and Electroconvulsive Shock on Locus Coeruleus
Electrophysiologic Activity
Michael M. Grant and Jay M. Weiss
Background: The locus coeruleus (LC) is the major noradrenergic cell body group in the brain. Although previous studies have examined changes in electrophysi-ologic activity of LC neurons produced by antidepressant drugs, only a small number have examined changes that occur with chronic drug administration, which is the therapeutically effective regimen, and only one group of investigators has assessed effects on activated (or “burst”) firing of LC neurons under such treatment conditions. The present study assessed changes produced in rats by effective antidepressant treatments—several drugs given chronically (two tricyclic antidepressants, two selective serotonin reuptake inhibitors, and a monoamine oxidase inhibitor) as well as a series of electroconvulsive shocks (ECSs)—in single-unit electrophysiologic activity of LC neurons, measuring effects on spontaneous depo-larization rate and also on sensory-evoked burst firing. Methods: Drugs were administered via osmotic minipumps for either 14 or 30 days; ECSs were adminis-tered five times, with a 72-hour interval between each administration. Electrophysiologic recording of LC activ-ity took place under halothane anesthesia on the last day of drug treatment or following a delay of 1 or 5 days after the final ECS.
Results: A common effect of all drugs tested and ECS treatment was to decrease LC spontaneous and sensory-evoked burst firing.
Conclusions: The clinical efficacy of antidepressant med-ication and ECS may be mediated, in part, through reduction of LC neural activity. The findings reported here are consistent with recent indications that LC neurons are hyperactive in depressed individuals and with suggestions that some behavioral changes seen in depression can arise from consequences of rapidly depolarizing LC terminals, such as release of peptides. Biol Psychiatry 2001;49: 117–129 © 2001 Society of Biological Psychiatry
Key Words: Antidepressant, locus coeruleus,
electro-physiology, tricyclic, SSRI, electroconvulsive shock
Introduction
T
he suggestion that noradrenergic neurons in the brain are involved in depression has been with us for many years. The original catecholamine hypothesis of depres-sion, advanced more than 30 years ago (Bunney and Davis 1965; Schildkraut 1965; Schildkraut and Kety 1967), proposed that depression arose from a deficiency of norepinephrine (NE) in the brain. But when various predictions derived from this hypothesis were not con-firmed (e.g., depressed individuals did not appear to have lower levels of NE or metabolites in brain, and tricyclic antidepressants required many days to reverse depression despite elevating synaptic NE immediately after adminis-tration), initial enthusiasm for the catecholamine hypoth-esis gave way to attempts to develop formulations that did not focus on NE.Despite prodigious efforts to develop alternatives, evi-dence has continued to point to the involvement of NE in depression and the action of antidepressant drugs. For example, when effective antidepressant drugs were dis-covered that did not block NE reuptake or directly increase NE release, this seemed to further compromise the cate-cholamine hypothesis until it was subsequently discovered that these drugs downregulate beta-adrenergic receptors, an action that could derive from, or was a consequence equivalent to, prolonged action of NE in the synapse (Vetulani et al 1976a, 1976b; Vetulani and Sulser 1975). A survey of antidepressant drugs that appeared shortly there-after concluded that almost all antidepressant drugs poten-tiated NE action in the synapse (Richelson and Pfenning 1984). Even drugs developed more recently that seem to suggest the primacy of neurotransmitters other than NE have been found to significantly affect noradrenergic systems. For instance, the antidepressant drugs classified as selective serotonin reuptake inhibitors (SSRIs) produce marked increases in extracellular NE in the brain as well From the Department of Psychology, Georgia State University (MMG) and the
Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine (JMW), Atlanta, Georgia.
Address reprint requests to Jay M. Weiss, Ph.D., Emory University School of Medicine, Emory West Campus, 1256 Briarcliff Road NE, Atlanta GA 30306. Received January 22, 2000; revised May 2, 2000; accepted May 16, 2000.
© 2001 Society of Biological Psychiatry 0006-3223/01/$20.00
as serotonin when given systemically as occurs in clinical use (Cosford 1995; Jordan et al 1994). Further-more, the antidepressant drug bupropion blocks dopa-mine reuptake more effectively than any other
antide-pressant drug presently in widespread use, but
preclinical studies have found that antidepressantlike behavioral changes produced by bupropion parallel changes in brain NE produced by this drug rather than changes in dopamine (Cooper et al 1994). Yet another example implicating NE is provided by the Flinders Sensitive rat strain that was selectively bred for sensi-tivity to cholinergic drugs and has been proposed as a model for depression (reviewed in Overstreet 1993). Although these animals were bred for sensitivity to cholinergic drugs, their depression-related behavior has been found to respond to drugs that affect brain NE rather than acetylcholine (Schiller et al 1992). In summary, despite various inconsistencies regarding the role of NE in depression, research continues to produce evidence that activity of noradrenergic neurons is im-portant in this disorder.
The study described here examined changes in electro-physiologic single-unit activity of neurons of the major noradrenergic cell-body group in the brain, the locus coeruleus (LC). This study assessed the changes produced by effective antidepressant treatments, both chronic ad-ministration of antidepressant drugs and electroconvulsive shock. The terminals of LC neurons supply nearly 70% on the total NE in the brain, giving rise to most of the NE in
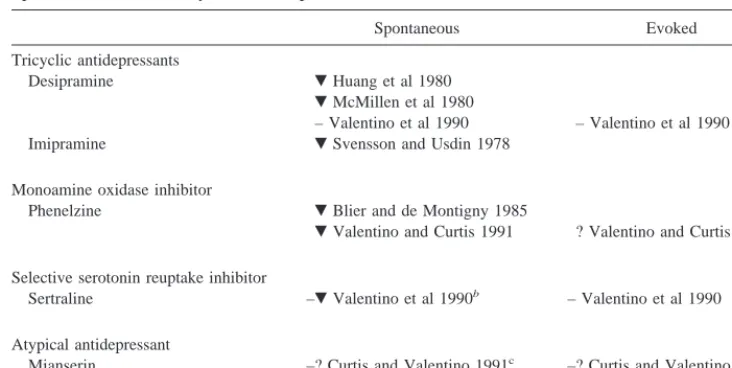
the forebrain and all of the NE in cortex and hippocampus. Studies have examined the effects of antidepressant drugs on electrophysiologic activity of LC neurons, but most of these studies have reported effects of acute drug adminis-tration. Because antidepressant drugs require prolonged or chronic administration to have therapeutic effects, changes of greater clinical interest would be those observed after these drugs have been given for a therapeutically relevant period of time. Also, LC neurons have two (or at least two) modes of firing: they depolarize at a spontaneous rate but also fire more rapidly in response to excitatory stimuli. Few studies have examined effects of antidepressant treatment on rapid firing (or “burst” firing) that occurs in response to an excitatory stimulus. The previously re-ported effects of chronic administration of antidepressant drugs on spontaneous depolarization rate and burst firing to an excitatory stimulus are summarized in Table 1. Examination of this table will reveal that the reported effects have not been consistent. To clarify these effects, we assessed spontaneous depolarization rate and sensory-evoked burst firing in rats treated chronically with several different types of antidepressant drugs or a series of electroconvulsive shocks.
