www.elsevier.com/locate/ibmb
Inhibition of insect juvenile hormone epoxide hydrolase:
asymmetric synthesis and assay of glycidol-ester and epoxy-ester
inhibitors of Trichoplusia ni epoxide hydrolase
Russell J. Linderman
a,*, R. Michael Roe
b, Shannon V. Harris
a, Deborah M.
Thompson
baDepartment of Chemistry, North Carolina State University, Raleigh, NC 27695, USA bDepartment of Entomology, North Carolina State University, Raleigh, NC 27695, USA Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
Juvenile hormone (JH) undergoes metabolic degradation by two major pathways involving JH esterase and JH epoxide hydrolase (EH). While considerable effort has been focussed on the study of JH esterase and the development of inhibitors for this enzyme, much less has been reported on the study of JH-EH. In this work, the asymmetric synthesis of two classes of inhibitors of recombi-nant JH-EH from Trichoplusia ni, a glycidol-ester series and an epoxy-ester series is reported. The most effective glycidol-ester inhibitor, compound 1, exhibited an I50of 1.2×1028 M, and the most effective epoxy-ester inhibitor, compound 11, exhibited an
I50of 9.4×1028M. The potency of the inhibitors was found to be dependent on the absolute configuration of the epoxide. In both
series of inhibitors, the C-10 R-configuration was found to be significantly more potent that the corresponding C-10 S-configuration. A mechanism for epoxide hydration catalyzed by insect EH is also presented. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Juvenile hormone; Epoxide hydrolase; JH metabolism; Trichoplusia ni; Inhibitors; Enantioselective
1. Introduction
The insect juvenile hormones (JHs) are methyl esters of farnesoic acid 10,11-epoxide (JH III) and analogous compounds, which function as important regulatory fac-tors in embryogenesis, larval and adult development, metamorphosis, reproduction, diapause, migration, poly-morphism, and metabolism (Nijhout, 1994; Roe and Venkatesh, 1990; de Kort and Granger, 1996; Hammock, 1985). The two primary metabolic degradation pathways of JH in insects are ester hydrolysis by JH esterase and epoxide hydration by an epoxide hydrolase (EH). The development of specific inhibitors of JH esterase has been instrumental in demonstrating a biological role for this enzyme in insects (Hammock et al., 1982, 1984; Prestwich et al., 1984; Abdel-Aal and Hammock, 1985;
* Corresponding author. Tel.:+1-919-515-3616; fax:+ 1-919-515-8920.
E-mail address: russell[email protected] (R.J. Linderman).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 4 8 - 5
Linderman et al., 1987, 1989). In contrast, significantly less information is available on JH epoxide hydrolase (JH-EH) (Roe and Venkatesh, 1990; de Kort and Granger, 1996).
The complete coding region for an EH has been cloned and sequenced by our laboratories from a fat body (L5D3) cDNA library derived from last stadium
Trichoplusia ni, GenBank accession no. U73680 (Roe et
al., 1996; Harris et al., 1999). The full length fat body EH cDNA was 1887 bp in length and consisted of an 81 bp 59 untranslated region (UTR), a 1389 bp ORF encoding 463 amino acids, and a 416 bp 39 UTR. The predicted protein amino terminus is hydrophobic sug-gesting that the protein encoded by U73680 is a microso-mal enzyme. A cDNA sequence encoding a JH-EH, showing significant similarity to vertebrate mEH, has also been reported by Prestwich, Hammock and co-workers (Wojtasek and Prestwich, 1996; Debernard et al., 1998). The two insect EH cDNAs from T. ni and
Manduca sexta appear to code for microsomal enzymes.
ident-ical at the amino acid level with mEH from rat and human, respectively; and 46% identical with M. sexta JH-EH (Wojtasek and Prestwich, 1996). The T. ni fat body EH also contains the identical amino acid catalytic triad (Glu-403, Asp-226 and His-430) found in M. sexta EH, microsomal EHs from rat (Falany et al., 1987), human (Skoda et al., 1988) and rabbit (Hassett et al., 1989), and a haloalkane dehalogenase (HLD1,
Xanthob-acter autotrophicus) (Janssen et al., 1989).
