Lipoprotein (a) and anticardiolipin antibodies are risk factors for
clinically relevant restenosis after elective balloon percutaneous
transluminal coronary angioplasty
Ludia Chiarugi *, Domenico Prisco, Emilia Antonucci, Monia Capanni, Sandra Fedi,
Agatina Alessandrello Liotta, Massimo Margheri, Ignazio Simonetti,
Gian Franco Gensini, Rosanna Abbate
Istituto di Clinica Medica Generale e Cardiologia,Uni6ersity of Florence,Viale Morgagni85,50134Florence,Italy
Received 3 August 1999; received in revised form 24 January 2000; accepted 18 February 2000
Abstract
Recent reports have shown the importance of new risk factors for cardiovascular disease. We investigated the relationship between Lp(a), fibrinolytic parameters and anticardiolipin antibodies (aCL) and the occurrence of clinical recurrence owing to restenosis after elective balloon percutaneous transluminal coronary angioplasty (PTCA) without stenting. In 167 patients, undergoing PTCA, Lp(a) plasma levels, aCL, euglobulin lysis time (ELT), plasminogen activator inhibitor-1 (PAI-1) activity and tissue-type plasminogen activator (t-PA) plasma levels were evaluated before the procedure. During follow-up 29 patients underwent clinical recurrence due to restenosis. Lp(a) levels were significantly higher in patients with restenosis in comparison to those without (PB0.05); an earlier restenosis was observed in patients with Lp(a) values \450 mg/L. Kaplan – Meier survival estimate showed an earlier occurrence of restenosis in patients with base-line Lp(a)\300 mg/l associated with aCL positivity. High Lp(a) plasma levels play a role in the occurrence of clinical recurrence due to restenosis after elective balloon PTCA without stenting; the association with aCL accelerates the development of restenosis. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Lp(a); Anticardiolipin antibodies; Fibrinolysis; PTCA; Restenosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
High levels of lipoprotein (a) (Lp(a)) have been found to predict myocardial infarction, coronary artery disease and vein graft stenosis after bypass procedures [1 – 3]. Lp(a) is structurally related to important proteins involved in fibrinolysis, coagulation and cellular mito-genesis [4,5]. These observations suggest a relevant role of Lp(a) in both atherogenesis and thrombogenesis. Less clear is the role of Lp(a) in restenosis after percu-taneous transluminal coronary angioplasty (PTCA). Whereas some studies suggested that Lp(a) levels are an independent predictor of restenosis [6 – 10], others re-ported that restenosis is unrelated to Lp(a) levels [11 –
16]. However, as recently pointed out [16], most of those studies either were partly or completely retrospec-tive, based on assays of stored blood samples, or in-cluded patients with recent myocardial infarction, all factors capable of confounding data interpretation. Controversial results have also been reported [6 – 8,11,13,16,17] on the association between lipid parame-ters (total cholesterol, triglycerides, HDL, apolipoproteins, etc.) and restenosis after PTCA.
Recent reports have shown that antiphospholipid antibodies are a risk factor for cardiovascular disease [18,19] and that the thrombotic risk is higher when elevated Lp(a) levels coexist with antiphospholipid anti-bodies positivity [20]. The present prospective study was designed to evaluate the relationship between Lp(a), fibrinolytic parameters and anticardiolipin anti-bodies (aCL) and the occurrence of clinically relevant restenosis after elective balloon PTCA without stenting. * Corresponding author. Tel.: +39-055-4279417; fax: +
39-055-4279418.
E-mail address:[email protected] (L. Chiarugi).
