www.elsevier.com / locate / chroma
Short communication
D
etermination of carotenoids in tomato juice by liquid
chromatography
*
C.H. Lin, B.H. Chen
Department of Nutrition and Food Sciences, Fu Jen University, Taipei 24205, Taiwan
Received 3 April 2003; received in revised form 26 June 2003; accepted 26 June 2003
Abstract
A high-performance liquid chromatography method was developed to determine the various carotenoids in tomato juice. A C30 column and a mobile phase of acetonitrile–1-butanol (7:3, v / v) (A) and methylene chloride (B) with the following gradient elution were used: 99% A and 1% B intitally, increased to 4% B in 20 min, 10% B in 50 min and returned to 1% B in 55 min. Sixteen carotenoids, including all-trans-lutein, all-trans-b-carotene, all-trans-lycopene and their 13 cis isomers were identified and resolved within 52 min with flow-rate at 2.0 ml / min and detection at 476 nm. Of the various extraction solvent systems, the best extraction efficiency of carotenoids in tomato juice was achieved by employing ethanol–hexane (4:3, v / v). Lycopene was found to be present in largest amount in tomato juice, followed byb-carotene and lutein.
2003 Elsevier B.V. All rights reserved.
Keywords: Fruit juices; Food analysis; Carotenoids
1
. Introduction degeneration and skin cancer have been well
docu-mented [5].
Epidemiological studies have shown that the in- Lycopene, an acyclic carotenoid containing 11
creased consumption of tomato and tomato-based conjugated double bonds, is naturally present in trans
products may reduce the risk of a certain type of form in raw tomato [6]. Because of presence of
cancers such as prostate and stomach cancer[1].One long-chain conjugated double bonds, lycopene has
of the major phytochemicals in tomato products been reported to possess antioxidative activity and is
contributing to the anti-carcinogenic function has superior to lutein or b-carotene [7]. In addition,
been attributed to lycopene [2,3]. In addition to lycopene may exhibit other physiological activities
lycopene, both lutein andb-carotene are also present such as suppression of proliferation of human cancer
in tomato in a much smaller amount [4]. The cells [8]. However, all-trans-lycopene may be
con-application of lutein andb-carotene in the treatment verted to its cis configuration during food processing
of chronic diseases such as age-related macular [9]. Several reports have demonstrated that the cis
isomers of lycopene could be absorbed into body more easily and played a more important role in
biological function than all-trans-lycopene[10,11].
*Corresponding author. Tel.: 1886-229-031-111; fax: 1
886-The consumption of tomato juice in Taiwan has
229-021-215.
E-mail address: [email protected](B.H. Chen). increased rapidly in recent years. However, the
104 C.H. Lin, B.H. Chen / J. Chromatogr. A 1012 (2003) 103–109
amount and variety of cis carotenoids remain unclear evaporator (model N-1) was from Eyela (Tokyo,
in processed tomato juice due to lack of an appro- Japan).
