www.elsevier.nlrlocateraqua-online
Evaluation of diets for culture of the calanoid
copepod Gladioferens imparipes
M.F. Payne
), R.J. Rippingale
School of EnÕironmental Biology, Curtin UniÕersity of Technology, GPO Box U1987, Perth, Western Australia 6845, Australia
Accepted 9 December 1999
Abstract
Provision of copepod nauplii as food increases larval survival in many fish species that are difficult to rear using standard practices. Gladioferens imparipes is a temperate estuarine calanoid copepod with culture potential. Diets were tested to determine which would maximise copepod production. Copepods were fed five diets: Isochrysis galbana, Chaetoceros muelleri, Dunaliella
tertiolecta, Nannochloropsis oculata and baker’s yeast. Copepod survival, time to maturity,
maturation rate, nauplius production and female length were recorded at 208C and 258C. By most criteria, Isochrysis was the most efficacious diet, followed by Chaetoceros and then Dunaliella.
Nannochloropsis and yeast resulted in little or no survival. Fatty acid profile of each diet was
determined. In general, copepod production was positively related to the ratio of DHA:EPA in their diet. Poor survival on Nannochloropsis was probably a result of the small cell size and poor digestibility of this species.q2000 Elsevier Science B.V. All rights reserved.
Keywords: HUFA; Calanoid; Copepod; Algae; Culture; Diet
1. Introduction
For many fish species, hatchery production is uneconomical or impossible using either rotifers or Artemia as larval diets. This is mostly a result of poor survival at the first feeding stage. The provision of copepod nauplii in the early larval diet often
)Corresponding author. Tel.:q61-8-9266-7915; fax:q61-8-9266-2495.
Ž .
E-mail address: [email protected] M.F. Payne .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
increases survival in these fish. Species in which this has been recently demonstrated
Ž . Ž .
include red snapper Doi et al., 1994 and some of the groupers Doi et al., 1997 . Also, the provision of copepods in the early larval diet decreases malpigmentation in halibut ŽMcEvoy et al., 1998 and increases stress resistance in mahi-mahi Kraul et al., 1993 .. Ž . These improvements are mostly attributed to increased feeding by fish on copepods and the high nutritional content of copepods.
Copepod culture must demonstrate reliable and prolific production to be accepted as part of commercial operations. Both calanoid and harpacticoid copepods have been cultured for use in fish larviculture. Harpacticoids are amenable to high-density culture conditions, hence, they are the subject of most studies on intensive copepod culture Že.g., Sun and Fleeger, 1995; Støttrup and Norsker, 1997; Nanton and Castell, 1998 .. However, harpacticoid copepods are predominantly benthic and so are not readily
Ž .
available to fish larvae that feed in the water column Kitajima, 1973 . In contrast, calanoid copepods are almost entirely planktonic, making them more suitable for feeding to larval fish. However, production from calanoid copepod cultures is typically much
Ž .
lower than that from harpacticoid cultures Støttrup et al., 1986; Schipp et al., 1999 . Calanoid copepod production is limited by difficulties in maintaining broodstock at high densities. Stress caused by crowding decreases fecundity in the calanoid
Cen-Ž .
tropages typicus Miralto et al., 1996 . Also, cannibalism of nauplii by adults reduces
Ž .
production in dense cultures. Rippingale and MacShane 1991 have identified a temperate estuarine species, Gladioferens imparipes, that exhibits high nauplius produc-tion at relatively high broodstock densities when cultured on a small scale and does not consume its own nauplii. While late copepodid and adult stages of this calanoid species
Ž .
adhere to surfaces, nauplii are entirely pelagic Rippingale, 1994 .
To increase the scale of G. imparipes cultivation, algal diets that maximise copepod growth, survival and fecundity must be identified. Diets that are high in highly
Ž .
unsaturated fatty acids HUFAs maximise fecundity in the calanoid Acartia tonsa ŽStøttrup and Jensen, 1990 and are generally the choice of diet for calanoid cultivation. Že.g., Rippingale and MacShane, 1991; Klein Breteler and Laan, 1993; Schipp et al.,
. Ž .
1999 . More specifically, the dietary ratio of two HUFAs, docosahexaenoic acid DHA
Ž . Ž
and eicosapentaenoic acid EPA , may affect copepod fecundity Støttrup and Jensen, .