2. Methods
2.1. Patients
Three hundred and fifty nine consecutive patients referred to our Department for elective PTCA were considered after having obtained their informed con-sent. Six patients were excluded for at least one of the following exclusion criteria: enzymatic or ECG evi-dence of acute myocardial infarction (AMI), clinical evidence of recent MI (less than 6 months), unstable angina class III (according to Braunwald) [21], surgical or invasive procedures in the month preceding the study, neoplastic disease, malar rash, discoid rash, oral or pharyngeal ulceration, frank arthritis; pleuritis in the absence of pulmonary embolism or left-side heart fail-ure, pericarditis in the absence of myocardial infarction or uremia; persistent proteinuria greater than 0.5 g per day, due to biopsy-proven immune complex related glomerulonephritis, lymphopenia less than 1000/ml, an-tibodies to native DNA, extractable nuclear anti-gen antibodies, antinuclear antibodies of more than 1:320, treatment with drugs known to induce aCL. One hundred seventy nine patients who underwent stent placement in addition to PTCA were excluded. Seven patients (5 males and 2 females), who underwent reoc-clusion of the angioplastied vessel during the first week, were also excluded from the study. Among the 167 patients included in the analysis, 43 patients were af-fected by stable effort angina, 77 by unstable angina, class II B according to Braunwald (they had been previously hospitalised for unstable angina in intensive care unit and then discharged), 47 had no symptoms but had a stress test positive for ischemia. The charac-teristics of the patients investigated are shown in Table 1.
Patients who had given up smoking since less than six months prior to the study were considered among smokers.
2.2. Angioplasty procedure
PTCA was performed through the femoral approach with steerable balloon catheter using the Judkins tech-nique [22]. All the patients were on chronic oral treat-ment with aspirin (ASA, 325 mg/day) in addition to antianginal standard medications (nitrates and/or cal-cium blockers and/or beta-blockers). During the proce-dure all patients received heparin (10 000 – 15 000 IU i.v.), ASA 500 mg i.v. and an i.v. infusion of nitro-glyc-erine (5 mg/min) plus an intracoronary bolus of nitro-glycerine (250 mg) or isosorbide dinitrate (200 mg). Post-PTCA drug regimen included heparin infusion (1000 – 1200 IU/h) for 12 – 18 h, followed by subcuta-neous administration (12 500 IU/day) for a week, oral treatment with ASA (325 mg/day) and antianginal therapy.
Angiograms were analysed with a quantitative visual assessment according to a modification of Brown – Dodge method [23] as previously reported [24]. The variability of measurement reproducibility of this method is 6.7% [24]. All angiograms were reviewed by three experienced angiographers who were not involved in the performance of the procedures and were blinded to the laboratory results and to the clinical outcome. With regard to coronary disease before PTCA, 70 patients had one-vessel disease, 76 had two-vessel dis-ease and 21 had three-vessel disdis-ease. The median and range values of the diameter stenosis of the treated coronary vessels before PTCA were 90% and 75 – 100%, respectively. After PTCA these values were 15% and 0 – 40%, respectively. Successful angioplasty was defined as the restoration of normal flow with less than 50% residual stenosis without major complications.
2.3. Blood Sampling