priate high-performance liquid chromatography
(HPLC) technique [12], though a gradient mobile 2 .3. Extraction of carotenoids from tomato juice
phase of acetone and water was developed to
de-termine the various carotenoids in tomato peel[12]. Five solvent systems were used for comparison of
Also, the effect of various solvent systems on the extraction efficiency: (1) ethanol–hexane (4:3, v / v),
extraction efficiency of carotenoids in tomato juice (2) acetone–hexane (3:5, v / v), (3) ethanol–acetone–
need to be compared. The objectives of this study hexane (2:1:3, v / v / v), (4) ethyl acetate–hexane (1:1,
were to develop a suitable extraction and separation v / v) and (5) ethyl acetate (100%). These solvent
method for determination of carotenoids in tomato systems were selected based on several reports of
juice by HPLC. previous studies [13–15]. Initially a 8 g sample of
tomato juice was placed in a 60 ml vial and mixed with 0.2 g magnesium carbonate and 40 ml
ex-2
. Experimental traction solvent as described above. The mixture was
shaken in a shaker at 140 rev. / min for 30 min. The 2
.1. Materials upper phase was collected and poured into a 500-ml
flask. The lower phase was extracted again with the
Fresh tomatoes (Tan-Tai Lan T93 ) were pur- same solvent (32 ml) and shaken for 30 min. The
chased from a local farm in Taichung county. All- upper phase was also collected and poured into the
trans-lutein and all-trans-b-carotene standards with same flask. The lower phase was repeatedly
ex-purities greater than 95% were obtained from Sigma tracted with 15 ml hexane and shaken for 20 min,
(St. Louis, MO, USA). All-trans-lycopene standard followed by 5 ml hexane and homogenized at 12 000
was from Extrasynthese (France). The HPLC-grade rev. / min for 5 min. After filtration through a
What-solvents, including ethanol, acetone, tetrahydrofuran, man No. 1 filter paper, the filtrates were pooled and
n-hexane, methanol, acetonitrile, ethyl acetate, poured into the same flask. Distilled water (150 ml)
methylene chloride and methyl tert.-butyl ether and 100 ml 10% aq.NaCl solution were added for
(MTBE) were from Mallinckrodt (Paris, KY, USA). partition, and the supernatant was collected. The
1-Butanol and sodium chloride were from Riedel-de lower phase was again extracted with 20 ml hexane.
¨
Haen (Barcelona, Spain). The deionized water was The filtrates were combined and evaporated to
made using a purified-water system (Millipore, Bed- dryness in a flask under vacuum. The residue was
ford, MA, USA). Magnesium carbonate was from dissolved in 1 ml methylene chloride and transferred
J.T. Baker (Pillipsburg, NJ, USA). A C30 column to a vial. The solution was filtered through a 0.2-mm
(25034.6 mm I.D, 5 mm particle) was from YMC membrane filter and 20 ml was injected for HPLC
(Tokyo, Japan). analysis.
Tomato juice was processed using a HTST
(High-Temperature-Short-Time) system and was obtained 2 .4. Determination of recovery
from a pilot plant located in the Hsin-Chu county of
Taiwan. For recovery test, a concentration of 30 mg / ml
all-trans-lutein and 100 mg / ml all-trans-b-carotene
2
.2. Instrumentation were added to 8 g tomato juice separately for
extration of carotenoids. For all-trans-lycopene, a
The HPLC system is composed of a Jasco MD- concentration of 250mg / ml was added to 2 g tomato
915 photodiode-array detector, a Rheodyne model juice instead of 8 g for extraction. This is because
7161 injector (Rheodyne, CA, USA) and a Sanwa that a large concentration was found for
all-trans-Tsusho DP-4010 degasser (Sanwa all-trans-Tsusho, Tokyo, lycopene in tomato juice, and the amount of
all-Japan). The homogenizer (model PT-MR 3000) was trans-lycopene standard incorporated would be much
recovery of each all-trans form of carotenoids was great difference in concentration was found between
obtained by dividing the calculated concentration by all-trans and cis form of lycopene in tomato juice.
the added concentration. The recoveries of all-trans- Six concentrations, 7, 70, 140, 280, 560 and 700
lutein, all-trans-b-carotene and all-trans-lycopene mg / ml were prepared for one curve, whereas 2, 4, 8,
were found to be 76, 84 and 94%, respectively. 10, 20 and 40 mg / ml were prepared for the other.
Because of absence of commercial standards of cis Each cis isomer of carotenoids was quantified based
form of carotenoids, the recoveries of cis isomers on the standard curve of all-trans carotenoids
be-were assessed to be equivalent to those of all-trans cause of similarity in extinction coefficient [9,17].
form of carotenoid standards. Duplicate analyses were performed and the mean
value was determined. The regression equation and 2
2
.5. HPLC separation of carotenoids in tomato correlation coefficient (r ) were obtained using a
juice Microsoft Excel 2000 software. A high correlation
2
coefficient (r .0.98) was achieved for
all-trans-b-Various binary and ternary solvent systems in carotene ( y50.0141x10.8907), all-trans-lutein ( y5
isocratic or gradient mode were compared with 0.0083x20.0807) and all-trans-lycopene ( y5
respect to the separation efficiency of carotenoids in 0.0068x119.833 for high concentration and y5
tomato juices. These solvent systems were selected 0.0069x11.0442 for low concentration). The data
and modified based on several previous studies by were subjected to analysis of variance and Duncan’s
Lee and Chen [6] and Emenhiser et al. [16]. A multiple range test using SAS [18].