The development of inhibitors for mammalian EHs has been somewhat successful. Hammock and co-work-ers developed chalcone oxides as effective inhibitors for mammalian soluble EH (sEH) (Morisseau et al., 1998; Borhan et al., 1995; Pinot et al., 1995). Trichloropropene oxide, cyclohexene oxide and glycidyl derivatives have been shown to be microsomal EH (mEH) inhibitors (Tzeng et al., 1996). Nearly all of the examples of EH inhibition involve epoxides which typically serve as competitive (alternative substrate) inhibitors, with the exception of recent work by Hammock and co-workers (Morisseau et al., 1999) that revealed ureas and carbam-ates could function as inhibitors of murine sEH. The inhibitors of insect EHs are generally not effective and exhibit considerable variation in activity depending on the insect and substrate used for the assay. Hammock and co-workers examined several glycidyl ethers and epoxy-alcohol JH analogs as inhibitors, but only the JH analogs provided significant levels (µM) of inhibition (Casas et al., 1991; Harshman et al., 1991). Typically, mammalian mEH or sEH inhibitors are not as effective against insect EH.
We previously investigated several potential inhibitors for T. ni JH-EH using crude microsomal enzyme prep-arations (Roe et al., 1996; Harris et al., 1999). Inhibitors designed to mimic a polarized (or ionic) transition state were only modest competitive inhibitors of T. ni JH-EH while non-juvenoid long chain aliphatic epoxides were the most potent competitive substrates to JH III. The most effective inhibitor identified in our earlier studies, methyl 10,11-epoxy-11-methyldodecanoate (MEMD), exhibited an I50of 80µM. Mimicry of the length of the JH backbone and of the methyl ester functional group of JH was found to be important in substrate binding. We now report a more extensive structure activity relationship (SAR) study of glycidol-ester and epoxy-ester inhibitors of JH-EH which further examines epox-ide substitution patterns, and addresses the question of enantioselectivity in epoxide hydration by recombinant
T. ni JH-EH.
2. Materials and methods
2.1. Insects
Larvae of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae), were reared on an artificial
diet at 27±1°C and a 14 h light: 10 h dark cycle (Roe et al., 1982). Gate I last instars and specifically aged last stadium larvae were selected as previously described (Kallapur et al., 1996). The last stadium in our studies was 4 days in duration with larvae wandering on day 3, becoming prepupae on day 4 and undergoing ecdysis to pupae by the next day. cDNA library construction from fat body tissue and library screening as described in Harris et al. (1999) provided plasmid pG6-1 containing the full-length EH insert, TmEH-1.
2.2. Baculovirus expression
Baculovirus expression of the full length epoxide hydrolase was obtained using the Autographica
califor-nica multiple nuclear polyhedrosis virus (AcMNPV)
vector system. The pG6-1 plasmid was sequentially digested with BamHI (Promega) and Xho1 (Stratagene), providing a fragment containing the full-length epoxide hydrolase message. This fragment (1908 bp) was ligated using T4 DNA ligase (Novagen) with the viral transfer vector pBacPak8 (pBP8, CLONTECH, Palo Alto, CA), previously treated with BamHI (Promega) and Xho1 (Stratagene), which provided the recombinant baculo-virus plasmid (pBP8–G6-1). Recombinant baculobaculo-virus was obtained by co-transfection of Spodoptera
frugip-erda (Sf9) insect cells with pBP8–G6-1, containing the
full-length EH, and Bsu361-digested BacPak6 viral DNA (CLONTECH), providing the virus vG6-1. Suc-cessful co-transfection and expression was verified by Northern blot analysis, using TmEH(237) (Roe et al., 1996) as a probe, of EH infected Sf9 cell RNA. All virus manipulations were according to O’Reilly et al. (1992).