Venous blood samples were collected by venipunc-ture technique using a 19 G butterfly, 1 h before PTCA in the morning (8 – 10:00 a.m.). Blood was drawn di-rectly into plastic tubes containing sodium citrate 0.129 M (1/10, v/v) for the determination of Lp(a) plasma levels, euglobulin lysis time (ELT), plasminogen activa-tor inhibiactiva-tor-1 (PAI-1) activity and tissue-type plasmi-nogen activator (t-PA) concentration. Plasma samples obtained after centrifugation were stored at −80°C except for Lp(a) and ELT determinations, which were performed on fresh samples. Sera for testing aCL were obtained by centrifugation of blood collected without anticoagulant at 1300×g for 10 min and stored at −20°C. Stored plasma and serum were assayed within 15 days for the determination of all parameters. Table 1
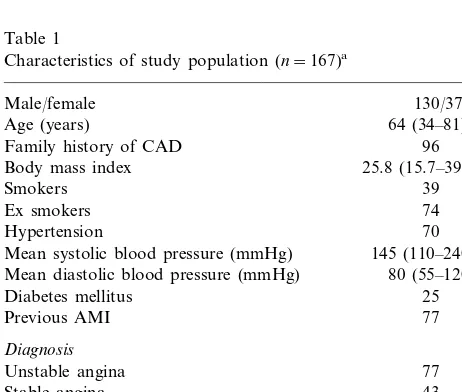
Characteristics of study population (n=167)a
130/37 Male/female
64 (34–81) Age (years)
Family history of CAD 96
25.8 (15.7–39.5)
Mean systolic blood pressure (mmHg) 145 (110–240) Mean diastolic blood pressure (mmHg) 80 (55–120)
Diabetes mellitus 25 Positive stress test for ischemia
Total cholesterol (mg/dl) 221 (126–500) HDL cholesterol (mg/dl) 45 (25–79) Triglycerides (mg/dl) 180 (56–666)
Table 2
Base-line Lp(a) and haemostatic factorsa
No restenosis related recurrence Restenosis related recurrence P* All patients
121 (10–1200)
Lp(a) (mg/l) 126 (10–1260) 193 (10–1174) 0.05
ELT (h) 6 (2–10.5) 6 (2–10.5) 6.5 (2-10.5) ns
12.1 (3–27.9)
t-PA (ng/mL) 12.4 (3–27.2) 12 (4–27.9) ns
7.4 (3–38.1) 7.6 (3–25) ns
7.4 (3–38.1) PAI-1 (IU/mL)
aELT, euglobolin lysis time; Lp(a), lipoprotein (a); PAI-1, plasminogen activator inhibitor-1 activity; t-PA, tissue-type plasminogen activator
antigen.
* comparison between patients with and wihout restenosis related recurrence.
2.4. Laboratory test
Lp(a) was assayed by ELISA (TintElyze Lp(a), Bio-pool, Umea, Sweden); (90th percentile of our control population=450 mg/l). PAI-1 activity was evaluated according to Chmielewska [25] by a chromogenic method (Spectrolyse (fibrin), Biopool) (control range 2 – 15 IU/ml). The t-PA antigen plasma concentration (control range 1.7 – 10 ng/ml) was assayed by ELISA (TintElyze t-PA, Bio-Pool). Euglobulin lysis time (con-trol range 2.5 – 5 h) was determined according to Chakrabarti [26]. The aCL assay was performed by ELISA (First Cardiolipin, Eurospital, Trieste, Italy) and aCL levels were reported in GPL units (for IgG) and in MPL units (for IgM). On the basis of analysis of several hundreds of normal sera performed in our laboratory in the past, and according to the literature, values above 20 IU for either IgG or IgM were consid-ered abnormal [27,28].
2.5. Follow-up
Patients were clinically followed up for a mean time of 20 months. During follow-up they were invited to contact the cardiologists when symptoms referable to cardiac ischemia occurred and to refer to the hospital for any subsequent clinical evaluation. Ergometric tests were performed 1, 3 and 6 months after PTCA. During the follow-up period, for ethical reasons, angiography was again performed only in patients with clinical recurrence defined as either recurrent anginal attacks, a positive treadmill test (Bruce protocol) [29], or a posi-tive stress test with 201Tl-scintigraphy. Restenosis was defined by the presence of a decrease \50% of gain in luminal diameter achieved by PTCA [30].
2.6. Statistical analysis
The results are expressed as median and range be-cause of their skewed distribution. Preliminary statisti-cal analysis was performed using the Wilcoxon’s signed rank test, Fisher’s exact test or Kruskal – Wallis test. Correlation coefficients were calculated with Spear-man’s rank test. Multivariate analysis was assayed by
logistic or linear regression. Survival estimate was ob-tained by Kaplan – Meier test. P valuesB0.05 were considered significant.
3. Results
All the 167 patients showed up for the non-invasive follow-up. Thirty-three patients underwent angiography for clinical recurrence and in 29/167 (17%) restenosis of the angioplastied artery was demonstrated. The mean time of restenosis was 593 months.