proper solvent strength was controlled for each
mobile phase by calculating the polarity index. The 2 .7. Determination of limits of detection and
most appropriate solvent system was found to be quantification
composed of 1-butanol–acetonitrile (30:70, v / v) (A)
and methylene chloride (B) with the following Both the limit of detection (LOD) and limit of
gradient elution: 99% A and 1% B initially, in- quantification (LOQ) were determined using a
meth-creased to 4% B in 20 min, 10% B in 50 min and od described by International Conference on
Har-returned to 1% B in 55 min. The flow-rate was 2.0 monization [19]. Due to difference in detector
re-ml / min with detection at 476 nm and sensitivity at sponse, three concentrations (1, 2 and 4 mg / ml)
0.005 AUFS. The separation efficiency was evalu- were prepared for lutein and
all-trans-ated on the basis of retention factor (k). lycopene separately, while 3, 6 and 18 mg / ml were
for all-trans-b-carotene. Both LODs and LOQs were 2
.6. Identification and quantification of carotenoids calculated based on a formula in a previous study
in tomato juice [6]. The LODs for lutein, b-carotene and lycopene
were 0.64, 0.65 and 0.09 mg / g, respectively, while
The identification of all-trans carotenoids was the LOQs were 1.94, 1.98 and 0.27 mg / g.
carried out by comparing the retention times and absorption spectra with reference standards, as well
as co-chromatography with added standards. In 3 . Results and discussion
addition, the cis isomers of carotenoids were
iden-tified based on absorption spectrum characteristics 3 .1. HPLC analysis of carotenoids in tomato juice
and Q ratios as described in the literature[6,20–23].
Because of an absence of suitable internal stan- After various studies, a gradient mobile phase as
dard, six concentrations of 1, 2, 4, 8, 10 and described in the method section was developed,
20mg / ml were used to prepare the standard curve of which was able to resolve 16 carotenoids in tomato
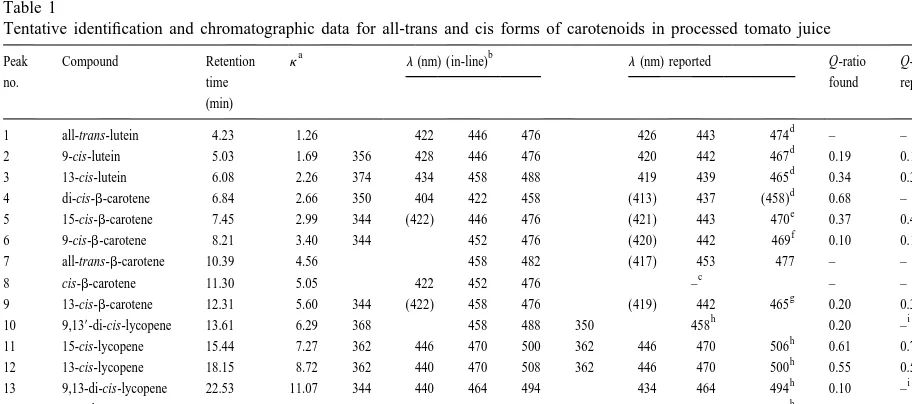
all-trans-lutein. Likewise, six concentrations of 3, 6, juice (Fig. 1). Table 1 shows the chromatographic
18, 36, 48 and 60 mg / ml were prepared for the data for all-trans and cis forms of lutein,b-carotene
standard curve of all-trans-b-carotene. For all-trans- and lycopene in tomato juice. With the exception of
carot-106 C.H. Lin, B.H. Chen / J. Chromatogr. A 1012 (2003) 103–109
Fig. 1. HPLC chromatogram of carotenoids in processed tomato juice by employing a gradient system of 1-butanol, acetonitrile and methylene chloride. Chromatographic conditions described in text. SeeTable 1for peak idenification.