1990
A wide range of algal species from different taxonomic groups are in common use in aquaculture, each with specific nutritional characteristics. Four algal species from different groups were fed to copepods in the present work: the prymnesiophyte
Ž .
Isochrysis galbana T-Iso , the diatom Chaetoceros muelleri, the chlorophyte Dunaliella
Ž
tertiolecta and the eustigmatophyte Nannochloropsis oculata. Baker’s yeast
Sac-.
charomyces cereÕisiae was also included as a copepod diet. I. galbana, C. muelleri and
Ž .
N. oculata each have a high HUFA content review by Brown et al., 1997 , whereas D.
Ž
tertiolecta and yeast contain little or no HUFAs Støttrup and Jensen, 1990; Whyte and
. Nagata, 1990 .
2. Materials and method
2.1. General methods
Nauplii were collected from 30-l copepod broodstock cultures maintained on a diet of
I. galbana at 228C and 27‰ salinity. Screening nauplii through 100mm mesh provided
Ž .
animals of approximately uniform age class -24 h old . These nauplii were mixed
Ž .
with filtered 1.2 mm seawater diluted to 27‰ to give a density of 1 naupliusrml. Nauplii were then randomly allocated to each of 36 150-ml plastic containers such that each contained approximately 80 nauplii. Volumes were made up with filtered seawater diluted to 27‰. Two groups of 18 containers each were placed in separate water-baths maintained at 20"0.58C and 25"0.58C. Lids on the water-baths shaded the containers from direct light. Five treatment diets were each randomly allocated to three containers in both groups, with the remaining three left unfed. Diets consisted of the algal species
Ž 1. Ž . Ž . Ž .
I. galbana 67 fl , C. muelleri 90 fl , D. tertiolecta 153 fl and N. oculata 16 fl and
Ž .
fresh baker’s yeast S. cereÕisiae; 83 fl . Hereafter, algal species will be referred to by
their generic name. Algal cultures were originally obtained from the CSIRO Marine Laboratories in Hobart, Tasmania, as non-axenic cultures, and maintained as batch cultures in 5-l Pyrexw flasks using Guillard’s fr2 medium. Algae was fed to copepods only when in mid to late logarithmic phase.
Nauplii were fed daily with equivalent concentrations by volume of the treatment diets. The mean cell volumes of each food organism were determined using a Coulter
Ž .
Counter Model D . Prior to feeding each day, the turbidity of each algal culture was
Ž .
measured using a Hach Kit Drelr5 and used to calculate cell density. The correlation equation used in this process was recalculated weekly. Suspensions of fresh yeast in deionised water were stored at 48C and renewed every 2 days. A haemocytometer was used to determine cell densities of each new suspension. Cell volume and density were then used to calculate a quantity of food such that treatments received 1 ppm food by volume each day up to day 4, increasing to 2 ppm for the duration of the trials. These quantities ensured excess food in all treatments, as indicated by the presence of uneaten algal or yeast cells in containers at the next feed. Every third day, 90% of the volume in each container was replaced with clean water.
2.2. Fatty acid analyses of copepod diets
Ž .
Fatty acids were analysed using techniques modified from Dunstan et al. 1992 . For each diet, between 250 and 500 ml of dense cell suspension was concentrated on Whatmanw
GFrC filter paper under gentle vacuum. The paper was immersed in
Ž . Ž .
dichloromethane DCM :methanol:water 1:2:0.8 vrvrv; Bligh and Dyer, 1959 , mashed with a glass rod and subject to ultrasonification for 10 min. It was then covered and stored in a refrigerator overnight. This sample was filtered under gentle vacuum and
Ž .
rinsed with DCM:methanol:water 50 ml . Lipids were extracted in DCM from a
1
Ž y1 5 .
Ž . w
mixture of DCM:water 1:1 vrv . The solvent was removed using a Bucci rotary
Ž .
evaporator and the remaining lipids dissolved in methanol 5 ml . Samples were
Ž . Ž
transferred to 20 ml reaction tubes, combined with acidified methanol 4 ml , toluene 2
. Ž Ž ..
ml and an internal standard nonadecanoic acid 0.5 mg and heated to 508C for 16 h.