2.3. Assay of EH activity
regression analysis from at least four different inhibitor concentrations which bracketed the I50 and fell within the linear range immediate to the I50. At each inhibitor concentration, the assays were run in triplicate. Inhibi-tors were dissolved in ethanol, and the concentration of ethanol due to the addition of inhibitor never exceeded 1% of the reaction volume. Inhibition assays were always compared with ethanol controls.
2.4. Chemical synthesis
Chemical reagents were purchased from Aldrich Chemical Co (Milwaukee) and purified prior to use by either distillation or chromatography. Alkyllithium reagents were titrated in toluene using 1,10-phenanthro-line as an indicator. Reactions were typically carried out under an inert atmosphere of argon using solvents that were distilled immediately prior to use.1H,13C, and19 F-NMR spectra were recorded on a 300 MHz spectrometer in CDCl3. Chromatography was performed on silica gel 60, 230–400 mesh ASTM, obtained from EM Science. Elemental analyses were carried out by Atlantic
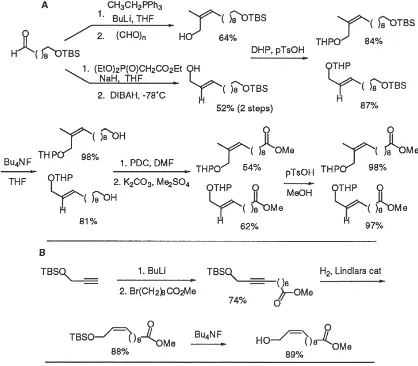
Fig. 1. A: Stereospecific synthesis of trisubstituted and trans-disubstituted allylic alcohols, precursors for Sharpless asymmetric epoxidation reac-tion. B: Stereospecific synthesis of cis-disubstituted allylic alcohol precursors for asymmetric epoxidareac-tion.
Microlab Inc. (Norcross, GA). High resolution and rou-tine mass spectra were obtained at the Mass Spec-troscopy Laboratory for Biotechnology at NC State Uni-versity. All new compounds were fully characterized by spectroscopic methods and combustion or mass spectro-scopic analysis. A description of the chemical synthesis of the inhibitors is given in Section 3.
3. Results
and chemoselective reduction of the unsaturated ester to the alcohol. The cis allylic alcohols, leading to the cis epoxide analogs, were obtained by stereospecific partial reduction of the corresponding alkyne. The synthetic routes are detailed in Fig. 1. Asymmetric epoxidation of each alkene was then carried out using (+) and (2) tar-trate esters as the chiral auxiliary to independently pro-vide each enantiomer of the epoxide. The enantiomeric excess was determined to be greater than or equal to
Fig. 2. A: Asymmetric epoxidation of trisubstituted allylic alcohols using the Sharpless methodology to provide glycidol-ester inhibitors of JH-EH, and conversion of the glycidol-esters to epoxy-esters by chemoselective reduction. B: Asymmetric epoxidation of the cis-disubstituted allylic alcohols and conversion to the epoxy-ester inhibitors. The trans-disubstituted allylic alcohols were converted to the corresponding epoxides by the same synthetic sequence. All asymmetric epoxidation reactions occurred in$95%ee.