T able 1
Tentative identification and chromatographic data for all-trans and cis forms of carotenoids in processed tomato juice
a b
Peak Compound Retention k l(nm) (in-line) l(nm) reported Q-ratio Q-ratio
no. time found reported
(min)
d
1 all-trans-lutein 4.23 1.26 422 446 476 426 443 474 – –
d 21 d
2 9-cis-lutein 5.03 1.69 356 428 446 476 420 442 467 0.19 0.12(8.6 )
d -1 d
3 13-cis-lutein 6.08 2.26 374 434 458 488 419 439 465 0.34 0.38(2.6 )
d
4 di-cis-b-carotene 6.84 2.66 350 404 422 458 (413) 437 (458) 0.68 –
e -1 e
5 15-cis-b-carotene 7.45 2.99 344 (422) 446 476 (421) 443 470 0.37 0.43(2.3 )
f -1 f
6 9-cis-b-carotene 8.21 3.40 344 452 476 (420) 442 469 0.10 0.12(8.2 )
7 all-trans-b-carotene 10.39 4.56 458 482 (417) 453 477 – –
c
8 cis-b-carotene 11.30 5.05 422 452 476 – – –
g -1 g
9 13-cis-b-carotene 12.31 5.60 344 (422) 458 476 (419) 442 465 0.20 0.35(2.8 )
h i
10 9,139-di-cis-lycopene 13.61 6.29 368 458 488 350 458 0.20 –
h h
11 15-cis-lycopene 15.44 7.27 362 446 470 500 362 446 470 506 0.61 0.75
h h
12 13-cis-lycopene 18.15 8.72 362 440 470 508 362 446 470 500 0.55 0.55
h i
13 9,13-di-cis-lycopene 22.53 11.07 344 440 464 494 434 464 494 0.10 –
h h
14 9-cis-lycopene 27.47 13.71 362 446 470 500 362 446 470 500 0.12 0.12
h h
15 5-cis-lycopene 30.92 15.56 344 446 476 506 368 452 476 506 0.05 0.06 h
16 all-trans lycopene 47.37 24.37 452 476 506 452 476 506 – –
a
k, retention factor.
b
A gradient mobile phase of 1-butanol–acetonitrile (30:70, v / v) and methylene chloride was used.
c
‘‘–’’ Data not available.
d
A mobile phase of methanol–methylene chloride (99:1, v / v) was used by Chen et al.[23].
e
A mobile phase of acetonitrile–methanol (90:10, v / v) was used by Chen and Chen[22].
f
A mobile phase of acetonitrile–methanol–methylene chloride was used by Saleh and Tan[21].
g
A mobile phase of acetone–hexane (3:97, v / v) was used by Tsukida et al.[20].
h
A mobile phase of 1-butanol–acetonitrile–methylene chloride (30:70:10, v / v / v) was used by Lee and Chen[6].
i
.H
.
Lin
,
B
.H
.
Chen
/
J.
Chromatogr
.
A
1012 (2003) 103
–109
107
T able 2
a
Concentrations (mg / g) of lutein,b-carotene and lycopene in tomato juice after extraction with various solvent systems
Treatment Lutein b-carotene Lycopene
f
All-trans 9-cis 13-cis Total 15-cis 9-cis All-trans cis 13-cis Total Di-cis(9,139) 15-cis 13-cis Di-cis(9,13) 9-cis 5-cis All-trans Total
b A A B A A A A A A A AB A A A A A A A
A 0.37 0.14 0.13 0.64 1.03 0.33 4.05 0.37 0.25 6.03 0.18 0.51 2.57 1.03 0.80 2.70 113.87 121.66
c A AB A A C B C AB AB C BC C C C C D E E
B 0.37 0.13 0.19 0.69 0.82 0.28 3.43 0.34 0.23 5.1 0.17 0.28 0.99 0.76 0.66 1.71 67.17 71.74
d B A B AB B A B A A B A B B B B B B B
C 0.32 0.14 0.13 0.59 0.87 0.32 3.79 0.36 0.25 5.59 0.18 0.37 1.59 0.88 0.72 2.19 90.20 96.13
e C B C C C C D B B D C B BC C C C D D
D 0.26 0.10 0.08 0.44 0.83 0.26 3.21 0.32 0.21 4.83 0.16 0.35 1.42 0.79 0.65 1.89 72.62 77.88
f BC B B BC C C CD AB AB CD C C C C C C C C
E 0.30 0.10 0.12 0.52 0.81 0.26 3.27 0.34 0.22 4.9 0.17 0.29 0.96 0.79 0.69 1.88 77.84 82.62
a A – E
Mean of duplicate analyses. Symbols bearing different letters in the same column are significantly different (P,0.05).