3.1. Lp(a), fibrinolysis and a CL
The results of base-line Lp(a) and haemostatic parameters are reported in Table 2. Base-line Lp(a) levels were \300 mg/l in 55/167 patients. Lp(a) levels were negatively correlated with PAI-1 activity (r= − 0.22, PB0.01) but this correlation was no longer present after adjustment for the presence of aCL, dia-betes mellitus and smoking habits, or the levels of total cholesterol and triglycerides. No correlation was ob-served between Lp(a) and the other haemostatic fac-tors. However, Lp(a) plasma levels \300 mg/l showed a trend to be associated with prolonged ELT (]5 h,
P=0.06). Eighteen patients had positivity for aCL and six of them showed Lp(a)\300 mg/l. No significant correlation was found between aCL positivity and Lp(a) levels.
3.2. Lp(a), clinical and angiographic factors
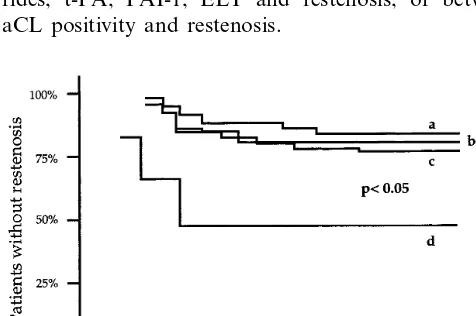
Fig. 1. Cumulative frequency of restenosis in patients with: a= Lp(a)B450 mg/l; b=Lp(a)\450 mg/l.
4. Discussion
In this study we report that high Lp(a) levels repre-sent a risk factor for restenosis-related clinical recur-rence after elective balloon PTCA and that the contemporary presence of aCL increases this risk in patients with Lp(a)\300 mg/l. Our study is hardly comparable with most of previous investigations on the role of Lp(a) levels in angiographic restenosis. Those studies have various limitations including retrospective analysis, small sample size, inclusion of patients within 4 weeks of acute myocardial infarction, blood sampling after PTCA, or assay of stored samples which can yield artificially low levels of Lp(a) [8,11 – 13,17,31,32].
4.1. Role of Lp(a) and lipids in restenosis-related clinical recurrence after PTCA
We found no significant relationship between either cholesterol or triglycerides levels and clinical recurrence after PTCA. Contradictory results have been reported over the last years on this issue and our data confirm the absence of consistent data to support a role of lipid levels as risk factor for restenosis.
The correlation between restenosis and Lp(a) levels observed by us is in agreement with data found by Desmarais et al. [17]. However, they measured the Lp(a) levels 4 weeks after PTCA in patients who had a history of myocardial infarction within 1 month of PTCA and these Lp(a) measurements after PTCA may have confounded the analysis. In a recent well-designed investigation, Alaigh et al. did not find any difference in Lp(a) levels between restenosis and no restenosis pa-tients after PTCA [16]. However, the end-point of our study was clinical recurrence due to restenosis and not restenosis per se so that the two studies are not com-parable. Plasma Lp(a) has recently been demonstrated to be not a predictor for restenosis after elective high-pressure coronary stenting [33]. However, due to differ-ences in the pathogenesis of restenosis after coronary stenting and after balloon PTCA, the two studies are not comparable.
It has been reported that patients with unstable angina and recent myocardial infarction have a tran-sient increase in the plasma levels of Lp(a) during acute phases of disease [34]. We did not find any significant difference in Lp(a) levels between patients with unstable and those with stable angina, whereas patients with recent infarction were excluded from our study. By univariate, but not by multivariate analysis we found an inverse correlation between PAI-1 activity and Lp(a). This relationship had been already reported in healthy controls [35], but had not been confirmed in other studies [36].
