Industrial Crops and Products 12 (2000) 39 – 46
Chemical and physical characterization of water- and
dew-retted flax fibers
W.H. Morrison III
a,*, D.D. Archibald
a, H.S.S. Sharma
b, D.E. Akin
aaR.B.Russell Agricultural Research Center,PO Box5677,USDA-ARS,Athens,GA30604,USA bDepartment of Applied Plant Science,The Queens Uni6ersity of Belfast,Belfast BT9 5PX,UK
Received 17 August 1999; accepted 17 November 1999
Abstract
The composition of dew-retted and water-retted flax fibers were evaluated by chemical and mass spectral analyses to determine their chemical differences. Phenolics, waxes, cutin, and carbohydrates were determined by gas liquid chromatography. Water-retted fibers contained more residual wax and lower arabinose content than the dew-retted and were finer and stronger. Pyrolysis mass spectrometric analysis differentiated water- and dew-retted fibers. Principal component analysis of the chemical data including both strength and fineness measurements produced a grouping of the water-retted samples distinct from the dew-retted fibers. Principal component analysis of the mass spectral data produced the same grouping based mass markers characteristic of the chemical components that were associated with the initial grouping with fineness and strength measurements. Published by Elsevier Science B.V.
Keywords:Flax; Retting; Mass spectrometry; Phenolics; Sugars; Lignin
www.elsevier.com/locate/indcrop
1. Introduction
Flax (Linum usitatissimum), the source for linen, is an important commercial crop in Europe and is gaining attention in the United States (Colombo, 1994). Retting, the process to free bast fibers from the core and epidermis/cuticle, is tra-ditionally accomplished by water- or dew-retting. More recently, commercial enzymes have been
used for retting. Both bacteria and fungi produce pectinase enzymes, which are important for ret-ting flax. While polyendogalacturonase, lyases, and pectin methyl esterases are especially impor-tant for retting, it has been shown that xylanases and cellulases also contribute to fiber release (Sharma and Van Sumere, 1992; Sharma et al., 1992).
In water-retting, a variety of anaerobic bacteria are present and are considered as the primary agents responsible for fiber release. Several species of bacteria have been identified and investigated during tank retting, of which spore-forming Clostridium spp. have been shown to contribute
* Corresponding author. Tel.: +1-706-5463361; fax: + 1-706-5463607.
E-mail address: [email protected] (W.H. Morrison III)
considerably to pectin-degrading activity and, therefore, retting. It is suspected that sources for retting bacteria are adhering soil particles, stem dust, air, and water. Bacterial flora are generally similar in various investigations (Sharma et al., 1992).
Currently, dew-retting is the method of choice for obtaining flax fiber due to the cost and pollu-tion arising from water-retting (Sharma et al., 1992; Van Sumere, 1992). In dew-retting, the plant is pulled from the ground and allowed to lay in the field. Naturally occurring fungi are primarily responsible for retting, with moisture and temperature conditions substantially affecting fungal activity and the quality of retting (Sharma et al., 1992). As with bacteria in water-retting, fungi responsible for dew-retting produce pecti-nases that disintegrate the flax stem to release fibers. With the switch from water- to dew-retting after World War II, the regions around Nor-mandy in France became the most important producers of flax in the world mainly due to the mild weather during the growing and retting period.
Generally, water-retting produces better quality fiber than dew-retting. Consistent conditions for bacterial growth and activity exist in water-ret-ting, and the evenness of inoculum and coloniza-tion by bacteria are likely to results in a more uniform and consistent ret. In contrast, fungal colonization for dew-retting, that is obviously af-fected by the environment, is likely to be less uniform on the stems. In addition, during dew-retting, cellulolytic fungi such asEpicoccul nigrum (Akin et al., 1998) can proliferate and effect over retting and weaken fibers.
Efficient retting results in the easy removal of particles of shive, cuticle, and surface waxes. Residual shive and cuticle can affect bleaching and dyeing characteristics, whereas excess wax results in poor dyeing characteristics (Kernaghan and Keikens, 1992). Therefore, it is particularly important to determine the effects of retting on surface active constituents.