Ž .
The cooled reaction mixture was transferred to hexane 20 ml , washed twice with
Ž .
deionised water 20 ml and dried over anhydrous sodium sulphate.
Ž .
The relative fatty acid methyl ester FAME composition of these solutions was
Ž . Ž
determined by gas chromatography GC with mass selective detection Hewlett
w Ž .
Packard HP a5890 Series II Gas Chromatograph with a HP a5971 Series Mass
. Ž
Selective Detector . The GC was fitted with a DB23 column J & W, 30 m=0.25 mm .
i.d.=0.25 mm film thickness with helium at 25 kPa used as the carrier gas. Each analytical run commenced with 5 min at 508C, increasing 58C each minute up to 2408C, with this temperature being maintained for the final 5 min. Retention times were determined relative to that of the internal standard and identification of the FAMEs was carried out by the comparison of the mass spectral analysis of individual eluted peaks with the Wiley electronic database. Identification of EPA and DHA was confirmed by comparisons to authentic FAME standards.
2.3. Copepod surÕiÕal
Copepod survival was assessed daily using a stereo microscope until egg production commenced. Containers in which-10% copepods survived were considered to have no copepod survival and were removed from the trial. Adult copepods were counted at the conclusion. Results were analysed using one-way ANOVAs and Tukey’s W procedure.
2.4. Maturation
Female copepods were judged to be mature when carrying their first clutch of embryos. Newly mature females were removed individually from containers daily using a stereo microscope and pipette. Females were counted and placed in a separate container with other females from the same treatment. Daily collection continued until all females had reached maturity and had been removed from the experimental contain-ers. Males were not removed from the containers as they reached maturity. Remaining males were counted and all males from the three replicates of each treatment pooled. Pooled males and females continued to be fed the treatment diets.
Cumulative proportions of mature females reared on treatment diets were logit transformed and regressed against time. The time taken for 50% of females to reach maturity on each diet was predicted from these regressions and compared using one-way ANOVAs and Tukey’s W procedure. Maturation rate was defined as the time between the collection of the first and last mature female, with a shorter time indicating a faster rate and resulting in a regression line with a steeper gradient. Gradients were compared using dummy variables.
2.5. Nauplius production and female growth
males and three embryo-bearing females were transferred to each of four containers and fed treatment diets to excess. Survival of the adult copepods was monitored daily. After exactly 96 h, nauplii and adult copepods were preserved for later enumeration. Produc-tions, expressed as naupliirfemalerday, were compared using one-way ANOVAs and Tukey’s W procedure.
Prosome length of females used in the nauplius production trial were measured using an ocular graticule. Results were analysed in the same manner as above.
3. Results
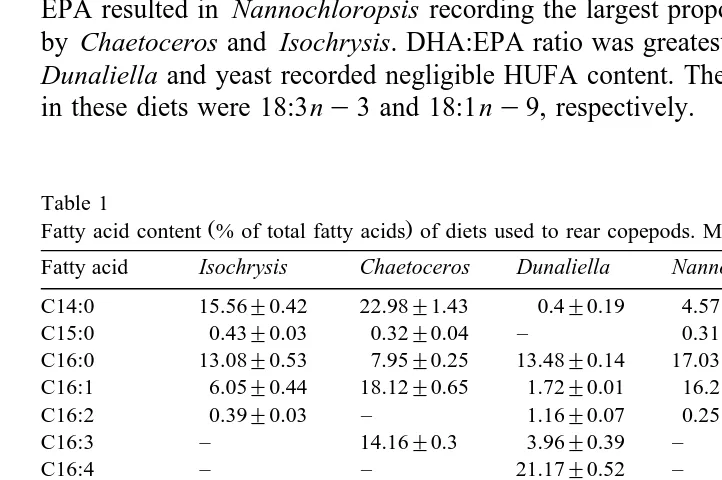
3.1. Fatty acid analyses of copepod diet
Ž .
Table 1 shows that Isochrysis contained the greatest proportion of DHA 22:6 ny3 , followed by Chaetoceros. This fatty acid was not detected in the other diets. EPA Ž20:5ny3 was the most abundant fatty acid in Nannochloropsis and Chaetoceros..