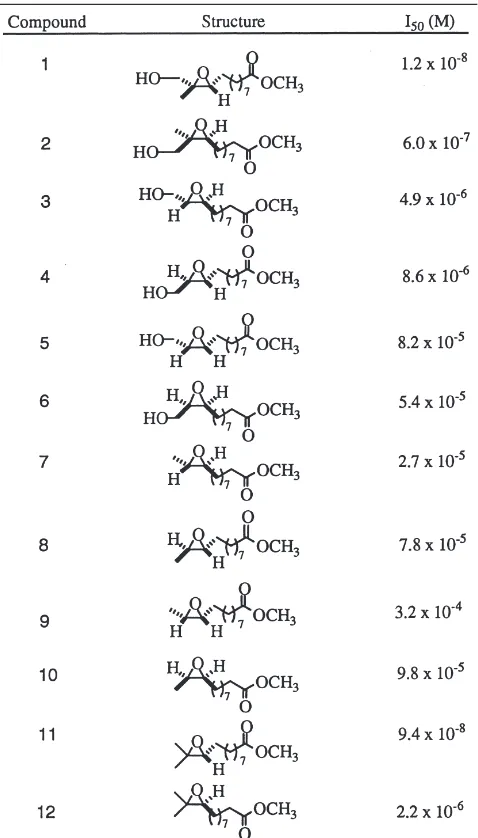
95% (by 19F-NMR analysis of the Mosher ester deriva-tives (Dale et al., 1969)) in all cases. The epoxy-ester inhibitors 7–12 were finally obtained by chemoselective deoxygenation of the glycidols 1–6 by initial conversion to the iodide and reduction with sodium cyanoborohyd-ride (Hutchins et al., 1977) (see Fig. 2). The results of the inhibition assays of the glycidol-esters 1–6 and the epoxy-ester inhibitors 7–12 are given in Table 1.
Table 1
I50data for enantiopure MEMD and MEMD analogsa
aI
50is the molar inhibitor concentration that reduces recombinant
T. ni JH-EH activity by one-half. The I50values were determined as described in Section 2. The r2was$0.98.
using crude cell homogenates prepared from TmEH-1 infected Sf9 cells as the enzyme source, [3H]-JH III as the substrate, with OTFP added as an inhibitor of JH esterase as described in Section 2. All of the assays were completed within the linear range of enzyme activity with respect to time and protein concentration. Complete JH esterase inhibition was confirmed by TLC analysis of the assay products. Several general trends were observed from the data in Table 1. In terms of the epoxide substi-tution pattern, as expected, the trisubstituted epoxides 1,
2, 11 and 12 were the most effective inhibitors of T. ni
JH-EH. The I50values for these compounds were gener-ally an order of magnitude less than the I50 values for the corresponding disubstituted epoxides, 3, 4, 5–10. Of
the disubstituted epoxides, the trans-disubstituted epox-ides 3, 4, 8 and 7 were more effective inhibitors than the corresponding cis-epoxides. Most significantly, the
R enantiomer of MEMD 11, I50=9.4×1028 M, proved to be a more effective inhibitor than the S enantiomer 12,
I50=2.2×1026 M. The trend of the C-10 R-configuration affording the highest degree of inhibitory efficacy is noted throughout this series of compounds. All of the glycidol-esters 1–6 examined were also effective tors of T. ni JH-EH and tended to be more potent inhibi-tors than the corresponding epoxy-esters 7–12, by an order of magnitude in most cases. Compound 1, the gly-cidol analog of R-MEMD, was the overall most effective inhibitor determined in this study, I50=1.2×1028 M. As with the epoxy-esters, the R absolute configuration at C-10 was a more potent inhibitor than the C-C-10 S com-pounds for the entire series of comcom-pounds.