Different mechanisms may link high Lp(a) levels to restenosis after PTCA. Lp(a), in addition to inhibiting 3.3. Restenosis-related clinical recurrence
Base-line Lp(a) median levels were significantly higher in patients with recurrence and restenosis in comparison to those without (respectively, 193 vs. 121 mg/l, PB0.05; see Table 2). A multivariate logistic regression showed an association of diabetes mellitus with restenosis (8 of the 25 diabetic patients underwent restenosis) and confirmed that Lp(a) is an independent predictor of restenosis-related clinical recurrence, after adjustments for the presence of aCL, total cholesterol, triglycerides, diabetes mellitus, smoking habitus.
Kaplan – Meier survival estimate showed an earlier occurrence of restenosis in patients with Lp(a)\450 mg/l (Fig. 1,PB0.05) as well as in patients with Lp(a) levels \300 mg/l if associated with aCL positivity (Fig. 2,PB0.05). No relationship was found either between levels of total cholesterol, HDL cholesterol, triglyce-rides, t-PA, PAI-1, ELT and restenosis, or between aCL positivity and restenosis.
plasminogen binding to the surface of endothelial cells and reducing the activity of t-PA [4], is able to promote human smooth muscle cells proliferation by interfering with transforming growth factor activation [5].
4.2. Co-operation of the presence of aCL and high Lp(a) le6els in restenosis-related clinical recurrence
after PTCA
In this study no relation was observed between the presence of aCL and clinical restenosis but we demon-strated that the contemporary presence of aCL and elevated Lp(a) levels markedly increased the risk of restenosis-related clinical recurrence after PTCA. An-tiphospholipid antibodies have been found to be associ-ated with arterial and vein thrombosis [37,38]. However, conflicting results have been reported on the role of aCL in coronary artery disease [39,40] and the mechanism by which aCL arise and their relationship to the thrombotic events have not been clearly eluci-dated [38].
A higher risk for arterial thrombosis in aCL positive patients with high Lp(a) levels has been reported in clinical conditions such as Systemic lupus erithematosus [20] and Rheumatoid Arthritis [41]. Recently, increased levels of Lp(a) have been reported to be associated with a low fibrinolytic activity in patients with the antiphos-pholipid syndrome [42]. In our patients we could not observe a clear-cut relationship among Lp(a) levels, aCL and the fibrinolytic parameters. However, a re-duced fibrinolytic activity is just one of the possible mechanisms linking the presence of both aCL and elevated Lp(a) levels to an increased risk of clinical recurrence after PTCA. Lp(a) has been found to be related to the acute phase reaction. Thus, the increased risk for restenosis in patients with high Lp(a) levels and aCL could suggest a role for an inflammatory condition predisposing to an enhanced reaction to the balloon injury.
4.3. Limitations of the study
One limitation of this study is the fact that we did not determine Lp(a) polymorphisms. Apo(a) shows a size polymorphism, with individual isoforms ranging in apparent molecular weights from about 250 – 800 KD. Small apo(a) has a significant association with coronary artery disease and the combination of high Lp(a) levels and small size apo(a) isoform has been reported to be the greatest risk factor for coronary artery disease in hypercholesterolemic patients [43] and to be associated with very low fibrinolytic activity [44]. So we cannot exclude that this combination could be a more relevant risk factor for restenosis after PTCA than Lp(a) levels alone. Another limitation may be the absence of sys-tematic follow-up angiography. As specified in Section
2, in the present study angiography has been performed only in patients with clinical recurrence or inducible ischemia. Thus, we cannot draw any conclusion on the effect of Lp(a) and aCL on restenosis ‘per se’. However, we chose a clinically relevant definition of recurrence (that required recurrence symptoms or positive stress test) with angiographically demonstrated restenosis. This definition seems appropriate because borderline luminal diameter narrowing scarcely correlates to clini-cal symptoms and prognosis [45]. Our policy, strictly limiting invasive procedures, is in agreement with recent ACC/AHA guidelines for coronary angiography [46]. Finally, it should be remembered that the results of this study are relevant only for patients undergoing elective balloon PTCA without stent implantation.