Isochrysis contained only a small proportion of this fatty acid. The high proportion of
EPA resulted in Nannochloropsis recording the largest proportion of HUFAs, followed by Chaetoceros and Isochrysis. DHA:EPA ratio was greatest in Isochrysis. In contrast,
Dunaliella and yeast recorded negligible HUFA content. The most abundant fatty acids
in these diets were 18:3ny3 and 18:1ny9, respectively.
Table 1
Ž .
Fatty acid content % of total fatty acids of diets used to rear copepods. Means of 3 replicates"SD Fatty acid Isochrysis Chaetoceros Dunaliella Nannochloropsis Yeast C14:0 15.56"0.42 22.98"1.43 0.4"0.19 4.57"0.38 0.63"0.06 C15:0 0.43"0.03 0.32"0.04 – 0.31"0.02 0.23"0.04 C16:0 13.08"0.53 7.95"0.25 13.48"0.14 17.03"0.4 8.83"0.28 C16:1 6.05"0.44 18.12"0.65 1.72"0.01 16.2"0.28 34.25"4.6
C16:2 0.39"0.03 – 1.16"0.07 0.25"0.05 –
C16:3 – 14.16"0.3 3.96"0.39 – –
C16:4 – – 21.17"0.52 – –
C18:0 0.38"0.06 0.41"0.03 0.72"0.45 0.44"0.08 2.86"0.12
C18:1ny7 2.48"0.06 0.51"0.15 – – 1.85"0.22
C18:1ny9 16.69"0.61 0.63"0.01 – 5.48"0.26 43.39"2.75 C18:2 ny6 2.25"0.23 0.69"0.08 4.22"0.05 1.73"0.03 6.9"1.59
C18:2 ny8 – – 2.24"0.06 – –
C18:3ny3 – – 47.25"0.49 – –
C18:3ny6 – 1.74"0.25 3.2"0.09 0.42"0.08 –
C18:4 ny3 26.03"0.74 0.84"0.12 – – –
C20:4 ny6 – 3.72"0.14 – 7.58"0.32 –
C20:5ny3 0.31"0.21 25.15"2.65 – 44.26"0.53 –
C22:0 0.1"0.03 – – – –
C22:6 ny3 16.2"0.93 2.44"0.49 – – –
C24 – 0.35"0.08 0.43"0.16 1.13"0.69 0.9"0.31 Total HUFA 16.51"0.75 31.31"2.1 tr 52.45"0.49 0
Ž . Ž .
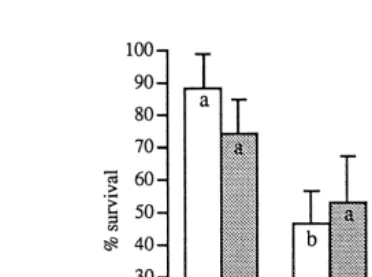
Fig. 1. Copepod G. imparipes survival mean"SD to maturity fed different diets at 208C and 258C.
Ž .
Different letters denote a significant difference between values at the same temperature P-0.05 .
3.2. Copepod surÕiÕal
Fig. 1 shows that, at both temperatures, survival was generally greatest on a diet of
Isochrysis, followed by Dunaliella, Chaetoceros and Nannochloropsis. At 208C, sur-vival was significantly greatest on a diet of Isochrysis and lowest on a diet of
Nannochloropsis. Other results were not significant. Copepods did not survive to
maturity on a diet of Nannochloropsis at 258C or on a diet of yeast at either temperature. However, some copepods survived 9 days longer than unfed controls on a diet of yeast at 208C.
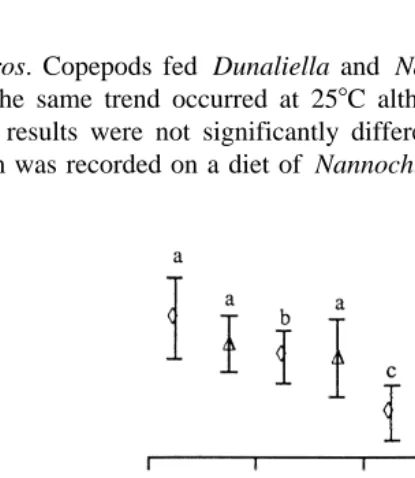
3.3. Maturation
Fig. 2 shows that at 208C, the time taken for 50% of female copepods to mature was significantly shortest with a diet of Isochrysis. Time to maturity increased with a diet of
Ž . Ž .