In earlier work using chemical and instrumental analyses and pyrolysis mass spectrometry, we demonstrated the possible relationship of waxes and residual cutin to the quality of the fiber and
yarn (Morrison and Archibald, 1998; Morrison et al., 1999b). Fiber and yarn samples graded low in quality had more wax and cutin markers than the higher quality samples. High quality fibers con-tained greater amounts of protein and nucleic acids indicating greater or deeper invasion of the retting organisms, and therefore, more effective retting.
In the current study, we evaluated a series of water- and dew-retted flax samples by chemical and pyrolysis mass spectrometric analysis to as-certain the action of the retting organisms on the chemical constituents related to quality. In addi-tion, chemical and measurements related to qual-ity such as fineness and strength were evaluated using multivariate analysis to relate chemical data and properties important to industrial use of flax.
2. Materials and methods
Flax fibers consisted of five water- and 11 dew-retted flax from Holland, Poland, Ireland, France, Germany and Belgium were cut to about 2 mm length and ground to a fine powder in a Spex 5100 mixer/mill (SPEX Certiprep, Metuchen, NJ).
2.1. Chemical analysis
W.H.Morrison III et al./Industrial Crops and Products12 (2000) 39 – 46 41
2.2. In-source pyrolysis mass spectrometry
In-source pyrolysis mass spectrometry (PyMS) was performed on a Finnigan GCQ equipped with a direct exposure probe (rhenium loop). Analyti-cal conditions were: ionization energy, 20 eV; mass range 50 – 500; scan time 1 s; temperature rise, ca. 10 – 675°C; ion source temperature 175°C. Finely ground samples were analyzed by prepar-ing a suspension of the sample in distilled water using a glass mortar and pestle. A small amount of the suspension was placed on the loop and water evaporated under vacuum. Each sample was run in triplicate.
2.3. Fiber strength and fineness
Test fibers were conditioned for a minimum of 12 h at 20°C and 65% RH. The tensile strength of the fibers was calculated as the mean of 50 indi-vidual fiber bundles of 1 cm test length measured according to the recommended methods of the British Standards Institute using a tensiometer (Testometric, model no M250-2.5 KN, Rochdale, England). Fiber fineness was measured by air flow meter (Wira fineness meter, Reynolds and Bran-son, Ltd, Leeds, England) using 2.5 g of fiber samples and fineness was calculated as the mean of 16 measurements per sample. Each treatment was assessed in triplicate.
2.4. Statistical analysis
Samples were analyzed by t-test differences identified atP50.05. The statistical analysis used on the pyrolysis mass spectral data has been described by Morrison and Archibald (1998).
3. Results and discussion
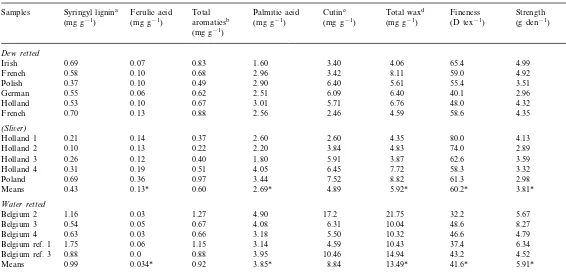
The chemical degradation products of the 4 M NaOH treatment are shown in Table 1. Aromat-ics, an indication of total lignin, are the sum of the ferulic acid, guaiacyl and syringyl lignin moi-eties. Lignin content is quite low in flax fiber (0.22 – 1.66 mg g−1, Table 1) compared to other
bast tissues such as kenaf, which ranges from 7.3
to 8.9 mg g−1(Morrison et al., 1999a). The major
concentration of lignin in the flax plant was found in the shive or core, and higher lignin contents reflect less effective retting due to poor separation of fiber and shive. Generally, the total aromatics were lower in dew-retted samples containing 0.60 mg g−1than the 0.92 mg g−1 in the water-retted
samples. Dew-retted fiber had lower syringyl lignin content, 0.43 mg g−1, than water-retted
fiber with a syringyl content of 0.86. mg g−1. This
observation may reflect the role of phenolic acids in strengthening flax fiber. Ferulic acid is associ-ated with lignin and in the crosslinking between lignin and hemicellulose in the cell wall carbohy-drates (Hartley et al., 1988, 1990; Hartley and Ford, 1990). However, ferulic acid content in water- and dew-retted samples was low at 0.034 and 0.13 mg g−1, respectively, and significantly
different.
