www.elsevier.com / locate / bres
Short communication
Schwann cell-induced loss of synapses in the central nervous system
*
Terry J. Sims , Shirley A. Gilmore
Department of Anatomy, University of Arkansas for Medical Sciences, 4301 West Markham, Little Rock, AR 72205, USA
Accepted 8 August 2000
Abstract
Synaptophysin immunostaining of areas of spinal gray matter occupied by radiation-induced intraspinal Schwann cells revealed a loss of immunoreactivity from the neuropil. In contrast, synaptophysin immunoreactivity was preserved on the somata and proximal dendrites of motor neurons. The present study extended these observations to the ultrastructural level and confirmed the absence not only of synapses but also of astrocytes and small- and medium-sized dendrites. These neural elements were abundant and appropriately organized in contiguous areas of irradiated neuropil not occupied by Schwann cells. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Formation and specificity of synapses
Keywords: Synapse; Schwann cell; Dendrite; Astrocyte; Radiation
Exposure of the immature spinal cord to ionizing tions suggested that the loss of immunoreactivity from the radiation results in an altered pattern of development of neuropil was related to the presence of Schwann cells and central glia, including oligodendrocytes [5,6], and in the not to the exposure to radiation. The present study extends occurrence of Schwann cells within the intraspinal en- these observations to the ultrastructural level and focuses vironment [7–9,12,16]. The intraspinal Schwann cells on the status of synapses in the Schwann cell-occupied appear to occur in two patterns and temporal phases. regions of the ventral gray matter to determine whether Within the first 3 weeks following irradiation, the Schwann synapses or synaptic elements have indeed disappeared cells ensheathe or myelinate areas in the dorsal funiculi from these regions or whether synaptic contacts persist but and extend somewhat into the gray matter of the dorsal are modified in some way as to alter expression of horn in essentially all irradiated animals [7,12,16]. Later, synaptophysin.
usually 2–3 months following irradiation, Schwann cells Charles River CD rats were used in this study. On the occur in the ventral gray matter in approximately 40% of 3rd post-natal day, pups were X-irradiated over the lum-the animals [11]. In assessing lum-the ventral gray matter bosacral spinal cord with a single dose of 40 Gray, as immunohistochemically with an antibody to synap- described previously [12]. Spinal cords from the irradiated tophysin, a marker for pre-synaptic elements [20], it was rats between 23 and 103 days of age were examined. These evident that there was a loss of immunoreactivity from the animals were perfused with a fixative composed of 2% neuropil occupied by Schwann cells with a preservation of paraformaldehyde and 2% glutaraldehyde in 0.12 M immunoreactivity on the neuronal perikarya and proximal Sorensen’s buffer at pH 7.4. Following overnight storage dendrites of motor neurons [10]. In contrast, synaptophysin of the irradiated spinal cords in fixative at 48C, the spinal immunoreactivity in the neuropil of irradiated regions of cords were cut into 1 mm slabs in the transverse plane and spinal cord lacking Schwann cells was not distinguishable post-fixed in 2% OsO solution for 1.5 h at 44 8C. The tissue from that of non-irradiated spinal cords. These observa- was then dehydrated, infiltrated with Poly / Bed 812 plastic (Polysciences, Inc.), and cast in blocks for sectioning on a Sorvall MT6000 ultramicrotone. Thick (1m) sections from *Corresponding author. Fax:11-501-686-6382.
E-mail address: [email protected] (T.J. Sims). each block were examined. Thin sections from selected
222 T.J. Sims, S.A. Gilmore / Brain Research 882 (2000) 221 –225
blocks were contrasted with uranyl acetate and lead citrate and examined and photographed using a JEOL 100CX electron microscope. In addition, synaptophysin immuno-stained sections from animals used for an earlier study [10] were re-assessed to evaluate certain aspects relevant to the present study. These sections were derived from 22 irradiated and five non-irradiated rats ranging from 60 to 150 days of age.
