzie WR, Schell WL, Blair KA,
et al. Massive outbreak of waterborne cryp-tosporidium infection in Milwaukee, Wis-consin: recurrence of illness and risk of sec-ondary transmission. Clin Infect Dis 1995; 21:57-62.
109. Quiroz ES, Bern C, MacArthur JR, et al.
An outbreak of cryptosporidiosis linked to a foodhandler. J Infect Dis 2000;181:695-700.
110. Steketee RW, Reid S, Cheng T, Stoebig
JS, Harrington RG, Davis JP. Recurrent out-breaks of giardiasis in a child day care cen-ter, Wisconsin. Am J Public Health 1989;79: 485-90.
111. Lengerich EJ, Addiss DG, Juranek DD.
Severe giardiasis in the United States. Clin Infect Dis 1994;18:760-3.
112. Harter L, Frost F, Grunenfelder G,
Per-kins-Jones K, Libby J. Giardiasis in an infant and toddler swim class. Am J Public Health 1984;74:155-6.
113. Esfandiari A, Swartz J, Teklehaimanot
S. Clustering of giardiosis among AIDS pa-tients in Los Angeles County. Cell Mol Biol (Noisy-le-grand) 1997;43:1077-83.
114. Owen RL. Direct fecal-oral
transmis-sion of giardiasis. In: Erlander SL, Meyer EA, eds. Giardia and giardiasis: biology, patho-geneses, and epidemiology. New York: Ple-num Press, 1984:329-39.
115. Addiss DG, Stewart JM, Finton RJ, et
al. Giardia lamblia and Cryptosporidium in-fections in child day-care centers in Fulton County, Georgia. Pediatr Infect Dis J 1991; 10:907-11.
116. Gatti S, Cevini C, Bruno A, Novati S,
Scaglia M. Transmission of Entamoeba his-tolytica within a family complex. Trans R Soc Trop Med Hyg 1995;89:403-5. [Erra-tum, Trans R Soc Trop Med Hyg 1995;89: 537.]
117. Vreden SG, Visser LG, Verweij JJ, et al.
Outbreak of amebiasis in a family in The Netherlands. Clin Infect Dis 2000;31:1101-4.
118. Steere AC, Mallison GF. Handwashing
practices for the prevention of nosocomial infections. Ann Intern Med 1975;83:683-90.
119. Larson EL, Gomez-Duarte C, Lee LV,
Della-Latta P, Kain DJ, Keswick BH.
Micro-bial flora of hands of homemakers. Am J In-fect Control 2003;31:72-9.
120. Ansari SA, Sattar SA, Springthorpe VS,
Wells GA, Tostowaryk W. In vivo protocol for testing efficacy of hand-washing agents against viruses and bacteria: experiments with rotavirus and Escherichia coli. Appl Environ Microbiol 1989;55:3113-8.
121. Lin CM, Wu FM, Kim HK, Doyle MP,
Michael BS, Williams LK. A comparison of hand washing techniques to remove Esche-richia coli and caliciviruses under natural or artificial fingernails. J Food Prot 2003;66: 2296-301. [Erratum, J Food Prot 2004;67(3): following table of contents.]
122. de Wit JC, Kampelmacher EH.
Micro-biological aspects of washing hands in slaughter-houses. Zentralbl Bakteriol Mik-robiol Hyg [B] 1982;176:553-61.
123. Black RE, Dykes AC, Anderson KE, et
al. Handwashing to prevent diarrhea in day-care centers. Am J Epidemiol 1981;113:445-51.
124. Mohle-Boetani JC, Stapleton M,
Fin-ger R, et al. Communitywide shigellosis: control of an outbreak and risk factors in child day-care centers. Am J Public Health 1995;85:812-6.
125. Gibson LL, Rose JB, Haas CN, Gerba
CP, Rusin PA. Quantitative assessment of risk reduction from hand washing with anti-bacterial soaps. J Appl Microbiol 2002;92: Suppl:136S-143S.
126. Larson EL, Lin SX, Gomez-Pichardo C,
Della-Latta P. Effect of antibacterial home cleaning and handwashing products on in-fectious disease symptoms: a randomized, double-blind trial. Ann Intern Med 2004; 140:321-9.
127. Bresee JS, Widdowson MA, Monroe
SS, Glass RI. Foodborne viral gastroenteri-tis: challenges and opportunities. Clin In-fect Dis 2002;35:748-53.
Copyright © 2004 Massachusetts Medical Society.
apply for jobs electronically at the nejm careercenter
Physicians registered at the NEJM CareerCenter can apply for jobs electronically using their own cover letters and CVs. You can keep track of your job-application history with a personal account that is created when you register with the CareerCenter and apply
for jobs seen online at our Web site. Visit www.nejmjobs.org for more information.