The plant cuticle is made up of cutin, which is a polyester derived entirely from aliphatic monomers imbedded in soluble waxes (Kolat-tukuty and Espelie, 1985). Palmitic acid, a wax component shown to be associated with lower fiber quality (Morrison and Archibald, 1998; Morrison et al., 1999b), was similar (P]0.05) among the samples. However, the dew-retted sam-ples tended to be lower in palmitic acid with values of 2.69mg g−1 compared to 3.85 mg g−1
for the water-retted samples. A t-test showed that dew- and water-retted samples were different (P50.05). A cutin marker, 8,16-dihydroxyhex-adecanoic acid, also associated with poor quality fiber (Morrison et al., 1999b), was lower in the dew-retted samples with values of 4.89 mg g−1
compared to 8.84 mg−1for the water-retted
sam-ples, indicating less efficient removal of cuticle from water-retted samples. Total wax was signifi-cantly lower in dew-retted samples with a value of 5.92 mg g−1
compared to 13.49 mg g−1
for the water-retted samples. The sum of wax and cutin values was 10.81 mg g−1 for dew-retted samples
compared to 22.33 mg g−1 for the water-retted
samples.
W
Wax, cutin and aromatic constituents and fineness and strength of dew and water retted flax fibers
Strength
Syringyl lignina Palmitic acid
Samples Ferulic acid Total Cutinc Total waxd Fineness
(D tex−1)
Irish 0.69 0.07 0.83 1.60 3.40
8.11 59.0 4.92
3.42 2.96
French 0.58 0.10 0.68
2.90 6.40 5.61 55.4 3.51
Polish 0.37 0.10 0.49
6.40 40.1 2.96
0.53 0.10 0.67 3.01 6.76 48.0 4.32
Holland
French 0.70 0.13 0.88 2.56 2.46 4.59 58.6 4.35
(Sli6er)
4.35 80.0
0.14 0.37 4.13
0.21
Holland 1 2.60 2.60
4.83 74.0 2.89
Holland 2 0.10 0.13 0.22 2.20 3.84
3.87 62.6 3.59
5.91
0.26 0.12
Holland 3 0.40 1.80
6.45
0.31 0.19 0.51 4.05 7.72 58.3 3.32
Holland 4
3.44 7.52 8.82 61.3 2.98
Poland 0.69 0.36 0.97
2.69* 4.89 5.92* 60.2* 3.81*
0.43
Belgium 2 4.90 17.2
10.04
Belgium 3 0.54 0.05 0.67 4.08 6.31 48.6 8.27
10.32 46.6 4.79
5.50 3.18
0.66
Belgium 4 0.63 0.03
4.59
1.75 0.06 1.15 3.14 10.43 37.4 6.34
Belgium ref. 1
14.94 43.2
Belgium ref. 3 0.88 0.0 0.88 3.95 10.46 4.52
13.49* 41.6* 5.91*
8.84 0.034*
0.99 0.92 3.85*
Means
aSyringyl lignin is the sum of syringladehyde, acetosyringone, syringic acid and ferulic acid.
bTotal aromatics are the sum of vanillin, acetovanillone, vanillic acid, syringaldehyde, acetosyringone, syringic acid, andp-coumaric. cCutin marker: 8,16-dihydroxyhexadecanoic acid.
dSum of palmitic acid and C 22–30 straight chain alcohols and C 24–28 straight chain fatty acids.