5. Conclusion
This study shows for the first time that aCL positiv-ity and high Lp(a) levels act synergistically in increasing the risk of clinically relevant restenosis after elective balloon PTCA. These results are relevant because, al-though stenting is largely used, a significant proportion of patients have still indication to PTCA without stent-ing. At present, Lp(a) plasma levels are not modifiable by drugs commonly used in clinical practice, but the effects of new agents able to lower Lp(a) concentration or apheresis could be interestingly explored in this high risk clinical condition [9]. Moreover, symptomatic pa-tients with antiphospholipid antibodies have been demonstrated to take advantage from oral anticoagu-lant therapy [47 – 50]. These results suggest an opportu-nity to design studies aimed at elucidating the effect of more aggressive therapeutic strategies in selected groups of patients in whom the risk of restenosis related recur-rence is particularly high in relation to the contempo-rary presence of high Lp(a) plasma levels and aCL positivity.
References
[1] Rosengren A, Wilhelmsen L, Eriksson E, Risberg B, Wedel H. Lipoprotein (a) and coronary heart disease: a prospective case-control study in a general population sample of middle aged men. Br Med J 1990;301:1248 – 51.
[2] Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. Lipoprotein(a) interactions with lipid and non lipid risk factors in early familial coronary artery disease. Arte-rioscler Thromb Vasc Biol 1997;17:2783 – 92.
[3] Terres W, Tatsis E, Pfalzer B, Beil U, Beisiegel U, Hamm CW. Rapid angiographic progression of coronary artery disease in patients with elevated lipoprotein (a). Circulation 1995;91:948 – 50.
[5] Grainger DJ, Kirschenlohr HL, Mecalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein (a). Science 1993;260:1655 – 8. [6] Hearn JA, Donohue BC, Ba’albaki H, Douglas JS, King SB, III,
Lembo NJ, et al. Usefulness of serum lipoprotein (a) as a predictor of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol 1992;69:736 – 9.
[7] Tenda K, Saikawa T, Maeda T, Sato Y, Niwa H, Inouse T, et al. The relationship between serum lipoprotein(a) and restenosis after initial elective percutaneous transluminal coronary angio-plasty. Jpn Circ J 1993;57:789 – 95.
[8] Yamamoto H, Imazu M, Yamabe T, Ueda H, Hattori Y, Yamakido M. Risk factors for restenosis after percutaneous transluminal coronary angioplasty: role of lipoprotein (a). Am Heart J 1995;130:1168 – 73.
[9] Low-density Lipoprotein Apheresis Angioplasty Restenosis Trial Group, Daida H, Lee YJ, Yokoi H, Kanoh T, Ishiwata S, Kato K, et al. Prevention of restenosis after percutaneous transluminal coronary angioplasty by reducing lipoprotein (a) levels with low-density lipoprotein apheresis. Am J Cardiol 1994;73:1037 – 40.
[10] Miyata M, Biro S, Arima S, Hamasaki S, Kaieda H, Nakao S, et al. High serum concentration of lipoprotein (a) is a risk factor for restenosis after percutaneous transluminal coronary angio-plasty in Japanese patients with single vessel disease. Am Heart J 1996;132:269 – 73.
[11] Schumacher N, Tiran A, Eber B, Toplak H, Wilders-Truschnig M, Klein W. Lipoprotein(a) is not a risk factor for restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol 1992;69:572.
[12] Shah PK, Amin J. Low high density lipoprotein level is associ-ated with increased restenosis rate after coronary angioplasty. Circulation 1992;85:1279 – 85.
[13] Cooke T, Sheahan R, Foley D, Reilly M, D’Arcy G, Jauch W, et al. Lipoprotein (a) in restenosis after percutaneus transluminal coronary angioplasty and coronary artery disease. Circulation 1994;89:1593 – 8.
[14] Bussiere JL, de Bourayne J, Monsegu J, Corbe´ H, Berville JD, Lancelin B, et al. Le taux plasmatique de lipoprotein(a) permet-il de predire la reste´nose apre`s angioplastie? Arch Mal Coeur 1996;89:425 – 9.