Fig. 2. Cumulative maturation logit transformed over time of female copepods G. imparipes fed Isochrysis
Ž .e, Chaetoceros Ž^., Dunaliella Ž`. and Nannochloropsis Ž\. at 208C. Dotted lines represent time
Žx-axis taken for 50% cumulative maturation y-axis . Days and gradients. Ž . Žb1.with different superscripts are
Ž .
Ž . Ž .
Fig. 3. Cumulative maturation logit transformed over time of female copepods G. imparipes fed Isochrysis
Ž .e, Chaetoceros Ž^. and Dunaliella Ž`. at 258C. Dotted lines represent time Žx-axis taken for 50%.
Ž . Ž .
cumulative maturation y-axis . Days and gradients b1 with different superscripts are significantly different
ŽP-0.05 ..
Chaetoceros. Copepods fed Dunaliella and Nannochloropsis took the longest time to
Ž .
mature. The same trend occurred at 258C although copepods matured faster Fig. 3 . However, results were not significantly different at this temperature and no copepod maturation was recorded on a diet of Nannochloropsis.
Ž .
Fig. 4. G. imparipes nauplius production per female per day and female prosome length both mean"SD fed different diets at 208C and 258C. Letters show significance of data at the same temperature, different letters
Ž .
Copepod maturation rate, as indicated by the regression gradients, was the same for
Ž .
animals on different diets at 208C Fig. 2 . At 258C, this rate was significantly greater
Ž .
for Isochrysis fed copepods compared to those with the other diets Fig. 3 .
3.4. Nauplius production and female length
Fig. 4 shows that at both 208C and 258C, daily nauplius production was greatest for females on a diet of Isochrysis. At 208C, there was no difference in production on the other diets, whereas at 258C, production on a diet of Chaetoceros was greater than on a diet of Dunaliella.
Prosome length was greatest for females reared at 208C with a diet of Isochrysis and least with a diet of Dunaliella. At 258C, copepods fed Isochrysis and Chaetoceros were longer than those fed Dunaliella. For those copepods fed Isochrysis, Chaetoceros and
Dunaliella, there appeared to be a positive correlation between female prosome length
and nauplius production at each temperature. In these copepods, length and nauplius production appeared to be determined more by diet than by temperature.
4. Discussion
The fatty acid profiles of algae used in copepod diets compare well with published data. Table 1 shows that Isochrysis contained the highest proportion of DHA, and had a
Ž . Ž .
high DHA:EPA ratio, as was recorded by Pillsbury 1985 and Dunstan et al. 1993 . Ž
Chaetoceros is rich in EPA and recorded a low DHA:EPA ratio Napolitano et al.,
.
1990 and Dunaliella is most often recorded as containing little or no HUFAs but a
Ž .
substantial proportion of 18:3ny3 Pillsbury, 1985; Norsker and Støttrup, 1994 . A high EPA content in Nannochloropsis is consistent with the findings of Dunstan et al. Ž1993 and Sukenik et al. 1993 . Baker’s yeast contains no HUFAs but large quantities. Ž .
Ž .
of the fatty acids 16:1 and 18:1ny9 Watanabe et al., 1983; Whyte and Nagata, 1990 .
Ž . Ž .
Copepod survival Fig. 1 and nauplius production Fig. 4 were greatest, and time to
Ž .
maturity Fig. 2 shortest with a diet of Isochrysis at 208C. At 258C, nauplius production ŽFig. 4 was greatest on this diet. Of the experimental diets fed to G. imparipes,.
Isochrysis was clearly the most efficacious. This supports the work of Arnott et al.
Ž1986 , who recorded high survival to maturity in a similar copepod species, G..
pectinatus, fed this algal species. Further, I. galbana was the only algal diet that
sustained viable egg production through multiple generations at 188C. In contrast, D.
tertiolecta did not sustain viable egg production in this species. These workers
specu-lated that Isochrysis contained essential micronutrients not present in Dunaliella. There is now abundant evidence that these micronutrients include HUFAs, particularly DHA.