b
A5ethanol–hexane (4:3, v / v).
c
B5acetone–hexane (3:5, v / v).
d
C5ethanol–acetone–hexane (2:1:3, v / v / v).
e
D5ethyl acetate–hexane (1:1, v / v).
e
E5ethyl acetate (100%).
f
108 C.H. Lin, B.H. Chen / J. Chromatogr. A 1012 (2003) 103–109
enoids were higher than 90%. Thek values of all 16 cis carotenoids were present at a much lower level.
As most carotenoids are present naturally in trans carotenoids were between 1.26 and 24.37, indicating
form in plants, the formation of cis isomers is that a proper solvent strength of mobile phase was
probably due to processing. Taungbodnitham et al.
controlled. Thek values observed in this study were
[26] compared different combinations of solvent
a bit higher, mainly because a large variety of
systems for extraction efficiency of carotenoids in carotenoid isomers were present in tomato juice, and
fruits and vegetables and concluded that both ace-a greace-at hydrophobic interace-action could occur between
tone–hexane (4:6, v / v) and ethanol–hexane (4:3,
C30 stationary phase and carotenoid isomers, which
v / v) resulted in a better extraction yield of lycopene.
in turn resulted in a prolonged retention time [17].
However, no difference was found forb-carotene. In
Peaks 1, 7 and 16 were positively identified as
our study the highest yield of lycopene, lutein and lutein, al-trans-b-carotene and
all-trans-b-carotene in tomato juice could be simultaneously lycopene, respectively, based on the criteria
de-obtained by using ethanol–hexane (4:3, v / v) as scribed in the Method section. Peaks 2 and 3 were
extraction solvent. The amounts of all-trans and cis tentatively identified as 9-cis-lutein and 13-cis-lutein,
forms of carotenoids in tomato juice could be respectively, because a low and high intensity of cis
affected by many factors such as variety and maturi-peak occurred for the former and the latter. In
ty of tomatoes as well as processing condition. In addition, a hypsochromic shift of 8 nm was found for
addition, the extraction and separation techniques 13-cis-lutein. Peaks 4, 5, 6, 8 and 9 were tentatively
used by the other authors may also cause this identified as di-cis-, 15-cis-, 9-cis-, cis- and
13-cis-difference. Shi and Maguer [27] reported that the
b-carotene, respectively, because a hypsochromic
content of lycopene ranged 5.8–9.0 mg / 100 g in shift of 36, 12, 6, 6 and 6 nm occurred and the
tomato juice produced in Israel and 9.70–11.84 mg / Q-ratios were similar to those reported in the
litera-100 g in Brasil on a wet basis. These values were a ture [20–23].No cis position was assigned to peaks
bit lower than that shown in our study, in which the 4 and 8 since no Q-ratio in the literature could be
lycopene content (all-trans plus cis form) in tomato compared. However, a large hypsochromic shift and
juice was 12.17 mg / 100 g. exclusion of the sterically-hindered isomers such as
In conclusion, the most appropriate HPLC method 7-cis- and 11-cis-b-carotene may indicate the
pres-for separation of 16 carotenoids in tomato juice was ence of di-cis-b-carotene as reported by Chen et al.
accomplished by employing a C column with a
[24].Peaks 10, 11, 12, 13, 14 and 15 were tentative- 30
gradient mobile phase of acetonitrile–1-butanol (7:3, ly identified as 9,139-di-cis-, 15-cis-, 13-cis-,
9,13-v / 9,13-v) and methylene chloride. A sol9,13-vent system of di-cis-, 9-cis- and 5-cis-lycopene, respectively, based
ethanol–hexane (4:3, v / v) was found to result in the on a hypsochromic shift of 16, 6, 6, 12, 6 and 0 nm
highest yield of carotenoids extracted from tomato
and the Q-ratios [6,25], as well as a reference by
juice.