[15] Kotamaki M, Laustiola K, Syvanne M, Heikkila J. Influence of continued smoking and some biological risk factors on restenosis after percutaneous transluminal coronary angioplasty. J Intern Med 1996;240:293 – 301.
[16] Alaigh P, Hoffman CJ, Korlipara G, Neuroth A, Dervan JP, Lawson WE, et al. Lipoprotein (a) level does not predict resteno-sis after percutaneous transluminal coronary angioplasty. Arte-rioscler Thromb Vasc Biol 1998;18:1281 – 6.
[17] Desmarais RL, Sarembock IJ, Ayers CR, Vernon SM, Powers ER, Gimple LW. Elevated serum lipoprotein (a) is a risk factor for clinical recurrence after coronary balloon angioplasty. Circu-lation 1995;91:1403 – 9.
[18] Hamsten A, Norberg R, Bjorkholm M, De Faire U, Holm G. Antibodies to cardiolipin in young survivors of myocardial in-farction: an association with recurrent cardiovascular events. Lancet 1986;1:113 – 6.
[19] Vaarala O, Manttari M, Manninen V, Tenkanen L, Puurunen M, Aho K, et al. Anticardiolipin antibodies and risk of myocar-dial infarction in a prospective cohort of middle aged men. Circulation 1994;91:23 – 7.
[20] Yamazaki M, Asakura H, Jokaji H, Saito M, Uotani C, Kuma-bashiri I, et al. Plasma levels of lipoprotein (a) are elevated in patients with the antiphospholipid antibody syndrome. Thromb Haemost 1994;71:424 – 7.
[21] Braunwald E. Unstable angina: a classification. Circulation 1989;80:410 – 4.
[22] Dotter CT, Judkins MP. Transluminal treatment of atheroscle-rotic obstruction: description of a new technique and a prelimi-nary report of its application. Circulation 1964;30:654 – 70. [23] Brown BG, Bolson E, Frimer M, Dodge HT. Quantitative
coronary angiography: estimation of dimensions of hemody-namic resistance, and atheroma mass of coronary artery lesions using arteriogram and digital computation. Circulation 1977;55:329 – 37.
[24] Simonetti I, De Caterina R, Marzilli M, de Nes M, L’Abbate A. Coronary vasodilatation by nitrates is not mediated by the prostaglandin system: an angiographic and hemodynamic study. Z Kardiol 1983;72(3S):40 – 5.
[25] Chmielewska J, Ranby H, Wiman B. Evidence for a rapid inhibitor to tissue plasminogen activator in plasma. Thromb Res 1983;31:427 – 36.
[26] Chakrabarti R, Bielowiec M, Evans JF, Fearnley GR. Methodo-logical study and recommended technique for determining the euglobulin lysis time. J Clin Pathol 1968;21:698 – 704.
[27] Branch DW, Silver RM, Blackwell JL, Reading JC, Scott JR. Outcome of treated pregnancies in women with antiphospholipid antibodies. Br J Haematol 1990;74:1 – 9.
[28] Boddi M, Prisco D, Fedi S, Cellai AP, Alessandrello Liotta A, Parretti E, Mecacci F, et al. Antiphospholipid antibodies and pregnancy disorders in women with insulin-dependent diabetes. Thromb Res 1996;82:207 – 16.
[29] Fletcher GF, Balady G, Froelicher VF, et al. Exercise standards: a statement for healthcare professionals from the American Heart Association. Circulation 1995;91:580 – 615.
[30] De Groote P, Bauters C, McFadden E, Lablanche JM, Leroy F, Bertrand ME. Local lesion-related factors and restenosis after coronary angioplasty. Circulation 1995;91:968 – 72.
[31] Craig WY, Poulin SE, Forster NR, Neveux LM, Wald NJ, Ledue TB. Effect of sample storage on the assay of lipo-protein(a) by commercially available radial immunodiffusion and enzyme-linked immunoadsorbent assay kits. Clin Chem 1992;38:550 – 3.