Ž .
Ž .
in A. tonsa by Jonasdottir and Kiørboe 1996 . A similar trend was recorded in the current study, suggesting that the DHA:EPA ratio is a more important criterion than HUFA content when selecting a diet for maximising egg production in calanoid copepods.
After Isochyrsis, Chaetoceros was the next most beneficial diet for G. imparipes. Small quantities of DHA in this alga resulted in a lower DHA:EPA ratio than recorded
Ž .
in Isochrysis Table 1 . Copepods fed Chaetoceros tended to have a shorter time to maturity and produce more nauplii than those fed Dunaliella. Despite this, survival at both temperatures tended to be lower on this diet compared to Dunaliella, although not by a significant margin. There is conflicting evidence that diatoms such as Chaetoceros
Ž
have an inhibitory effect on calanoid copepod reproduction Ianora et al., 1995; .
Jonasdottir and Kiørboe, 1996 . While inhibition of reproduction was not observed in G.
Ž .
imparipes, the value of this alga or another diatom as a monodiet for intensive culture
requires further investigation.
By most criteria, Dunaliella was a less efficacious diet for G. imparipes than
Isochrysis in this study. Dunaliella contained no DHA, hence a DHA:EPA ratio could
Ž .
not be calculated Table 1 . This is consistent with the findings of Støttrup and Jensen Ž1990 mentioned previously. Also, Arnott et al. 1986 found that, unlike Isochrysis,. Ž .
Dunaliella did not sustain viable egg production in G. pectinatus.
No survival to maturity was observed in G. imparipes fed yeast. Like Artemia ŽCoutteau et al., 1990 , copepods may be unable to fully digest yeast cells. Some. copepods survived longer than the unfed controls at 208C, but not at 258C. At lower
Ž .
temperatures, copepod growth is depressed Klein Breteler and Schogt, 1994 as a result of lower metabolic activity. It is possible that G. imparipes received enough energy from yeast to sustain metabolism for a short time at 208C. The mean volume of yeast cells was comparable to that of the three most successful diets in this trial, Isochrysis,
Chaetoceros and Dunaliella. Hence, nauplii should have been able to ingest yeast.
Despite poor copepod survival, yeast may still have some potential as an indirect Ž food for G. imparipes. Yeast can be used effectively to culture dinoflagellates Kleppel
.
and Burkart, 1995 and G. imparipes will readily consume some species of
dinoflagel-Ž .
late S. Griffin, pers. comm. . Alternatively, dead yeast cells provide substrate for Ž bacteria and some calanoid copepod nauplii can utilise bacteria as a food source Roff et
.
al., 1995 . Either dinoflagellates or bacteria could have been available to copepods as food in yeast-fed treatments and may have contributed to limited survival of G.
imparipes at 208C. Some calanoid copepods exhibit high rates of growth and fecundity Ž
when fed dinoflagellates compared to microalgae or yeast alone Kleppel and Burkart, .
1995; Klein Breteler et al., 1999 , hence this requires investigation in G. imparipes. Ž A diet of Nannochloropsis resulted in no copepod survival to maturity at 258C Fig.
. Ž .
1 and reduced survival, development and egg production at 208C Figs. 1, 2 and 4 . Given the high HUFA content of this alga, this result is unexpected. However, the mean cell volume of Nannochloropsis was considerably smaller than cells of the other diets fed to copepods. This may make it too small for G. imparipes nauplii to capture from the water column. Also, Nannochloropsis has a hard cell wall that makes it indigestible
Ž .
alga and lower metabolic demands at a lower temperature resulted in limited survival at 208C. This prolific and nutritious alga is in common use in aquaculture as a diet for rotifers and its use in the mass culture of G. imparipes should not be discounted.
Female length appeared to correlate more with diet than with temperature in G.
Ž .
imparipes Fig. 4 . Thus, copepod growth on Chaetoceros, Dunaliella and
Nan-nochloropsis was nutrient-limited. Fig. 4 also shows a positive correlation between
female length and nauplius production. This relationship has been well established for
Ž . Ž
G. imparipes Rippingale and Hodgkin, 1974 and other calanoid copepods McLaren,
. Ž .