4. Discussion
et al., 1998). Significantly, the ester intermediate formed
from 2S-glycidyl-4-nitrobenzoate was found to
hydrolyze substantially slower than the 2R enantiomer. The formation of a more stable ester intermediate from the 2S enantiomer may be due to a conformational change of the enzyme induced by nucleophilic attack of the aspartate residue on the epoxide (Armstrong, 1999). Aside from the studies of Prestwich and co-workers on EH catalyzed epoxide hydration of disparlure (Graham and Prestwich, 1994), little data are available for insect EH enantioselectivity. Hammock showed that EH cata-lyzed hydration of JH by M. sexta EH was inhibited more by the 10S enantiomer of a JH analog than the 10R enantiomer (Casas et al., 1991). These data are in accord with the recent mammalian mEH mechanistic data reported by Armstrong and co-workers described above. From the alignment of the amino acid sequences for
T. ni and M. sexta JH-EHs with mammalian mEHs and
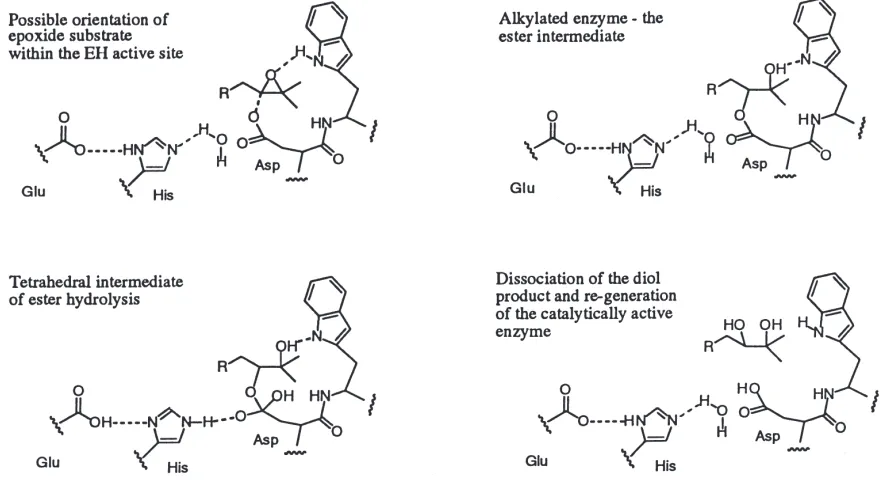
HLD1, all of these enzymes appear to possess the same catalytic triad of Asp/His/Glu. Mechanism studies in our laboratories using lyophilized T. ni microsomes indi-cated that epoxide hydration of JH III in 97.9% 18 O-labeled water resulted in only 91.0% 18O-incorporation at C10 (Linderman et al., 1995). These results are con-sistent with a multiple turnover reaction involving an ester intermediate. Based on these data and the proposed mammalian mEH and HLD1 mechanisms discussed above, we have illustrated a probable mechanism for insect JH-EH in Fig. 3. The active site Asp residue undergoes nucleophilic attack at the least substituted oxi-rane carbon of the substrate to produce an ester inter-mediate. The adjacent Trp residue may activate the epoxide toward nucleophilic attack (Muller et al., 1997);
Fig. 3. Proposed mechanism of insect epoxide hydrolase catalyzed epoxide hydration.
however, recent data from Armstrong and co-workers (Laughlin et al., 1998) implies that activation by a Trp residue may not be obligatory. Hydrolysis of the ester intermediate is then required for re-activation of the enzyme. The active site His residue is presumed to acti-vate water for this purpose with a Glu residue acting as the proton scavenger from His.
The observations of Armstrong (Tzeng et al., 1998) and Hammock (Casas et al., 1991) discussed above, led us to hypothesize that the relative rate of epoxide hydration of MEMD would be dependent upon the absolute configuration of the epoxide. In addition, we believed that the glycidol analogs of R- and S-MEMD and related compounds would be at least moderately potent inhibitors. As indicated in Section 3, these hypotheses were correct. Insect JH-EH appears to have a very similar if not identical dependence on the absolute configuration of the epoxide of the inhibitor as that observed in mammalian EHs. We believe that these data give further support to the premise that the insect and mammalian mEHs employ a very similar if not identical catalytic mechanism for epoxide hydration.
hydrolysis by inducing a conformational change in the enzyme, or could function to reduce the rate of dis-sociation of the diol product from within the enzyme active site. These novel inhibitors of insect EH may be useful in the elucidation of the functional role of EH in insect development.
Acknowledgements
We thank Douglas D. Anspaugh and Woodward D. Bailey for assistance in insect rearing. The USDA Com-petitive Grants Program (90-37263 and 93-01607 to RJL and RMR) supported this research.