Methods And Materials
Subjects
Male and female Sprague-Dawley rats (virus/antigen free, bred in our laboratory from stock originally obtained from Charles Table 1. Effects of Chronic Treatment (1 Week or Longer) with Antidepressant Drugs on
Spontaneous and Sensory-Evoked Depolarization Rate of Locus Coeruleus Neurons
Spontaneous Evoked
Tricyclic antidepressants
Desipramine Huang et al 1980
McMillen et al 1980
– Valentino et al 1990 – Valentino et al 1990 Imipramine Svensson and Usdin 1978
Monoamine oxidase inhibitor
Phenelzine Blier and de Montigny 1985
Valentino and Curtis 1991 ? Valentino and Curtis 1991a
Selective serotonin reuptake inhibitor
Sertraline –Valentino et al 1990b – Valentino et al 1990
Atypical antidepressant
Mianserin –? Curtis and Valentino 1991c –? Curtis and Valentino 1991c
The depolarization rate shown by animals treated with drug in comparison with animals receiving no drug was, decreased, or2, unchanged.
aEvoked response assessed, but results not reported for this measure. Report presents only the ratio of evoked to spontaneous
rate; because spontaneous rate was decreased by phenelzine, effect on evoked rate could not be determined from data presented.
bSpontaneous firing rate of sertraline-treated rats was not different from untreated rats but was significantly lower than that
of rats that received a similar schedule of injections of vehicle.
cSpontaneous and evoked firing rate of mianserin-treated rats was not different from untreated rats but was less than that of
River [Wilmington, MA]) aged 5 to 6 months were used. Animals were group housed (three to a cage) directly on bedding in solid-bottom polycarbonate cages. In studies of antidepressant drugs, animals in any single cage received the same drug, with control animals (i.e., animals receiving vehicle) distributed among drug groups and housed with them. Animals that received ECS and control animals for this manipulation were similarly housed. Food (lab chow) and water were available ad libitum. A 12:12 light:dark cycle and temperature of approximately 21°C was maintained in the colony room.
Drugs and Administration
The effects of five antidepressants were examined: desipramine HCl (DMI), a secondary amine dibenzazepine tricyclic NE reuptake inhibitor (Sigma, St. Louis); imipramine HCl (IMI), a tertiary amine dibenzazepine tricyclic NE reuptake inhibitor (Sigma); phenelzine sulfate (PHE), a nonselective monoamine oxidase inhibitor (Sigma); and fluoxetine HCl (FLU) and sertra-line HCl (SER), both heterocyclic SSRIs (Lilly and Pfizer, respectively). Antidepressants were administered via Alzet Os-motic Minipumps (Alza, Mountain View, CA), implanted sub-cutaneously in the animals, using Model 2ML2 pumps for 14-day administration and Model 2ML4 pumps for 30-day administra-tion. Minipumps, which provide continuous drug delivery begin-ning approximately 4 hours after pump implantation, were used to insure the presence of drug in the animal throughout the study and at the time that electrophysiologic recording took place. Use of minipumps also eliminated the need for repeated handling and injection of animals to administer drug chronically. Studies that have assessed effects of daily injection of vehicle while studying effects of antidepressant drugs have found that repeated injec-tions constitute a stressful procedure. For example, compared with no injections, repeated vehicle injections have been ob-served to produce 1) elevated brain norepinephrine release as measured by microdialysis (E. Abercrombie, unpublished data) and 2) increased stress-sensitive tumor growth (Garabal et al 1991). Consequences of daily handling and injection were therefore avoided in the present study.
Each animal’s body weight was determined before pump implantation, and the flow rate of mini-pumps (manufacturer’s specifications) was used to compute the drug concentration loaded in the pumps to achieve the desired dosage (i.e., DMI, IMI, FLU, and SER, 10 mg/kg/day; PHE, 5 mg/kg/day; also, an additional group was treated with SER 25 mg/kg/day to assess effects of a higher dose of an SSRI). The doses for the different drugs used in this study were chosen on the basis that the dose used had been administered to rats via minipump and was found to be effective in preclinical models for detection of antidepres-sant activity (West and Weiss, 1995, 1998). We prepared DMI, IMI, and PHE in sterile 0.9% saline solution, whereas FLU and SER were administered in 75% polyethylene glycol (PEG; Sigma) and 25% saline because of their low solubility in water. Pumps containing 0.9% saline or saline/PEG vehicle were installed in vehicle-treated control animals. Pumps were im-planted subcutaneously under halothane anesthesia in the dorsal rear flank region, and the wound was closed with stainless steel clips; this surgery, details of which can be found in West and
Weiss (1998), required approximately 10 min per animal. Fol-lowing surgery, animals were returned to the home cage and not disturbed until removed for the electrophysiologic recording session.
Electroconvulsive Shock Procedure
Electroconvulsive shock was applied using a 5:1 AC step-up transformer, with the primary voltage set to deliver 350 V from the output. With no additional resistance in series other than the animal, this output generated an approximately 50 mA current when administered to the animal transcranially (current intensity verified by oscilloscope recording during ECS delivery). For each ECS treatment, the animal was anesthetized with halothane for 5 min, after which an ECS was administered to the anesthetized animal for 500 msec through ear clips moistened with conducting cream. Rats treated with ECS immediately exhibited a tonic phase lasting 1 to 5 secs, followed by a clonic phase lasting 20 to 30 secs. Animals received the ECS treatment five times, with 72 hours between each treatment. Control animals for ECS treatment were anesthetized in the same manner as the ECS-treated rats for the five treatment sessions, but no ECS was given. Electrophysiologic recording was then conducted either 1 day or 5 days after the last ECS or control treatment.
Electrophysiologic Recording
has been shown to be little affected by the intensity of the paw compression; rather, a burst will occur when the intensity exceeds the threshold needed to elicit a burst response, with the number of depolarizations in a burst then being determined by factors unrelated to paw compression intensity such as the activation of receptors on the LC neuron, resting potential of the cell, and so forth (Simson and Weiss 1989). To determine the amount of sensory-evoked burst firing by a unit, several paw compressions were applied, each spaced at least 10 sec apart; the average was calculated as the response of the neuron. Following the last paw compression, spontaneous activity was recorded for 1 min (to verify that the cell returned to its normal baseline firing rate) after which the electrode was slowly moved to isolate another unit. Whenever possible, several units (3 to 5) were recorded in this manner from an animal.