1965 . Shortened time to maturity Fig. 3 and increased nauplius production on the two
Ž .
most successful diets Fig. 4 at 258C show that culture production would be maximised at this higher temperature.
Nutrition is such a complex issue that it is simplistic to consider one dietary factor in isolation. Components other than HUFAs are also important to copepod health and fecundity. Dietary content of essential amino acids has been correlated to egg production
Ž .
in A. tonsa by Kleppel et al. 1998 . Also, these workers recorded poor egg production in copepods fed with a strain of Isochrysis that was found to be unusually deficient in the amino acid histidine. This suggests that copepod amino acid requirements may be at least as important as their HUFA requirements and thus requires more attention.
This study sought to determine diets that improve production of G. imparipes in intensive culture. However, the nutritional adequacy of these copepods for use as food for larval fish has yet to be assessed. Feeding G. imparipes broodstock with a high HUFA diet such as I. galbana will be likely to result in HUFAs being incorporated in the lipid reserves of nauplii. Hence, these nauplii should be nutritious food for larval fish without the need for post-harvest enrichment.
Acknowledgements
Financial support was received from the Fisheries Research and Development
Ž .
Corporation Project No. 96r398 .
References
Arnott, G.H., Brand, G.W., Kos, L.C., 1986. Effects of food quality and quantity on the survival, development,
Ž . Ž .
and egg production of Gladioferens pectinatus Brady Copepoda: Calanoida . Aust. J. Mar. Freshwater Res. 37, 467–473.
Bligh, E.G., Dyer, W.J., 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 912–917.
Brown, M.R., Jeffrey, S.W., Volkman, J.K., Dunstan, G.A., 1997. Nutritional properties of microalgae for mariculture. Aquaculture 151, 315–331.
Coutteau, P., Lavens, P., Sorgeloos, P., 1990. Baker’s yeast as a potential substitute for live algae in aquaculture diets: Artemia as a case study. J. World Aquacult. Soc. 21, 1–9.
Dhont, J., Lavens, P., 1996. Tank production and use of ongrown Artemia. In: Lavens, P., Sorgeloos, P.
ŽEds. , Manual on the Production and Use of Live Food for Aquaculture. FAO Fisheries Technical Paper.
Doi, M., Singhagraiwan, T., Singhagraiwan, S., Ohno, A., 1994. An investigation of copepods being applied as initial food organisms for red snapper larvae. Thai Mar. Fish. Res. Bull. 5, 21–26.
Doi, M., Toledo, J.D., Golez, M.S.N., Santos, M.d.l., Ohno, A., 1997. Preliminary investigation of feeding performance of larvae of early red-spotted grouper, Epinephelus coioides, reared with mixed zooplankton. Hydrobiologia 358, 259–263.
Dunstan, G.A., Volkman, J.K., Barrett, S.M., Garland, C.D., 1993. Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J. Appl. Phycol. 5, 71–83.
Dunstan, G.A., Volkman, J.K., Jeffrey, S.W., Barrett, S.M., 1992. Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae: 2. Lipid classes and fatty acids. J. Exp. Mar. Biol. Ecol. 161, 115–134.
Ianora, A., Poulet, S.A., Miralto, A., 1995. A comparative study of the inhibitory effect of diatoms on the reproductive biology of the copepod Temora stylifera. Mar. Biol. 121, 533–539.
Jonasdottir, S.H., Kiørboe, T., 1996. Copepod recruitment and food composition: do diatoms affect hatching success? Mar. Biol. 125, 743–750.
Kitajima, C., 1973. Experimental trials on mass culture of copepods. Bull. Plankton Soc. Jpn. 20, 54–60. Klein Breteler, W.C.M., Laan, M., 1993. An apparatus for automatic counting and controlling density of
pelagic food particles in cultures of marine organisms. Mar. Biol. 116, 169–174.
Ž .
Klein Breteler, W.C.M., Schogt, N., 1994. Development of Acartia clausi Copepoda, Calanoida cultured at different conditions of temperature and food. Hydrobiologia 292r293, 469–479.