References
Abdel-Aal, Y.A.I., Hammock, B.D., 1985. 3-Octylthio-1,1,1-trifluoro-2-propanone, a high affinity and slow binding inhibitor of juvenile hormone esterase from Trichoplusia ni (Hubner). Insect Biochem. 15, 111.
Argiriadi, M.A., Morisseau, C., Hammock, B.D., Christianson, D.W., 1999. Detoxification of environmental mutagens and carcinogens: structure, mechanism, and evolution of liver epoxide hydrolase. Proc. Natl. Acad. Sci. USA 96, 10637–10642.
Armstrong, R.N., 1999. Kinetic and chemical mechanism of epoxide hydrolase. Drug Metab. Rev. 31, 71–86.
Beetham, J.K., Grant, D., Arand, M., Garbafino, J., Kiyosue, T., Pinot, F., Oesch, F., Belknap, W.R., Shinozaki, K., Hammock, B.D., 1995. Gene evolution of epoxide hydrolases and recommended nomenclature. DNA Cell Biol. 14, 61.
Borhan, B., Jones, A.D., Pinot, F., Grant, D.F., Kurth, M.J., Hammock, B.D., 1995. Mechanism of soluble epoxide hydrolase. J. Biol. Chem. 270, 26923.
Casas, J., Harshman, L.G., Hammock, B.D., 1991. Epoxide hydrolase activity on juvenile hormone in Manduca sexta. Insect Biochem. 21, 17.
Corey, E.J., Yamamoto, H., Herron, D.K., Achiwa, K., 1970. New stereospecific synthetic routes to trisubstituted olefins. J. Am. Chem. Soc. 92, 6635–6636.
Dale, J.A., Dull, D.L., Mosher, H.S., 1969. a -Methoxytrifluorome-thylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J. Org. Chem. 34, 2543–2551.
De Kort, C.A.D., Granger, N.A., 1996. Regulation of JH titers: the relevance of degradative enzymes and binding proteins. Arch. Insect Biochem. Physiol. 33, 1–26.
Debernard, S., Morisseau, C., Severson, T.F., Feng, L., Wojtasek, H., Prestwich, G.D., Hammock, B.D., 1998. Expression and charac-terization of the recombinant juvenile hormone epoxide hydrolase (JHEH) from Manduca sexta. Insect Biochem. Molec. Biol. 28, 409.
Falany, C.N., McQuiddy, P., Kasper, C.B., 1987. Structure and organi-zation of the microsomal xenobiotic epoxide hydrolase gene. J. Biol. Chem. 262, 5924.
Graham, S.M., Prestwich, G.D., 1994. Synthesis and inhibitory proper-ties of pheromone analogues for the epoxide hydrolase of the gypsy moth. J. Org. Chem. 59, 2956–2966.
Hammock, B.D., 1985. Regulation of juvenile hormone titer: degra-dation. In: Derkut, G.A., Gilbert, L.I. (Eds.), Comprehensive Insect Physiology, Biochemistry and Pharmacology, Volume 7. Pergamon Press, NY, pp. 431–472.
Hammock, B.D., Abdel-Aal, Y.A.I., Mullin, C.A., Hanzlik, T.N., Roe, R.M., 1984. Substituted thiotrifluoropropanones as potent selective inhibitors of juvenile hormone esterase. Pestic. Biochem. Physiol. 22, 209.
Hammock, B.D., Pinot, F., Beetham, J.K., Grant, D.F., Arand, M.E., Oesch, F., 1994. Isolation of a putative hydroxyacyl enzyme inter-mediate of an epoxide hydrolase. Biochem. Biophys. Res. Comm. 198, 850.
Hammock, B.D., Wing, K.D., McLaughlin, J., Lovell, V.M., Sparks, T.C., 1982. Trifluoromethylketones as possible transition state ana-log inhibitors of juvenile hormone esterase. Pestic. Biochem. Phy-siol. 17, 76.