Measurement of Blood Levels of Antidepressant Drugs
To confirm the presence of drug in circulation at the time of electrophysiologic measurement, blood levels of the drugs used were measured. Randomly selected male and female rats that had undergone electrophysiologic recording were sacrificed by de-capitation and truck blood collected. To permit comparison of blood levels of drug achieved in the present study when drug was administered by minipump with blood levels when drug is given by repeated intraperitoneal injection, additional rats (female) were given daily injections of drug, after which the animals were sacrificed by decapitation (following anesthetization with halo-thane) and truck blood was collected. The injection procedure used attempted to approximate the schedule of Valentino and colleagues (Curtis and Valentino 1991; Valentino et al 1990; Valentino and Curtis 1991); thus, drug was injected daily for 21 days, and sacrifice took place either 12 hours or 24 hours after the final injection (to bracket the time of recording in those studies, which is stated to have occurred between 12 and 20 hours after last injection). To provide additional information, blood levels at these time intervals after a single intraperitoneal (IP) injection of drug were also measured. Serum from samples was maintained frozen at280°C until analyzed. The levels of desipramine and imipramine were determined by the method of Mazhar and Binder (1989); the levels of fluoxetine, sertraline, and their metabolites were determined by the method described in Ritchie and Zhang (1996).
Statistical Analysis
Statistical analysis, which was carried out on the activity of the individual units, was conducted primarily using one-way analy-ses of variance (ANOVAs). If a significant main effect of treatment (p , .05) was obtained, the significance of the difference between each drug or ECS group and the control condition was then determined using Dunnett’s test. In cases where frequency distributions between conditions were com-pared,x2test was used.
Results
Effects of Antidepressant Drugs
Mean spontaneous and sensory-evoked burst firing rates of LC neurons in male and female rats given antidepres-sant drugs or vehicle (saline or saline/PEG) for 14 and 30 days are shown in Figures 2 and 3. To compare LC firing rates seen in the drug-treated groups with vehicle-treated animals, the vehicle-treated condition is represented by the combined data from all vehicle-treated animals because no differences were found among any of the different vehicle regimens (i.e., 14 vs. 30 days of vehicle administration or use of physiologic saline vs. PEG/saline as vehicle).
As can be seen in Figure 2 and 3, chronic administration of all antidepressant drugs tested caused a marked reduc-tion in the rate of depolarizareduc-tion of LC neurons. Effects on
spontaneous rate of depolarization are shown in Figure 2. When the data for the groups shown in this figure were
analyzed by one-way ANOVA (one ANOVA for male rats and one for female rats), a significant overall effect Figure 2. Spontaneous depolarization rate (spikes/sec [Hz]) of locus coeruleus (LC) neurons in adult male and female rats treated chronically with antidepressant drugs. Experimental groups were treated for 14 or 30 days (by subcutaneous minipump) with desipramine (DMI; 10 mg/kg/day), imipramine (IMI; 10 mg/kg/day), phenelzine (PHE; 5 mg/kg/day), fluoxetine (FLU; 10 mg/kg/day), or sertraline (SER; 10 mg/kg/day or 25 mg/kg/day). Distributed in the home cages amongst the drug-treated animals, control animals that received vehicle (VEH) were measured throughout the study; all control conditions (i.e., 14-day and 30-day treatment) are combined as there were no differences between them. Numbers of LC units represented in each bar of the graph (left to right) are as follows: for male animals, 39, 14, 10, 12, 11, 11, 7, 15, 10, 9, 11, 11, and 10; for female animals, 34, 8, 14, 19, 8, 12, 9, 15, 6, 29, 16, 16, and 16. Numbers of individual animals from which LC units were recorded in each group (left to right) are as follows: for male animals, 10, 3, 4, 5, 4, 4, 3, 6, 4, 4, 4, 4, and 4; for female animals, 10, 3, 6, 5, 3, 5, 4, 6, 3, 6, 6, 4, and 4. Means and standard errors are shown. *Statistically significant (at least p,.05) decrease from the spontaneous rate of the VEH condition of the same gender.
attributable to drug treatment was obtained for both male rats [F(12,156) 5 15.82, p , .001] and female rats [F(12,190) 5 14.08, p, .001]. Post hoc comparison of each individual group with the vehicle-treated condition showed that every antidepressant drug at either time of drug administration (i.e., 14 or 30 days) caused a signifi-cant suppression of spontaneous firing rate (at least p, .05) in both male and female rats.
Effects on the sensory-evoked burst firing are shown in Figure 3. When the data for these groups were also analyzed by one way ANOVA (one ANOVA for male rats and one for female rats), a significant overall effect attributable to drug treatment was obtained for both male
subjects [F(12,156) 5 11.43, p , .001] and female
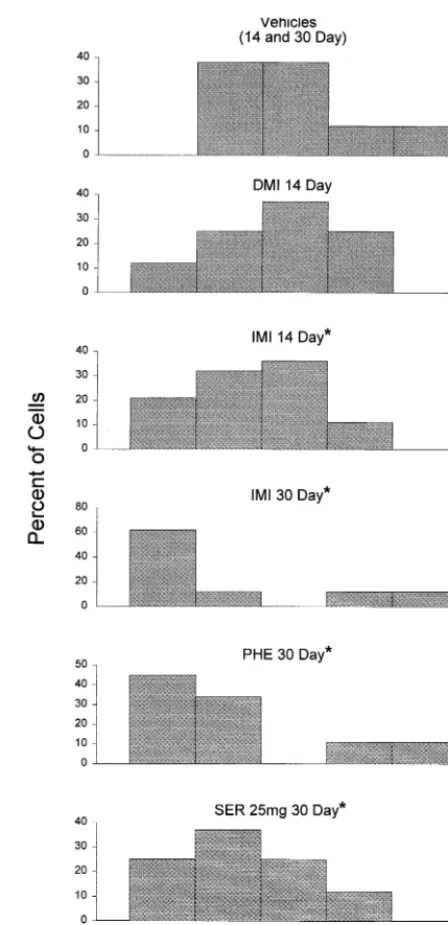
subjects [F(12,190)52.56, p,.004]. Post hoc compar-ison of each individual group with the vehicle-treated condition showed that every antidepressant drug at either time of administration (i.e., 14 or 30 days) caused a significant suppression of sensory-evoked burst firing in the male rats. In the female rats, however, this post hoc analysis indicated that seven of the drug groups differed significantly from the vehicle-treated condition, but five of the groups did not; the conditions that did not differ significantly were DMI and IMI at 14 days of administra-tion and IMI, PHE, and SER 25 mg at 30 days. Nonethe-less, close inspection of the data suggested that these five conditions might not have differed significantly from vehicle treatment because of the presence of a few fast-firing cells in these conditions. This possibility was tested by examining frequency distributions for the senso-ry-evoked burst firing rates of each of these groups, which are shown in Figure 4. Chi-square analysis comparing the frequency distribution for each of these drug groups with that of the vehicle-treated animals showed that the senso-ry-evoked burst activity of the cells in four of these five drug groups (IMI 14, IMI 30, PHE 30, and SER 25 mg 30) differed significantly from that of the vehicle-treated condition [x2(4) for these comparisons were, respectively, 9.6 (p,.05), 25.6 (p,.001), 18.6 (p,.001), and 11.0 (p , .05)]. As can be seen in Figure 4, these drug treatments resulted in an appreciable number of cells that had a low amount of burst firing but also a few cells that had either a normal or high amount of burst firing that apparently precluded reaching statistical significance us-ing a parametric statistical test. For the DMI 14 group, the frequency distribution did not differ significantly from the vehicle-treated condition, but even the DMI 14 group had more low-frequency burst firing cells than did the vehicle-treated condition.