Fig. 1 shows an area of spinal gray matter partially occupied by radiation-induced intraspinal Schwann cells as visualized in a section immunostained with the antibody against synaptophysin. The region of irradiated neuropil lacking Schwann cells appeared to be intensely and relatively uniformly immunostained. In contrast, portions of the neuropil in contiguous regions occupied by Schwann cells appeared to be devoid of immunoreactivity. The interface between the Schwann cell-occupied and Schwann cell-free regions (Fig. 1) lacked distinct borders or mor-phological modifications. The Schwann cell-occupied re-gion includes somata of large ventral motor neurons. As described previously [10], these somata and their large, proximal dendrites were outlined by the presence of synaptophysin immunoreactivity.
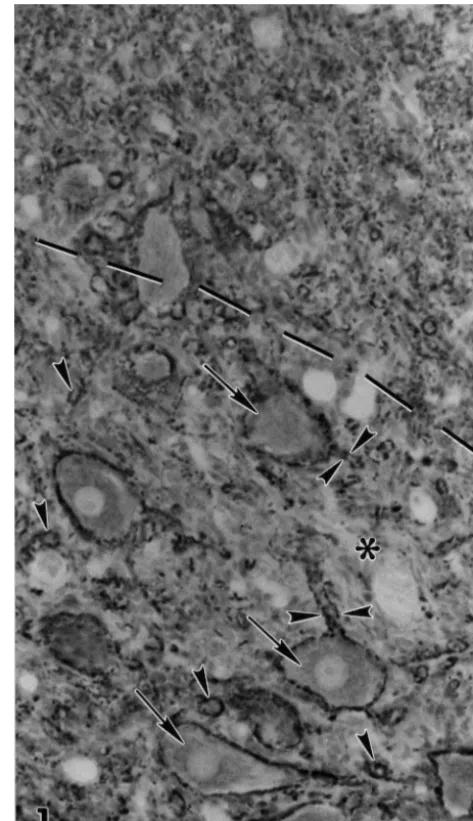
At the ultrastructural level, neuropil lacking Schwann cells was composed of numerous axons and dendrites, along with astrocyte processes (Fig. 2). Synaptic contacts between axons and dendrites were frequent, and astrocytic processes typically contacted the axon terminals at these sites. All of these neural elements were compactly ar-ranged with little observable extracellular space. In con-trast, the neuropil of Schwann cell-occupied regions was profoundly different in composition and organization. These areas contained many axons, myelinated or en-sheathed by Schwann cells (Fig. 3) surrounded, in turn, by basal lamina, and were no longer recognizable as typical
central nervous system neuropil. Notably absent from the Fig. 1. Photomicrograph of synaptophysin immunostained portion of ventral gray matter from a 63-day-old irradiated rat. The dashed line Schwann cell-occupied neuropil were dendrites, synapses
indicates the interface between neuropil occupied by Schwann cells and astrocytes processes (Fig. 3). In the absence of
(below dashed line) and neuropil without Schwann cells (above dashed astrocytes, Schwann cells appeared to border the blood
line). Note that there is no distinct boundary between the area occupied vessels (Fig. 3) as described previously [10,17,18]. Ex- by Schwann cells and that lacking Schwann cells. The Schwann cell-tracellular space was evident in areas occupied by occupied neuropil shows a reduction of synaptophysin immunostaining in contrast to neuropil without Schwann cells. Some areas (*) of the Schwann cells (Fig. 3), in contrast to its absence from
Schwann cell-occupied neuropil are essentially devoid of synaptophysin neuropil occupied by the neural elements typical of spinal
immunoreactivity. Motor neurons retain synaptophysin immunostained gray matter (Fig. 2).