W.H.Morrison III et al./Industrial Crops and Products12 (2000) 39 – 46 43
mg g−1 compared to water-retted fiber with a
value of 4.70 mg g−1. Because arabinose is
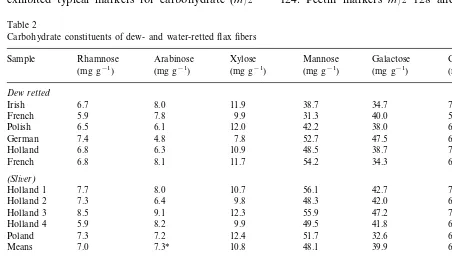
associ-ated with hemicellulose, these data indicassoci-ated that anaerobic bacteria degraded some hemicellulose in the water-retted samples. If non-structural hemicellulose is removed, fiber strength is not compromised as long as the structural hemicellu-lose, which exists in close association with pheno-lic acids and cellulose, is not removed during retting (Morvan et al., 1989a,b; Sharma et al., 1999). This is supported by the significantly higher strength of the finer water-retted samples compared to dew-retted fibers (Table 1). No sig-nificant differences were present in rhamnose, xy-lose, mannose, galactose and glucose among the fibers.
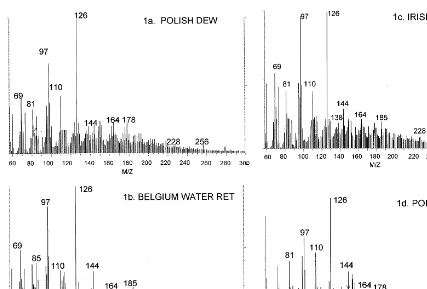
In-source, low-voltage pyrolysis mass spectra of typical water and dew retted samples are shown in Fig. 1. Lignin is not a major component of flax fiber (Akin et al., 1996) and its typical lignin markers were quite small (Table 3). All samples exhibited typical markers for carbohydrate (m/z
31, 32, 43, 55, 60, 72, 74, 82 57, 60, 73, 85, 86, 96, 98, 100, 102, 110, 112, 126, 144, 58, 85, 86, 114), myristic (m/z 228), and palmitic acids (m/z 256). The distinguishing feature of these mass spectra was the presence of a hexosan marker (m/z 144) in all water-retted samples and Irish dew-retted. All other dew-retted samples exhibited a similar and relatively low relative abundance of this marker.
Statistical treatment of PyMS data using multi-variate analysis of selected masses is shown in Fig. 2. Principal component analysis grouped water-retted samples separate from dew-water-retted samples sharing characteristics common to both retting treatments. The only exception was the Irish dew-retted sample that grouped with the water-dew-retted samples. The principal mass marker responsible for grouping wasm/z144, a marker characteristic of hexosans. Other markers associated with the dew-retted samples were cellulose markers (m/z, 110, 112, 126), and a guaiacyl lignin marker m/z 124. Pectin markers m/z 128 and 140 are also
Table 2
Carbohydrate constituents of dew- and water-retted flax fibers
Rhamnose Arabinose Mannose Galactose
Sample Xylose Glucose
French 31.3 40.0 585
38.0 618
6.1 12.0
Polish 6.5 42.2
47.5 687
German 7.4 4.8 7.8 52.7
38.7 709
6.8
Holland 6.3 10.9 48.5
666
Holland 1 56.1 42.7 716
Holland 2 7.3 6.4 9.8 48.3 42.0 680
Poland 7.3 12.4 51.7
7.0 7.3* 10.8 48.1
Means 39.9 672
Water retted 7.6
Belgium 2 4.9 14.7 43.5 40.6 713
7.0 4.8 11.1
Belgium 3 53.1 31.7 696
32.0
Belgium ref. 1 46.3 33.7 647
Belgium ref. 3 6.2 4.8 13.8 44.1 38.7 660
668
Fig. 1. Representative in-source pyrolysis low voltage (20 eV) of (A) Polish dew-retted flax; (B) Belgium water-retted flax; (C) Irish dew-retted flax; (D) Polish dew-retted sliver.
associated with dew-retted samples. The presence of pectin markers indicates that all samples were probably not completely retted fiber, which is also supported by the presence of waxes and cuticle in all samples. Chemical analysis showed no differ-ence in guaiacyl lignin with average values be-tween 0.26 and 0.04 mg g−1 (data not shown);
however, the presence of a guaiacyl lignin marker (m/z 124) in the PyMS spectrum suggested this lignin is important in the grouping of dew- and water-retted flax. Chemical analysis showed sy-ringyl lignin to be significantly higher in the wa-ter-retted samples but did not contribute to the PyMS groupings.