Effects of Electroconvulsive Shock
Mean spontaneous depolarization rate and sensory-evoked burst firing of LC neurons in male and female rats that
received ECS with the control animals, the data from all non-ECS animals were combined because no differences were found between such animals tested either 1 day or 5 days after having received preparatory procedures without ECS.
As can be seen in Figure 5, a series of electroconvulsive shocks caused a reduction in the rate of depolarization of LC neurons. The effect on spontaneous depolarization rate is shown in the top part of Figure 5. When the data for these groups were analyzed by one-way ANOVA (one ANOVA for male rats and one for female rats), a signif-icant overall effect attributable to treatment was obtained for male rats [F(2,53)519.13, p,.001] and female rats [F(2,68)523.02, p,.001]. Post hoc comparison of each
ECS-treated group with the control condition showed that ECS treatment resulted in a significant reduction of spontaneous depolarization rate at each time of measure-ment (1- or 5-day delay) in both male and female rats.
The effect of ECS on sensory-evoked burst firing is shown in the bottom part of Figure 5. When the data for these groups were analyzed by one-way ANOVA (one ANOVA for male rats and one for female rats), a signif-icant overall effect attributable to treatment was obtained for male rats [F(2,53)529.13, p,.001] and female rats [F(2,68)526.06, p,.001]. Post hoc comparison of each ECS-treated group with the control condition showed that ECS treatment resulted in a significant suppression of burst firing at each time of measurement (1- and 5-day delay) in both male and female rats.
Blood Levels of Antidepressant Drugs
To establish that antidepressant drugs were in circulation at the time electrophysiologic measures were taken and also to make possible informed comparison of the blood levels achieved by minipump administration with those achieved by repeated IP injection, blood levels of different antidepressant drugs were measured. Table 2 shows blood levels of the antidepressant drugs used in this study Figure 5. Effects of electroconvulsive shock (ECS) on
sponta-neous depolarization rate and sensory-evoked “burst” firing of locus coeruleus (LC) neurons. Adult male and female rats received ECSs (a series of five shocks under halothane anesthe-sia spaced 72 hours apart) whereas control rats received similar treatment without shock being given; electrophysiologic record-ing then took place 1 day (11 day) or 5 days (15 days) after the last ECS or control procedure. Control rats measured 1 day or 5 days after final treatment are combined as there were no differences between them. Numbers of LC units represented in each bar of the graph (left to right) are as follows: for male rats, 39, 10, and 8; for female rats, 49, 10, and 13. Numbers of individual animals from which LC units were recorded in each group (left to right) are as follows: for male rats, 8, 4, and 2; for female rats, 9, 5, and 4. Means and standard errors are shown. *Statistically significant (at least p,.05) decrease from control rats of the same gender.
Table 2. Blood Levels of Antidepressant Drugs (ng/mL) Resulting from Administration by Minipump
Drug (dosage)
Duration of Administration 14 days 30 days DMI (10 mg/kg/day) 669.76100.6 1874.26540.7 IMI (10 mg/kg/day) 553.26152.2 254.5652.6 FLU (10 mg/kg/day) 1923.06237.0 2618.86479.0 SER 10 (10 mg/kg/day) 369.06132.0 619.86154.6 SER 25 (25 mg/kg/day) 1684.06715.5 2251.86519.4
Blood was taken for measurement following electrophysiologic recording on the day of administration indicated. Means and standard errors are shown. N54 in each cell except for 14-day administration of DMI and IMI (n53) and 14-day administration of FLU (n52). The blood levels (ng/mL serum) reported above are as follows:2for DMI, desipramine; for IMI, imipramine1desipramine; for FLU, norfluoxetine1fluoxetine; for SER, des-sertraline1sertraline.
Table 3. Blood Levels of Antidepressant Drugs (ng/mL) Resulting from Administration by Intraperitoneal Injection
Time of measurement after last injection
Duration of administration Chronic
(i.e., daily injection for 21 days)
Acute (i.e., a single
injection) DMI (10 mg/kg) 12 hours 573.86277.4 334.56181.9
24 hours 422.0685.8 295.26204.0 SER (10 mg/kg) 12 hours 765.06172.9 329.5685.8
24 hours 287.0685.8 172.5620.0
(except for phenelzine, which was not assessed because of the unavailability of the method in our clinical laboratory). The blood levels shown in Table 2 were those present after administration of drug by osmotic minipump for 14 or 30 days. Blood levels were also determined after repeated IP injection of drug to permit comparison of levels produced by this technique with those resulting from minipump administration. Table 3 shows blood levels of DMI and SER following repeated IP injection. The blood levels shown in Table 3 were those present at 12 and 24 hours after the final injection of 21 days of daily drug injections and also at these times after a single IP injection.
Discussion
Several effective antidepressant treatments— chronic ad-ministration of five antidepressant drugs (two tricyclics, two SSRIs, and an MAO inhibitor) and a series of electroconvulsive shocks—all decreased electrophysi-ologic activity of LC neurons in halothane-anesthetized rats. Spontaneous depolarization of LC neurons was mark-edly decreased by all of the treatments tested. Sensory-evoked burst firing was also decreased, although in one of the groups (female rats that received DMI for 14 days), the change in this parameter did not reach statistical signifi-cance. It should be noted that despite the size and consistency of these effects, the magnitude of the changes shown in Figures 2 through 5 quite possibly underesti-mates the decrease in LC activity produced by the antide-pressant treatments. This is because LC units were diffi-cult to isolate and quantify in some of the animals that received antidepressant treatments. As a result, some of the animals that received an antidepressant treatment did not contribute to the data reported here because, during recording from these animals, repeated passes of the microelectrode resulted in no units that fired consistently or rapidly enough to be designated as LC neurons by the electrophysiologic criteria used (described in Electro-physiologic Recording in Methods and Materials). In several cases, a total absence of discernable single-unit electrophysiologic activity was encountered. Specifically, whereas LC single units were successfully isolated and quantified in all 37 of the vehicle-treated and control animals used during the course of the study, 23 out of 140 animals that received an antidepressant treatment were not represented in the data because of failure to isolate and measure any LC units in these animals. This difference between control and antidepressant-treated conditions is highly significant (p , .01 by x2). All antidepressant-treated conditions contributed to this result (i.e., animals in which no units could be isolated/animals from which recording was attempted 5 3/19 DMI; 5/22 IMI; 3/19 PHE; 4/23 FLU; 2/22 SER 10 mg; 2/18 SER 25 mg; and
4/19 ECS). It can be suggested that the animals in which no units could be isolated were ones in which electro-physiologic activity of LC neurons was so suppressed that their LC neurons did not depolarize sufficiently to permit identification of LC activity by the criteria employed. In this event, exclusion of these subjects from the data set means that the results shown above underestimate some-what the extent to which LC activity was suppressed by these treatments.