Klein Breteler, W.C.M., Schogt, N., Bass, M., Schouten, S., Kraay, G.W., 1999. Trophic upgrading of food quality by protozoans enhancing copepod growth: role of essential lipids. Mar. Biol. 135, 191–198. Kleppel, G.S., Burkart, C.A., 1995. Egg production and the nutritional environment of Acartia tonsa: the role
of food quality in copepod nutrition. ICES J. Mar. Sci. 52, 297–304.
Kleppel, G.S., Burkart, C.A., Houchin, L., 1998. Nutrition and the regulation of egg production in the calanoid copepod Acartia tonsa. Limnol. Oceanogr. 43, 1000–1007.
Kraul, S., Brittain, K., Cantrell, R., Nagao, T., Ako, H., Ogasawara, A., Kitagawa, H., 1993. Nutritional factors affecting stress resistance in the larval mahimahi Coryphaena hippurus. J. World Aquacult. Soc. 24, 186–193.
McEvoy, L.A., Naess, T., Lie, O., 1998. Lipid and fatty acid composition of normal and malpigmented atlantic
Ž .
halibut Hippoglossus hippoglosus fed enriched Artemia: a comparison with fry fed wild copepods.
Aquaculture 163, 237–250.
McLaren, I.A., 1965. Some relationships between temperature and egg size, body size, development rate and fecundity of the copepod Pseudocalanus. Limnol. Oceanogr. 10, 528–538.
Miralto, A., Ianora, A., Poulet, S.A., Romano, G., Laabir, M., 1996. Is fecundity modified by crowding in the copepod Centropages typicus? J. Plankton Res. 18, 1033–1040.
Nanton, D.A., Castell, J.D., 1998. The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use as a live food for marine fish larvae. Aquaculture 163, 251–261. Napolitano, G.E., Ackman, R.G., Ratnayaye, W.M.N., 1990. Fatty acid composition of three cultured algal
Ž .
species Isochrysis galbana, Chaetoceros gracilis and C. calcitrans used as food for bivalve larvae. J. World Aquacult. Soc. 21, 122–130.
Norsker, N.H., Støttrup, J.G., 1994. The importance of dietary HUFAs for fecundity and HUFA content in the harpacticoid, Tisbe holothuriae Humes. Aquaculture 125, 155–166.
Pillsbury, K.S., 1985. The relative food value and biochemical composition of five phytoplankton diets for
Ž .
Queen Conch, Strombus gigas Linne larvae. J. Exp. Mar. Biol. Ecol. 90, 221–231.
Rippingale, R.J., 1994. A calanoid copepod Gladioferens imparipes, holding to surfaces. Hydrobiologia 292r293, 351–360.
Rippingale, R.J., Hodgkin, E.P., 1974. Population growth of a copepod Gladioferens imparipes Thomson. Aust. J. Mar. Freshwater Res. 25, 351–360.
Rippingale, R.J., MacShane, M.G., 1991. A calanoid copepod for intensive cultivation. Mem. Queensl. Mus. 31, 457.
Schipp, G.R., Bosmans, J.M.P., Marshall, A.J., 1999. A method for hatchery culture of tropical calanoid copepods, Acartia spp. Aquaculture 174, 81.
Støttrup, J.G., Jensen, J., 1990. Influence of algal diet on feeding and egg-production of the calanoid copepod
Acartia tonsa Dana. J. Mar. Biol. Assoc. U. K. 141, 87–105.
Støttrup, J.G., Norsker, N.H., 1997. Production and use of copepods in marine fish larviculture. Aquaculture 155, 231–248.
Støttrup, J.G., Richardson, K., Kirkegaard, E., Pihl, N.J., 1986. The cultivation of Acartia tonsa Dana for use as a live food source for marine fish larvae. Aquaculture 52, 87–96.
Sukenik, A., Zmora, O., Carmeli, Y., 1993. Biochemical quality of marine unicellular algae with special emphasis on lipid composition: II. Nannochloropsis sp. Aquaculture 117, 313–326.
Sun, B., Fleeger, J.W., 1995. Sustained mass culture of Amphiascoides atopus a marine harpacticoid copepod in a recirculating system. Aquaculture 136, 313–321.
Watanabe, T., Kitajima, C., Fujita, S., 1983. Nutritional values of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34, 115–143.
Whyte, J.N.C., Nagata, W.D., 1990. Carbohydrate and fatty acid composition of the rotifer, Brachionus