terminals on their cell bodies (arrows) as do many of their primary These ultrastructural observations confirm the absence dendrites (double arrowheads). A large proportion of the staining ob-of synapses from the neuropil ob-of spinal gray matter served in the ventral Schwann cell-occupied regions appears to be on large dendrites (single arrowheads) probably originating from motor occupied by Schwann cells. These findings substantiate the
neurons. Scale bar540mm. earlier observation of a loss of synaptophysin
immuno-Fig. 2. An electron micrograph of ventral neuropil lacking Schwann cells from a 63-day-old irradiated rat. This neuropil contains an abundance of both dendrites (d) and axons (a). Synapses (arrows) between dendrites and axons are frequent and are commonly contacted by astrocyte processes (arrowheads). Note that these neural elements are compactly arranged, with little or no extracellular space. Scale bar51mm.
224 T.J. Sims, S.A. Gilmore / Brain Research 882 (2000) 221 –225
staining from Schwann cell-occupied regions of spinal [8,17,18]. Schwann cells appear to displace astrocytic end-gray matter [10]. That this synaptic loss is related to the feet from their perivascular location. This displacement presence of Schwann cells rather than to the exposure to could, in turn, alter the astrocyte-mediated exchange of radiation is supported by the observation of numerous glucose and metabolites between blood vessels and neuro-synapses in contiguous neuropil exposed to radiation but pil and markedly disrupt normal metabolic patterns. There-not occupied by Schwann cells. Immunohistochemical fore, alterations of astrocytes and their processes in observations indicate that the loss of synapses from the Schwann cell-occupied regions may, in turn, contribute to Schwann cell-occupied neuropil is not simply a physical or cause the observed loss of synapses. A similar case may displacement of synaptic elements due to the invasion and be made for the observed loss of dendrites of smaller occupation of neuropil by Schwann cells. If there were diameter. These dendrites, as well as the synapses, may such a displacement or re-organization, one would antici- have metabolic or trophic requirements that make them pate a concentration of synaptophysin immunoreactivity at highly dependent on astrocyte support.
the interface between Schwann cell-occupied and Schwann In summary, this ultrastructural study confirms that cell-free areas. This was not observed, reinforcing the idea synapses are absent from the neuropil of irradiated spinal that the synapses have, in fact, disappeared. This loss of gray matter occupied by Schwann cells. In addition to synapses from Schwann cell-occupied neuropil stands in pre-synaptic terminals, small- and medium-sized dendrites marked contrast to the preservation or sparing of synapses are also lost from these Schwann cell-occupied regions. on the somata and the large primary dendrites of motor Whether these intraspinal Schwann cells directly affect the neurons surrounded by Schwann cells [10]. Why synapses pre-synaptic terminals, the dendrites or both remains to be remain at these latter sites has yet to be determined. determined.
An unanticipated finding was the absence also of small-and medium-sized dendrites from the Schwann
cell-oc-cupied neuropil. This loss contrasts with the preservation Acknowledgements of large, primary dendrites on motor neurons in the
Schwann cell-occupied areas [10]. The absence or loss of The authors thank Napoleon Phillips for his excellent both of these elements, i.e. the synapses and the small- or technical assistance and Rosemary Cornett for her excel-medium-sized dendrites, may be interrelated since the loss lent secretarial support. This study was supported by NIH of one could, in turn, result in the loss of the other. For grant NS 04761.
example, dendrites may be dependent on synaptic contacts for their continued maintenance and structural integrity. Modifications of dendrites and their spines have been
References shown to occur following peripheral nerve axotomy [1,19]
and injuries within the central nervous system (CNS) that
[1] H. Aldskogius, J. Arvidsson, G. Grant, The reaction of primary result in loss of afferent input [2,13,14]. On the other hand, sensory neurons to peripheral nerve injury with particular emphasis loss of dendrites and their post-synaptic specializations on transganglionic changes, Brain Res. 357 (1985) 27–46.