Mechanisms can be suggested by which the antidepres-sant treatments tested produced inhibition of LC activity. Decreased LC activity caused by drugs that block NE reuptake (DMI and IMI) is consistent with a strong inhibitory action on LC firing that is exerted via stimula-tion of somatodendritic a2 receptors on LC neurons (Aghajanian and Vandermaelen 1982; Cedarbaum and Aghajanian 1976; Simson and Weiss 1987). Blockade of NE reuptake and resultant elevation of NE in the LC region will increase stimulation of these somatodendritic a2 receptors to potently inhibit depolarization of LC neurons. A similar influence can be suggested to account for the effects of SSRIs on LC activity. Serotonergic receptors are found on LC cell bodies (Pickel et al 1977), and activation of these receptors also inhibits LC activity (Segal 1979); thus, blockade of reuptake of 5-HT released from serotonergic terminals in the LC region will increase stimulation of these 5-HT receptors to inhibit LC depolar-ization. Because MAO inhibition increases the extracellu-lar levels of both NE and 5-HT, the MAOI tested (PHE) could decrease LC activity by increasing stimulation of both inhibitory adrenergic and serotonergic receptors on LC neurons. Whereas the effects of the drugs tested are not surprising, it is noteworthy that ECS also produced a decrease in activity of LC neurons at two time points after the final ECS had been given (i.e., 24 hours and 5 days afterward). The mechanism by which a series of electro-convulsive shocks causes a persisting inhibition of LC activity is not evident at this time and remains to be elucidated.
admin-istration of the SSRI sertraline changed evoked activity of LC neurons as was observed in the present study. They did observe, however, that spontaneous activity of LC was decreased by chronic administration of the MAO inhibitor phenelzine and also sertraline if comparison is made with rats that received a similar series of vehicle injections. Commenting on why decreased LC firing was not seen after chronic administration of DMI whereas it was ob-served after acute DMI, Valentino and colleagues sug-gested that “tolerance” develops to the acute effect of the drugs. In the case of DMI that potently blocks NE reuptake, a likely mechanism for “tolerance” would be downregulation of the inhibitory somatodendritic a2 -re-ceptors on LC neurons so that firing rate of LC neurons returns to normal despite elevation of NE in synapses in the LC region. The same rationale could be applied to the SSRI; chronic administration could be thought to down-regulate the inhibitory 5-HT receptors on LC neurons to reduce inhibitory effects. It was with this hypothesis in mind that we administered drug for 30 days as well as for 14 days in our studies. The rationale was as follows: if drug administration for 14 days resulted in a decrease in LC single-unit activity, it might be that drug administra-tion for this length of time was not sufficient to cause “tolerance” to occur (e.g., was not sufficient to produce enough downregulation of presynaptic inhibitory receptors to bring about recovery of LC activity). Were this the case, however, administering drug for a longer period (i.e., 30 days) should either produce the expected recovery or, at the least, cause some recovery from the amount of decrease seen with 14 days of drug administration as an indication that the proposed “tolerance” process was underway. The results found in the present study indicated that drug administration for 30 days did not bring about recovery of LC activity. Moreover, inhibition of LC activity was often more pronounced with 30 days of drug administration than it was with 14 days, so that there was no clear indication that a recovery process was underway that would eventually return LC activity to a normal level. It should be noted that we do not contend that downregu-lation of inhibitory presynaptic receptors does not occur with chronic stimulation of these receptors; rather, our conclusion is that when one delivers drug to rats via minipump so that drug is clearly in circulation at time of electrophysiologic measurement (see the following two paragraphs), the findings indicate that downregulation is not sufficient to cause return of LC activity to a normal level, and, consequently, antidepressant drug administra-tion results in an ongoing and pervasive decrease of LC activity.
To try to explain why we and other researchers ob-served decreases in LC activity with chronic administra-tion of certain antidepressant drugs whereas Valentino and
colleagues did not, we considered that blood levels of drug might be a significant factor. In the present study, antide-pressant drugs were administered by minipump not only to avoid subjecting the animals to repeated injections but also to have constant infusion of drug to insure that drug was in circulation at the time electrophysiologic recordings were made. The latter consideration was thought to be important because clinical practice indicates that sufficient circulat-ing levels of drug should be attained to produce a therapeutic result (Orsulak 1986; Preskorn 1989; Preskorn and Fast 1991; Van Brunt 1983). In contrast to minipump administration, Valentino and colleagues (i.e., Curtis and Valentino 1991; Valentino and Curtis 1991; Valentino et al 1990) gave antidepressant drugs by daily intraperitoneal injection for 21 days (usually 10 mg/kg) and then recorded LC activity 12 to 20 hours after the final injection. We therefore measured blood levels of drug to assess the possibility that these different methods of drug adminis-tration resulted in different circulating levels of drug.
the electrophysiologic findings of Valentino and col-leagues compared with those of the present study and other investigators do not appear explainable on the basis of blood levels of drug at the time of electrophysiologic recording. At present, we are unable to account for the difference between results of Valentino and colleagues in comparison with others.