Using selected analytical values, principal com-ponent analysis was conducted and the results shown in Fig. 3. Water-retted samples were grouped separately from the dew-retted samples. Generally, water-retted samples were
character-ized by greater amounts of total aromatics, waxes, cutin, xylose, finer fibers and greater strength.
W.H.Morrison III et al./Industrial Crops and Products12 (2000) 39 – 46 45
the past and current studies, clear relationships appear to exist that help establish criterion for evaluation retting efficiency. To date, few samples have been evaluated by chemical and physical methods, but it is clear that the measurements are sensitive to the various processes that take place during retting.
Fig. 3. Principal component analysis of chemical and physical measurement data for dew-retted flax (D); water-retted flax (W); Irish dew-retted (D(I)) and dew-retted slivers (D(s)). Compounds shown are those associated with each treatment. The first principal component explains 43% of the variance and the second principal component explains 16% of the variance.
Table 3
Pyrolysis low-voltage EI mass peaks
Compound Mass peaksa
31, 32, 43, 55, 60, 72, 74, 82 Non-specific polysaccharides
fragment ions
57, 60, 73, 85, 86, 96, 98, Hexose polymers
100, 102, 110, 112, 126, 144 Pentose polymers 58, 85, 86, 114
Rhamanose 128
Methylgalacturonan 140, 172 108 Phenols
Phenolic acids
p-coumaric 120, 147, 164
Ferulic 150, 177, 194
Guaiacyl lignin 124, 137, 138, 150, 152, 164, 166, 178, 180
Syringyl lignin 154, 167, 168, 180, 182, 194, 196, 208, 210
Nucleic acids and protein 81, 83, 91, 92,111, 117, 186 Fatty acids 171, 228, 236, 256, 284
aMass assignments from Van Arendonk et al., 1997.
4. Conclusions
Water- and dew-retted fibers had different fer-ulic acid, palmitic acid, total waxes, arabinose, fineness and strength values. Fiber with higher wax content also had higher total phenols, sy-ringyl lignin, and cutin. While more samples of each treatment will be needed for a more com-plete assessment, this study does show differences between dew- and water-retted flax, which suggest that high fiber strength in water-retted fiber is probably due to conservation of the cross-linking fractions. In addition, higher content of wax and cutin markers in the finer and stronger water-retted fibers does show the basic difference in the two retting methods.
References
Akin, D.E., Gamble, G.R., Morrison, W.H., Rigsby, L.L., 1996. Chemical and structural analysis of fiber and core from flax. J. Sci. Food Agric. 72, 155 – 165.
Akin, D.E., Rigsby, L.L., Henrikson, G., Eriksson, K-E.L., 1998. Structural effects on flax stems of three potential retting fungi. Textile Res. J. 68, 515 – 519.
Colombo, A., 1994. Linen — world market development key factors — USA. In: Anderson, J.F., Shiavonia, M.S. (Eds.), Proceedings World Flax Symposium, The Connect-icut Agricultural Experiment Station, New Haven, CT, USA, pp. 87 – 101.
Englyst, H.N., Cumming, J.H., 1988. Improved method for measuring dietary fiber as non-starch polysaccharides in plant foods. J. Assoc. Off. Anal. Chem. 71, 808 – 814. Englyst, H.N., Quigley, M.E., Hudson, G.J., 1994.
Determina-tion of dietary fiber as non-starchpolysaccharides with gas-chromatography, high-performance liquid chromato-graphic or spectrophotometric measurements of con-stituent sugars. Analyst 119, 1497 – 1509.
Hartley, R.D., Ford, C.W., 1990. Cyclodimers of p-coumaric and ferulic acids in the cell wall of tropical grasses. J. Sci. Food Agric., 29 – 43.