Harris, S.V., Thompson, D.M., Linderman, R.J., Tomalski, M.D., Roe, R.M., 1999. Cloning and expression of a novel juvenile hormone metabolizing epoxide hydrolase during larval–pupal metamor-phosis of the cabbage looper. Trichoplusia ni. Insect Mol. Biol. 8, 85–96.
Harshman, L.G., Casas, J., Dietze, E.C., Hammock, B.D., 1991. Epox-ide hydrolase activities in Drosophila melanogaster. Insect Biochem. 21, 887.
Hassett, C., Turnblom, S.M., Deangles, A., Omiecinski, C.J., 1989. Rabbit microsomal epoxide hydrolase: isolation and characteriz-ation of the xenobiotic metabolizing enzyme cDNA. Arch. Biochem. Biophys. 271, 380.
Hutchins, R.O., Kandasamy, D., Maryanoff, C.A., Masilamani, D., Maryanoff, B., 1977. Selective reductive displacement of alkyl hal-ides and sulfonate esters with cyanoborohydride reagents in hex-amethylphosphoramide. J. Org. Chem. 42, 82–91.
Janssen, D.B., Pries, F., Ploeg, J.V.D., Kazemier, B., Terpstra, P., Witholt, B., 1989. Cloning of 1,2-dichloroethane degradation genes of Xanthobacter autotrophicus GJ10 and expression and sequen-cing of the dh1A gene. J. Bacteriol. 171, 6791.
Kallapur, V.L., Majumder, C., Roe, R.M., 1996. In vivo and in vitro-tissue specific metabolism of juvenile hormone during the last stad-ium of the cabbage looper, Trichoplusia ni. J. Insect Physiol. 42, 181–190.
Katsuki, T., Sharpless, K.B., 1980. The first practical method for asym-metric epoxidation. J. Am. Chem. Soc. 102, 5974–5976. Lacourciere, G.M., Armstrong, R.N., 1993. The catalytic mechanism
of microsomal epoxide hydrolase involves an ester intermediate. J. Am. Chem. Soc. 115, 10466.
Lacourciere, G.M., Armstrong, R.N., 1994. Microsomal and soluble epoxide hydrolases are members of the same family of C–X bond hydrolase enzymes. Chem. Res. Toxicol. 7, 212.
Laughlin, L.T., Tzeng, H.F., Lin, S., Armstrong, R.N., 1998. Mech-anism of microsomal epoxide hydrolase. Semifunctional site-spe-cific mutants affecting the alkylation half-reaction. Biochemistry 37, 2897.
Linderman, R.J., Leazer, J., Venkatesh, K., Roe, R.M., 1987. The inhi-bition of insect juvenile hormone esterase by trifluoromethylke-tones: steric parameters at the active site. Pestic. Biochem. Physiol. 29, 266.
Linderman, R.J., Upchurch, L., Lonikar, M., Venkatesh, K., Roe, R.M., 1989. Inhibition of insect juvenile hormone esterase bya,b -unsatu-rated and a-acetylenic trifluoromethyl ketones. Pestic. Biochem. Physiol. 35, 291.
Linderman, R.J., Walker, E.A., Haney, C., Roe, R.M., 1995. Determi-nation of the regiochemistry of insect epoxide hydrolase catalyzed epoxide hydration of juvenile hormone by 18O-labeling studies. Tetrahedron 51, 10845.
Morisseau, C., Du, G.H., Newman, J.W., Hammock, B.D., 1998. Mechanism of mammalian soluble epoxide inhibition by chalcone oxide derivatives. Arch. Biochem. Biophys. 356, 214.
Muller, F., Arand, M., Frank, H., Siedel, A., Hinz, W., Winkler, L., Hanel, K., Blee, E., Beetham, J.K., Hammock, B.D., Oesch, F., 1997. Visualization of a covalent intermediate between microsomal epoxide hydrolase, but not cholesterol epoxide hydrolase, and their substrates. Eur. J. Biochem. 245, 490.