Although measuring blood levels of drug failed to clarify why one group of investigators obtained results that differ from the findings of the others, there is significant additional information that bears on the question of whether antidepressant treatment decreases LC activity. A number of human studies have attempted to assess renergic activity in human brain by measuring the norad-renergic metabolite MHPG in cerebrospinal fluid (CSF) in conjunction with antidepressant treatment. These studies, summarized in Table 4, have consistently found decreased MHPG in CSF of human patients taking antidepressant drugs. Although MHPG in CSF does not exclusively
reflect NE release in brain because MHPG in blood can diffuse freely into CSF (Kopin 1985), Scheinin reviewed this issue and concluded, “Drug induced alterations in the concentration of monoamine metabolites in human CSF probably reflect quite accurately the effects of several classes of drugs [inhibitors of MAO and antidepressant drugs included in this list] on monoamine turnover in the CNS” (Scheinin 1985, 10). One study (Sharma et al 1994) utilized a correction of CSF MHPG for potential contri-bution by plasma MHPG that was recommended by Kopin (1985) and reached the same conclusion as other studies that CSF MHPG was reduced as a result of antidepressant drug treatment. Considering that approximately 70% of the NE in brain derives from the LC, the findings shown in Table 4 are consistent with the influence of antidepres-sant drugs in humans decreasing LC neural activity, as was observed in the rats in the present study. Moreover, these data from humans also suggest that despite the high blood levels of antidepressant drug found in the animals of the
Table 4. Summary of the Effects of Chronically Administered Antidepressants on Levels of Cerebrospinal Fluid MHPG
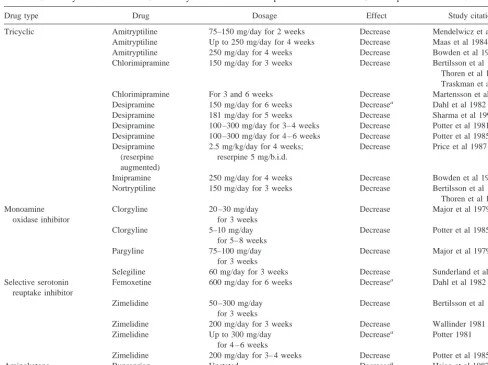
Drug type Drug Dosage Effect Study citation
Tricyclic Amitryptiline 75–150 mg/day for 2 weeks Decrease Mendelwicz et al 1982 Amitryptiline Up to 250 mg/day for 4 weeks Decrease Maas et al 1984 Amitryptiline 250 mg/day for 4 weeks Decrease Bowden et al 1985 Chlorimipramine 150 mg/day for 3 weeks Decrease Bertilsson et al 1974;
Thoren et al 1980; Traskman et al 1979 Chlorimipramine For 3 and 6 weeks Decrease Martensson et al 1991 Desipramine 150 mg/day for 6 weeks Decreasea Dahl et al 1982
Desipramine 181 mg/day for 5 weeks Decrease Sharma et al 1994 Desipramine 100 –300 mg/day for 3– 4 weeks Decrease Potter et al 1981 Desipramine 100 –300 mg/day for 4 – 6 weeks Decrease Potter et al 1985 Desipramine
(reserpine augmented)
2.5 mg/kg/day for 4 weeks; reserpine 5 mg/b.i.d.
Decrease Price et al 1987
Imipramine 250 mg/day for 4 weeks Decrease Bowden et al 1985 Nortryptiline 150 mg/day for 3 weeks Decrease Bertilsson et al 1974;
Thoren et al 1980 Monoamine
oxidase inhibitor
Clorgyline 20 –30 mg/day for 3 weeks
Decrease Major et al 1979
Clorgyline 5–10 mg/day for 5– 8 weeks
Decrease Potter et al 1985
Pargyline 75–100 mg/day for 3 weeks
Decrease Major et al 1979
Selegiline 60 mg/day for 3 weeks Decrease Sunderland et al 1994 Selective serotonin
reuptake inhibitor
Femoxetine 600 mg/day for 6 weeks Decreasea Dahl et al 1982
Zimelidine 50 –300 mg/day for 3 weeks
Decrease Bertilsson et al 1980
Zimelidine 200 mg/day for 3 weeks Decrease Wallinder 1981 Zimelidine Up to 300 mg/day
for 4 – 6 weeks
Decreasea Potter 1981
Zimelidine 200 mg/day for 3– 4 weeks Decrease Potter et al 1985
Aminoketone Buproprion Unstated Decreasea Hsiao et al 1987
Atypical Mianserin 30 – 60 mg/day for 2 weeks Decrease Mendelwicz et al 1982
present study, the results reported here are relevant to what occurs in humans undergoing antidepressant treatment.
The finding reported here that all antidepressant treat-ments tested cause a marked decrease in LC neuronal activity leads to the question of how such a change might be important for effective antidepressant treatment. Re-cently, other investigators have suggested that LC neuro-nal activity is abnormally elevated in depression and that antidepressant action consequently may occur through reduction of LC neuronal activity (Gold and Chrousos 1999; Zhu et al 1999). Of course, in considering the mechanism by which reduced LC activity might produce an antidepressant effect, it should be stated at the outset that decreased LC neuronal activity resulting from antide-pressant treatment could be an epiphenomenon unrelated to antidepressant action. For example, LC neuronal activ-ity might be decreased simply as a homeostatic adjustment because antidepressants increase NE in synapses through-out the brain and that potentiation of NE action throughthrough-out the brain (or resulting downregulation of B-adrenergic receptors) is what actually underlies antidepressant action. Also, the linkage of decreased LC activity to depression is possibly, or even probably, not a simple one, but, rather, the result of complex influences on neural transmission and information processing (e.g., Mongeau et al 1997). On the other hand, the study described in this article was prompted by a hypothesis we have offered recently that links LC activity directly to certain behavioral changes and processes seen in depression (Weiss et al 1996, 1998). This hypothesis suggests that certain salient behavioral changes seen in depression, particularly psychomotor retardation and failure to appreciate rewarding stimuli, can arise from abnormally high activity of LC neurons. The proposed mechanism is as follows: when LC neurons are highly active, the peptide galanin, which is colocalized in LC neurons, will be released from the LC terminals in addition to NE. When galanin release occurs from LC terminals in the ventral tegmentum, this peptide will exert a hyperpolarizing influence on dopaminergic cell bodies in this brain region, resulting in decreased release of dopa-mine in the forebrain. This in turn is hypothesized to cause behavioral changes that occur in depression, particularly psychomotor retardation and anhedonia. With respect to the findings described here, this hypothesis suggests that decreasing LC activity will reduce galanin release from LC terminals in the ventral tegmentum, thereby releasing dopaminergic cell bodies in this region from galanin-mediated inhibition. A reduction in LC activity, possibly through the mechanism described here, is proposed to underlie, at least in part, the effectiveness of currently used antidepressant treatments. An important study by Nestler et al published several years ago showed that antidepres-sants decrease synthesis of tyrosine hydroxylase in LC
neurons (Nestler et al 1990). Because tyrosine hydroxylase is an activity-dependent peptide, Nestler’s findings of a decrease in tyrosine hydroxylase activity with various antidepressant treatments also agrees with the results reported here indicating that LC activity is decreased by antidepressant drugs. Our thinking, however, is that al-though tyrosine hydroxylase may play a role in the antidepressant action of these drugs, a more critical element is another activity-dependent peptide localized in LC neurons, namely, galanin. It is our view that decreased LC activity similarly results in decreased galanin synthesis by LC cell bodies, the result of which is that transport of galanin to LC terminals in ventral tegmentum is decreased, which, according to the hypothesis we are presently evaluating, will decrease galanin release in this brain region to bring about antidepressant action.
This work was supported in part by Grant No. MH56602 from the National Institutes of Health. The authors gratefully acknowledge the expert assistance of Dr. James Ritchie in carrying out the assays for blood levels of the antidepressant drugs. They also thank Sandra Parks for her excellent assistance in preparation of the manuscript.
References
Aghajanian GK, Vandermaelen CP (1982): Alpha-2-adrenore-ceptor-mediated hyperpolarization of locus coeruleus neu-rons: Intracellular studies in vivo. Science 215:1394 –1396. Aston-Jones G, Bloom FE (1981): Norepinephrine-containing
locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1:887–900.