Hartley, R.D., Morrison, W.H., Himmelsbach, D.S., Borne-man, W.S., 1990. Cross-linking of cell wall phenolic ara-binoxylans in graminaceous plants. Phytochemistry 29, 3705 – 3709.
Hartley, R.D., Whatley, F.R., Harris, P.J., 1988. 4,4% -dihy-droxytruxillic acid as a component of cell walls ofLoium multiflorum. Phytochemistry 27, 349 – 351.
Hoebler, C., Barry, L.D., Delort-Laval, J., 1989. Rapid hy-drolysis of plant cell wall polysaccharides by gas – liquid chromatography. J. Agri. Food Chem. 37, 360 – 367. Kernaghan, K., Keikens, P., 1992. Bleaching and dying of
linen. In: Sharma, H.S.S., Van Sumere, C.F. (Eds.), The Biology and Processing of Flax. M Publications, Belfast, UK, pp. 343 – 345.
Kolattukuty, P.E., Espelie, K.E., 1985. Biosynthesis of cutin, suberin, and associated waxes. In: Higuchi, T. (Ed.), Biosynthesis and Biodegradation of Wood Components. American Academic Press, Orlando, FL, USA, pp. 161 – 207.
Morrison, W.H., Akin, D.E., Archibald, D.D., Dodd, R.B., Raymer, P.L., 1999a. Chemical and instrumental charac-terization of maturing kenaf. Ind. Crops Prod. 10, 21 – 34. Morrison, W.H., Akin, D.E., Himmeslbach, D.S., Gamble, G.R., 1999b. Chemical, microscopic, and instrumental analysis of graded flax fiber and yarn. J. Sci. Food Agric. 79, 3 – 10.
Morrison, W.H., Archibald, D.D., 1998. Analysis of graded flax fiber and yarn by pyrolysis mass spectrometry and pyrolysis gas chromatography mass spectrometry. J. Ag. Food Chem. 46, 1870 – 1876.
Morrison, W.H., Akin, D.E., Ramaswamy, G., Baldwin, B., 1996. Evaluating chemically retted kenaf using chemical, histochemical, and microspectrophotometric analysis. Tex-tile Res. J. 66, 651 – 656.
Morvan, O., Jauneau, A., Morvan, C., Voreux, H., Demarty, M., 1989a. Biosynthese de pectins et differenciations des fibers cellulosique au cours de la croissance lin. Can. J. Bot. 67, 135 – 139.
Morvan, C., Abdul-Hafez, A., Morvan, O., Jauneau, A., Demarty, M., 1989b. Estude physiocochimique et bio-chemique de polysaccharides extraits de lin sous-roui. Plant Physiol. Biochem. 27, 451 – 459.
Sharma, H.S.S., Faughey, G., Lyons, G., 1999. Comparison of physical, chemical and thermal characteristics of water-, dew-, and enzyme retted flax fibers. J. Appl. Polymer Sci. 74, 139 – 143.
Sharma, H.S.S., Lefevre, J., Boucaud, J., 1992. Role of micro-bial enzymes during retting and their affects on fiber characteristics. In: Sharma, H.S.S., Van Sumere, C.F. (Eds.), The Biology and Processing of Flax. M Publica-tions, Belfast, UK, pp. 157 – 198.
Sharma, H.S.S., Van Sumere, C.F., 1992. Enzyme treatment of flax. Genetic Engin. Biotechnol. 12, 19 – 23.
Van Arendonk, J.J.C.M., Niemann, G.J., Boon, J.J., 1997. The effect of enzymatic removal of proteins from plant leaf material as studied by pyrolysis-mass spectrometry: detec-tion of addidetec-tional protein marker fragment ions. J. Anal. Appl. Pyrolysis 42, 33 – 51.
Van Sumere, C.F., 1992. Retting of flax with special reference to enzyme retting. In: Sharma, H.S.S., Van Sumere, C.F. (Eds.), The Biology and Processing of Flax. M Publica-tions, Belfast, UK, pp. 157 – 198.