[32] Sgoutas DS, Tuten T. Effect of freezing and thawing of serum on the immunoassay of lipoprotein(a). Clin Chem 1992;38:1873 – 7.
[33] Ribichini F, Steffenino G, Della Valle A, Ferrero V, Camilla T, Giubergia S, et al. Plasma lipoprotein (a) is not a predictor for restenosis after elective high-pressure coronary stenting. Circula-tion 1998;98:1172 – 7.
[34] Maeda S, Abe A, Seishima M, Makino K, Noma A, Kawade M. Transient changes of serum lipoprotein (a) as an acute phase protein. Atherosclerosis 1989;78:145 – 50.
[35] Heinrich J, Sandkamp M, Kokott R, Schulte H, Assmann G. Relationship of lipoprotein (a) to variables on coagulation and fibrinolysis in a healthy population. Clin Chem 1991;37(11):1950 – 4.
[36] Etingin OR, Hajjar DP, Hajjar KA, Harpel PC, Nachman RL. Lipoprotein (a) regulates plasminogen acitvator inhibitor-1 ex-pression in endothelial cells. J Biol Chem 1991;266:2459 – 65. [37] The Antiphospholipid Antibodies in Stroke Study Group
(APASS). Clinical and laboratory findings in patients with an-tiphospholipid antibodies and cerebral ischemia. Stroke 1990;21:1286 – 93.
[38] Hughes GRV. The antiphospholipid syndrome: ten years on. Lancet 1993;342:341 – 4.
[39] Brenner B, Blumenfeld Z, Markiewicz W, Reisner SA. Cardiac involvement in patients with primary antiphospholipid syn-drome. J Am Coll Cardiol 1991;18:931 – 6.
[41] Seriolo B, Accardo S, Fasciolo D, Sulli A, Bertolini S, Cutolo M. Lipoprotein (a) and anticardiolipin antibodies as risk factors for vascular disease in rheumatoid arthritis. Thromb Haemost 1996;74:799 – 800.
[42] Atsumi T, Khamashta MA, Andujar C, Leandro MJ, Amengual O, Ames PRJ, et al. Elevated plasma lipoprotein (a) level and its association with impaired fibrinolysis in patients with antiphos-pholipid syndrome. J Rheumatol 1998;25:69 – 73.
[43] Bowden JF, Pritchard PH, Hill JS, Frohlich JJ. Lp(a) concentra-tion and apo(a) isoform size: relaconcentra-tion to the presence of coronary artery disease in familial hypercholesterolemia. Arterioscler Thromb 1994;14:1561 – 8.
[44] Falco` C, Estelles A, Dalmau J, Espana F, Aznar J. Influence of lipoprotein(a) levels and isoforms on fibrinolytic activity-study in families with high lipoprotein(a) levels. Thromb Haemost 1998;79:818 – 23.
[45] Gordon PC, Friedrich SP, Piana RN, Kugelmass AD, Leidig GA, Gibson CM, et al., @ ‘‘Is 40 to 70% diameter narrowing at
the site of previous stenting or directional coronary atherectomy clinically significant?’’ Am. J. Cardiol. 994; 74: 26-32.
[46] ACC/AHA Guide Lines for Coronary Angiography: Executive Summary and Recommendations. A report of the American College of Cardiology/American Heart Association Task Force on practice Guidelines (committee on coronary angiography). Circulation 1999;99:2345 – 57.
[47] Rosove MH, Brewer PMC. Antiphospholipid thrombosis: clini-cal course after the first thrombotic event in 70 patients. Ann Intern Med 1992;117:303 – 8.
[48] Lockshin MD. Which patients with antiphospholipid should be treated and how? Rheum Dis Clin North Am 1993;19:235 – 47. [49] Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ,
Hughes GRV. The management of thrombosis in the antiphos-pholipid syndrome. New Eng J Med 1995;332:993 – 997¯. [50] Espinoza LR. Antiphospholipid antibody syndrome: treatment.
Lupus 1996;5:456 – 7.