Bertilsson L, Asberg M, Thoren P (1974): Differential effect of chlorimipramine and nortriptyline on cerebrospinal fluid metabolites of serotonin and noradrenaline in depression. Eur
J Clin Pharmacol 7:365–368.
Bertilsson L, Tuck JR, Siwers B (1980): Biochemical effects of zimelidine in man. Eur J Clin Pharmacol 18:483– 487. Blier P, de Montigny C (1985): Serotonergic but not
noradren-ergic neurons in rat central nervous system adapt to long-term treatment with monoamine oxidase inhibitors. Neuroscience 14:949 –955.
Borsody MK, Weiss JM (1996): Influence of corticotropin-releasing hormone on electrophysiological activity of locus coeruleus neurons. Brain Res 724:149 –168.
Bowden CL, Koslow SH, Hanin I, Maas JW, Davis JM, Robins E (1985): Effects of amitriptyline and imipramine on brain amine neurotransmitter metabolites in cerebrospinal fluid.
Clin Pharmacol Ther 37:316 –324.
Bunney WE Jr, Davis JM (1965): Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry 13:483– 494. Cedarbaum JM, Aghajanian GK (1976): Noradrenergic neurons
of the locus coeruleus: Inhibition by epinephrine and activa-tion by the alpha-antagonist piperoxane. Brain Res 122:413– 419.
(1994): Evidence that the acute behavioral and electrophysi-ological effects of Bupropion (Wellbutrint) are mediated by a noradrenergic mechanism. Neuropsychopharmacology 11: 133–141.
Cosford RJO (1995): Development of a method for quantitative microdialysis under transient conditions with application to the analysis of extracellular monoamines. Doctoral disserta-tion, Emory University, Atlanta.
Curtis AL, Valentino RJ (1991): Acute and chronic effects of the atypical antidepressant, mianserin on brain noradrenergic neurons. Psychopharmacology 103:330 –338.
Curtis AL, Valentino RJ (1994): Corticotropin-releasing factor neurotransmission in locus coeruleus: A possible site of antidepressant action. Brain Res Bull 35:581–587.
Dahl LE, Lundin L, le Fe`vre HP, Dencker SJ (1982): Antide-pressant effect of femoxetine and desipramine and relation-ship to the concentration of amine metabolites in cerebrospi-nal fluid. A double-blind evaluation. Acta Psychiatr Scand 66:9 –17.
Foote SL, Aston-Jones G, Bloom FE (1980): Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad
Sci U S A 77:3033–3037.
Garabal MF, Nunez MJ, Balboa JL, Suarez JA, Belmonte A (1991): Effects of alprazolam on the development of MTV-induced mammary tumors in female mice under stress.
Cancer Lett 62:185–189.
Gold PW, Chrousos GP (1999): The endocrinology of melan-cholic and atypical depression: Relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians 111:22–34. Graham AW, Aghajanian GK (1971): Effects of amphetamine on single-cell activity in a catecholamine nucleus, the locus coeruleus. Nature 234:100 –102.
Hsiao JK, Agren H, Bartko JJ, Rudorfer MV, Linnoila M, Potter WZ (1987): Monoamine neurotransmitter interactions and the prediction of antidepressant response. Arch Gen Psychiatry 44:1078 –1083.
Huang YH, Maas JW, Hu GH (1980): The time course of noradrenergic pre- and postsynaptic activity during chronic desipramine treatment. Eur J Pharmacol 68:41– 47. Jordan S, Kramer GL, Zukas PK, Moeller M, Petty F (1994): In
vivo biogenic amine efflux in medial prefrontal cortex with imipramine, fluoxetine, and fluvoxamine. Synapse 18:294 – 297.
Kopin IJ (1985): Catecholamine metabolism: Basic aspects and clinical significance. Pharmacol Rev 37:333–364.
Korf J, Bunney BS, Aghajanian GK (1974): Noradrenergic neurons: Morphine inhibition of spontaneous activity. Eur
J Pharmacol 25:165–169.
Maas JW, Koslow SH, Katz MM, Bowden CL, Gibbons RL, Stokes PE, et al (1984): Pretreatment neurotransmitter me-tabolite levels and response to tricyclic antidepressant drugs.
Am J Psychiatry 141:1159 –1171.
Major LF, Murphy DL, Lipper S, Gordon E (1979): Effects of clorgyline and pargyline on deaminated metabolites of nor-epinephrine, dopamine, and serotonin in human cerebrospinal fluid. J Neurochem 32:229 –231.
Martensson B, Wagner A, Beck O, Brodin K, Montero D, Asberg M (1991): Effects of clomipramine treatment on
cerebrospi-nal fluid monoamine metabolites and platelet 3H-imipramine binding and serotonin uptake and concentration in major depressive disorder. Acta Psychiatr Scand 83:125–133. Mazhar M, Binder SR (1989): Analysis of benzodiazepines and
tricyclic antidepressants in serum using a common solid-phase clean-up and a common mobile solid-phase. J Chromatogr 497:201–212.
McMillen BA, Warnack W, German DC, Shore PA (1980): Effects of chronic desipramine treatment on rat brain norad-renergic responses toa-adrenergic drugs. Eur J Pharmacol 61:239 –246.
Mendlewicz J, Pinder RM, Stulemeijer SM, Van Dorth R (1982): Monoamine metabolites in cerebrospinal fluid of depressed patients during treatment with mianserin or amitritriptyline. J
Affect Disord 4:219 –226.
Mongeau R, Blier P, de Montigny C (1997): The serotonergic and noradrenergic systems of the hippocampus: Their inter-actions and the effects of antidepressant treatments. Brain Res
Rev 23:145–195.
Nestler EJ, McMahon A, Sabban EL, Tallman JF, Duman RS (1990): Chronic antidepressant administration decreases the expression of tyrosine hydroxylase in the rat locus coeruleus.
Proc Natl Acad Sci U S A 87:7522–7526.
Nyback HV, Walters JR, Aghajanian GK, Roth RH (1975): Tricyclic antidepressants: Effects on the firing rate of brain noradrenergic neurons. Eur J Pharmacol 32:302–312. Orsulak PJ (1986): Therapeutic monitoring of antidepressant
drugs: Current methodology and applications. J Clin
Psychi-atry 47:39 –50.
Overstreet DH (1993): The Flinders Sensitive line rats: A genetic animal model of depression. Neurosci Biobehav Rev 17:51– 68.
Pickel VM, Joh TH, Reis DJ (1977): A serotonergic innervation of noradrenergic neurons in nucleus locus coeruleus: Dem-onstration by immunocytochemical localization of the trans-mitter specific enzymes tyrosine and tryptophan hydroxylase.
Brain Res 131:197–214.