[2] G. Benshalom, E.L. White, Dendritic spines are susceptible to could, in turn, lead to the retraction of the pre-synaptic
structural alterations induced by degeneration of their presynaptic terminals. In the current study we were unable to
de-afferents, Brain Res. 443 (1988) 377–382. termine if there was a temporal sequence of involvement
[3] W.F. Blakemore, R.C. Patterson, Observations on the interactions of of the pre- and post-synaptic elements. Schwann cells and astrocytes following X-irradiation of neonatal rat
Another factor which could have a major influence on spinal cord, J. Neurocytol. 4 (1975) 473–485.
[4] R.S. Ghirnikar, L.F. Eng, Astrocyte–Schwann cell interactions in the structural integrity of both the synapses and dendrites
culture, Glia 11 (1994) 367–377. is their interaction with astrocytes. This glial cell type is
[5] S.A. Gilmore, The effects of X-irradiation on the spinal cords of reported to promote the formation and functional integrity
neonatal rats. II. Histological observations, J. Neuropath. Exp. of synapses [15]. A reduction of the astrocyte population in Neurol. 22 (1963) 294–301.
the model used in the present study has been well [6] S.A. Gilmore, Delayed myelination induced by X-irradiation of the neonatal rat spinal cord, Neurology 16 (1966) 749–753.
documented [3,16]. Astrocytes and their processes are rare
[7] S.A. Gilmore, D. Duncan, On the presence of peripheral-like in regions where Schwann cells become established but are
nervous and connective tissue within irradiated spinal cord, Anat. observed, although reduced in number [16], in adjacent
Rec. 160 (1968) 675–690.
regions not occupied by Schwann cells. One likely possi- [8] S.A. Gilmore, T.J. Sims, The role of Schwann cells in the repair of bility is that astrocytes and their processes retract from a glial cell deficits in the spinal cord, in: R.B. Wallace, G.D. Das region as the Schwann cell population becomes estab- (Eds.), Neural Transplantation and Regeneration, Springer-Verlag,
New York, 1986, pp. 245–269. lished. This possibility is supported by the observation that
[9] S.A. Gilmore, T.J. Sims, Glial–glial and glial–neuronal interfaces in astrocytes and Schwann cells segregate when co-cultured
radiation-induced, glia-depleted spinal cord, J. Anat. 190 (1997) [4], suggesting an incompatibility between these two cell 5–21.
[11] S.A. Gilmore, N.P. Phillips, P. White, T.J. Sims, Schwann cell cells and the cellular constituents normally occurring in the spinal induction in the ventral portion of the spinal cord, Brain Res. Bull. cord: An ultrastructural study in the irradiated rat, Brain Res. 276
30 (1993) 339–345. (1983) 17–30.
[12] S.A. Gilmore, T.J. Sims, J.K. Heard, Autoradiographic and ultra- [17] T.J. Sims, M.B. Durgun, S.A. Gilmore, Schwann cell invasion of structural studies of areas of spinal cord occupied by Schwann cells ventral spinal cord: the effect of irradiation on astrocyte barriers, J. and Schwann cell myelin, Brain Res. 239 (1982) 365–375. Neuropathol. Exp. Neurol. 57 (1998) 866–873.
[13] J. Hamori, Morphological plasticity of postsynaptic neurones in [18] T.J. Sims, M.B. Durgun, S.A. Gilmore, Transplantation of sciatic reactive synaptogenesis, J. Exp. Biol. 153 (1990) 251–260. nerve segments into normal and glia-depleted spinal cords, Exp. [14] C. Nitsch, R. Riesenberg, Synaptic reorganisation in the rat striatum Brain Res. 125 (1999) 495–501.
after dopaminergic deafferentation: an ultrastructural study using [19] B.E.H. Sumner, F.I. Sutherland, Quantitative electron microscopy glutamate decarboxylase immunocytochemistry, Synapse 19 (1995) on the injured hypoglossal nucleus in the rat, J. Neurocytol. 2
247–263. (1973) 315–328.