Nardini, M., Ridder, I.S., Rozeboom, H.J., Kalk, K.H., Rink, R., Janssen, D.B., Dijkstra, B.W., 1999. The X-ray structure of epoxide hydrolase from Agrobacterium radiobacter AD1. J. Biol. Chem. 274, 14579–14586.
Nijhout, H.F., 1994. Insect Hormones. Princeton University Press, Princeton, NY.
Ollis, D.L., Cheah, E., Cygler, M., Dijkstra, B., Frolow, F., Franken, S.M., Harel, M., Remington, S.J., Silman, I., Schrag, J., Sussman, J.L., Verschueren, K.H.G., Goldman, A., 1992. The alpha/beta hydrolase fold. Protein Engng 5, 197.
O’Reilly, D.R., Miller, L.K., Luckow, V.A., 1992. Baculovirus Expression Vectors: A Laboratory Manual. W.H. Freeman and Company, New York, NY.
Pinot, F., Grant, D.F., Beetham, J.K., Parker, A.G., Borhan, B., Landt, S., Jones, A.D., Hammock, B.D., 1995. Molecular and biochemical evidence for the involvement of the Asp-333–His-523 pair in the catalytic mechanism of soluble epoxide hydrolase. J. Biol. Chem. 270, 7968.
Prestwich, G.D., Eng, W.-S., Roe, R.M., Hammock, B.D., 1984. Syn-thesis and bioassay of isoprenoid 3-alkylthio-1,1,1-trifluoro-2-pro-panones: potent, selective inhibitors of juvenile hormone esterase. Arch. Biochem. Biophys. 228, 639.
Roe, R.M., Hammond, A.M. Jr., Sparks, T.C., 1982. Growth of larval
Diatraea saccharalis (Lepidoptera: pyralidae) on artificial diet and
synchronization of the last larval stadium. Ann. Entomol. Soc. Am. 75, 421–429.
Roe, R.M., Kallapur, V., Linderman, R.J., Viviani, F., Harris, S.V.,
Walker, E.A., Thompson, D.M., 1996. Mechanism of action and cloning of epoxide hydrolase from the cabbage looper,
Trichoplu-sia ni. Arch. Insect Biochem. Physiol. 32, 527.
Roe, R.M., Venkatesh, K., 1990. In: Morphogenetic Hormones of Arthropods, Volume I. Rutgers University Press, New Brunswick, pp. 126–179.
Schanstra, J.P., Ridder, I.S., Heimriks, G.J., Rink, R., Poelarends, G.J., Kalk, K.H., Dijkstra, B.W., Janssen, D.B., 1996. Kinetic charac-terization and X-ray structure of a mutant haloalkane dehalogenase with higher catalytic activity and modified substrate range. Bio-chemistry 35, 13186.
Share, M.D., Roe, R.M., 1988. A partition assay for the simultaneous determination of insect juvenile hormone esterase and epoxide hydrolase activity. Anal. Biochem. 169, 81–88.
Skoda, R.C., Demierre, A., McBride, O.W., Gonzalez, F., Meyer, U.A., 1988. Human microsomal xenobiotic epoxide hydrolase. Complementary DNA sequence, complementary DNA-directed expression in COS-1 cells, and chromosomal localization. J. Biol. Chem. 263, 1549.
Tzeng, H.F., Laughlin, L.T., Armstrong, R.N., 1998. Semifunctional site-specific mutants affecting the hydrolytic half-reaction of microsomal epoxide hydrolase. Biochemistry 37, 2905.
Tzeng, H.-F., Laughlin, L.T., Lin, S., Armstrong, R.N., 1996. The cata-lytic mechanism of microsomal epoxide hydrolase involves revers-ible formation and rate-limiting hydrolysis of the alkyl-enzyme intermediate. J. Am. Chem. Soc. 118, 9436.
Verschueren, K.H.G., Seljee, F., Roseboom, H.J., Kalk, K.H., Dijkstra, B.W., 1993. Crystallographic analysis of the catalytic mechanism of haloalkane dehalogenase. Nature 363, 693.