Potter WZ, Calil HM, Extein I, Gold PW, Wehr TA, Goodwin FK (1981): Specific norepinephrine and serotonin uptake inhibitors in man: A crossover study with pharmacokinetic, biochemical, neuroendocrine and behavioral parameters. Acta
Psychiatr Scand Suppl 290:152–165.
Potter WZ, Scheinin M, Golden RN, Rudorfer MV, Cowdry RW, Calil HM, et al (1985): Selective antidepressants and cere-brospinal fluid. Lack of specificity on norepinephrine and serotonin metabolites. Arch Gen Psychiatry 42:1171–1177. Preskorn SH (1989): Tricyclic antidepressants: The whys and
hows of therapeutic drug monitoring. J Clin Psychiatry 50(suppl):34 – 42.
Preskorn SH, Fast GA (1991): Therapeutic drug monitoring for antidepressants: Efficacy, safety, and cost effectiveness.
J Clin Psychiatry 52(suppl):23–33.
Price LH, Charney DS, Heninger GR (1987): Reserpine augmen-tation of desipramine in refractory depression: Clinical and neurobiological effects. Psychopharmacology 92:431– 437. Richelson E, Pfenning M (1984): Blockade by antidepressants
Ritchie JC, Zhang W (1996): Modification of the Bio Rad tricyclic antidepressant methodology to measure the serotonin reuptake inhibitors. Clin Chem 42:S222.
Scheinin M (1985): Monoamine metabolites in human cerebrospi-nal fluid: Indicators of neurocerebrospi-nal activity? Med Biol 63:1–17. Schildkraut JJ (1965): The catecholamine hypothesis of affective
disorders: A review of supporting evidence. Am J Psychiatry 122:509 –522.
Schildkraut JJ, Kety SS (1967): Biogenic amines and emotion.
Science 156:21–37.
Schiller GD, Pucilowski O, Wienicke C, Overstreet DH (1992): Immobility-reducing effects of antidepressants in a genetic animal model of depression. Brain Res Bull 28:821– 823. Scuvee-Moreau JJ, Dresse AE (1979): Effect of various
antide-pressant drugs on the spontaneous firing rate of locus coer-uleus and dorsal raphe neurons in the rat. Eur J Pharmacol 57:219 –225.
Segal M (1979): Serotonergic innervation of the locus coeruleus from the dorsal raphe and its action on responses to noxious stimuli. J Physiol (Lond) 286:401– 405.
Sharma RP, Javaid JI, Faull K, Davis JM, Janicak PG (1994): CSF and plasma MHPG, and the CSF MHPG index: Pretreat-ment levels in diagnostic groups and response to somatic treatments. Psychiatry Res 51:51–56.
Simson PE, Weiss JM (1987): Alpha-2 receptor blockade in-creases responsiveness of locus coeruleus neurons to excita-tory stimulation. J Neurosci 7:1732–1740.
Simson PE, Weiss JM (1989): Blockade of a2-adrenergic
recep-tors, but not blockade of gamma-aminobutyric acid-A,
sero-tonin, or opiate receptors, augments responsiveness of locus coeruleus neurons to excitatory stimulation.
Neuropharma-cology 28:651– 660.
Sunderland T, Cohen RM, Molchan S, Lawlor BA, Mellow AM, Newhouse PA, et al (1994): High-dose selegiline in treat-ment-resistant older depressive patients. Arch Gen Psychiatry 51:607– 615.
Svensson TH, Usdin T (1978): Feedback inhibition of brain noradrenaline neurons by tricyclic antidepressants: Alpha-receptor mediation. Science 202:1089 –1091.
Thoren P, Asberg M, Bertilsson L, Mellstrom B, Sjoqvist F, Traskman L (1980): Clomipramine treatment of obssessive-compulsive disorder. II. Biochemical aspects. Arch Gen
Psychiatry 37:1289 –1294.
Traskman L, Asberg M, Bertilsson L, Cronholm B, Mellstrom B, Neckers LM, et al (1979): Plasma levels of chlorimipramine
and its demethyl metabolite during treatment of depression.
Clin Pharmacol Ther 26:600 – 610.
Valentino RJ, Curtis AL (1991): Antidepressant interactions with corticotropin-releasing factor in the noradrenergic nucleus locus coeruleus. Psychopharmacol Bull 27:263–269. Valentino RJ, Curtis AL, Parris DG, Wehby RG (1990):
Anti-depressant actions on brain noradrenergic neurons. J
Phar-macol Exp Ther 253:833– 840.
Van Brunt N (1983): The clinical utility of tricyclic antidepres-sant blood levels: A review of the literature. Ther Drug Monit 5:1–10.
Vetulani J, Stawarz RJ, Dingell JV, Sulser F (1976a): A possible common mechanism of action of antidepressant treatments (reduction in the sensitivity of the noradrenergic cyclic AMP generating system in the rat limbic forebrain). Naunyn
Schmiedebergs Arch Pharmacol 293:109 –114.
Vetulani J, Stawarz RJ, Sulser F (1976b): Adaptive mechanisms of the noradrenergic cyclic AMP generating system in the limbic forebrain of the rat: Adaptation to persistent changes in the availability of norepinephrine (NE). J Neurochem 27: 661– 666.
Vetulani J, Sulser F (1975): Action of various antidepressant treatments reduces reactivity of noradrenergic cyclic AMP-generating system in limbic forebrain. Nature 257:495– 496. Wallinder J, Carlsson A, Persson R (1981): 5-HT reuptake inhibitors plus tryptophan in endogenous depression. Acta
Psychiatr Scand 63(suppl 290):179 –190.
Weiss JM, Bonsall RW, Demetrikopoulos MK, Emery MS, West CHK (1998): Galanin: A significant role in depression? In: Ho¨kfelt T, Bartfai T, Crawley J, editors. Annals of the New
York Academy of Sciences. New York: New York Academy
of Sciences, 364 –382.
Weiss JM, Demetrikopoulos MK, West CHK, Bonsall RW (1996): Hypothesis linking the noradrenergic and dopaminer-gic systems in depression. Depression 3:225–245.
West CHK, Weiss JM (1995): A new, sensitive and potentially selective test for antidepressant therapeutic agents. In:
Amer-ican College of Neuropsychopharmacology 34th Annual Meeting Abstracts. Nashville: The American College of
Neuropsychopharmacology, 247.
West CHK, Weiss JM (1998): Effects of antidepressant drugs on rats bred for low activity in the swim test. Pharmacol
Biochem Behav 61:67–79.
Zhu M-Y, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, et al (1999): Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol
![Figure 2. Spontaneous depolarization rate (spikes/sec [Hz]) of locus coeruleus (LC) neurons in adult male and female rats treatedchronically with antidepressant drugs](https://thumb-ap.123doks.com/thumbv2/123dok/3142660.1383360/5.612.95.508.448.654/figure-spontaneous-depolarization-spikes-coeruleus-neurons-treatedchronically-antidepressant.webp)