Oleser and Albert[12].
3
.2. Comparison of extraction efficiency
R
eferences
Table 2 shows the concentrations of lutein,
b-[1] E . Giovannucci, J. Natl. Cancer Inst. 91 (1999) 317.
carotene and lycopene in tomato juice after
ex-[2] S .K. Clinton, Nutr. Rev. 56 (1998) 35.
traction with various solvent systems. Lycopene was
[3] B . Watzl, A. Bub, M. Blockhaus, B.M. Herbert, P.M.
found to be present in largest concentration, followed Luhrmann, M. Neuhauser-Berthold, G. Rechkemmer, J.¨ ¨
by b-carotene and lutein. Of the various solvent Nutr. 130 (2000) 1719.
systems, ethanol–hexane (4:3, v / v) resulted in the [4] L .H. Tonucci, J.M. Holden, G.R. Beecher, F. Khachik, C.S.
Davis, G. Mulokozi, J. Agric. Food Chem. 43 (1995) 579.
highest yield of lycopene, followed by
ethanol–ace-[5] O . Aust, H. Sies, W. Stahl, M.C. Polidori, J. Chromatogr. A
tone–hexane (2:1:3, v / v / v), ethyl acetate (100%),
936 (2001) 83.
ethyl acetate–hexane (1:1, v / v) and acetone–hexane [6] M .T. Lee, B.H. Chen, Chromatographia 54 (2001) 613.
(3:5, v / v). The same trend also applied to lutein and [7] N .J. Miller, J. Sampson, L.P. Candeias, P.M. Bramley, C.A.
[8] J . Levy, E. Bosin, B. Feldman, Y. Giat, A. Mimster, M. [19] G uideline on the Validation of Analytical Procedures: Danilenko, Y. Sharoni, Nutr. Cancer 24 (1995) 257. Methology Q2B from International Conference on Harmoni-[9] M .T. Lee, B.H. Chen, Food Chem. 78 (2002) 425. zation, 1996.
[10] V . Bohm, R. Bitsch, Eur. J. Nutr. 38 (1999) 118. [20] K . Tsukida, K. Saiki, T. Takii, Y. Koyama, J. Chromatogr. [11] A .C. Boileau, N.R. Merchen, K. Wasson, C.A. Atkinson, J.W. 245 (1982) 359.
Erdman Jr., J. Nutr. 129 (1999) 1176. [21] M .H. Saleh, B. Tan, J. Agric. Food Chem. 39 (1991) 1438. [12] T . Olaser, K. Albert, On-line LC-NMR and Related Tech- [22] T .M. Chen, B.H. Chen, Chromatographia 39 (1994) 346.
niques, in: K. Albert (Ed.), Wiley, Chichester, 2002. [23] B .H. Chen, H.Y. Peng, H.E. Chen, J. Agric. Food Chem. 43 [13] A ssociation of Official Analytical Chemists (AOAC) Section (1995) 1912.
43.015, 1984. [24] B .H. Chen, T.M. Chen, J.T. Chien, J. Agric. Food Chem. 42 [14] M . Nguyen, S. Schwartz, Proc. Soc. Exp. Biol. Med. 218 (1994) 2391.
(1998) 101. [25] J . Schierle, W. Bretzel, I. Buhler, N. Faccin, D. Hess, K. [15] J . Shi, M. Le Maguer, Y. Kakuda, A. Liptay, Food Res. Int. Steiner, W. Schuep, Food Chem. 59 (1997) 459.
32 (1999) 15. [26] A .K. Tanugbodhitham, G.P. Jones, M.L. Wahlqvist, D.R. [16] C . Emenhiser, L.C. Sander, S.J. Schwartz, J. Chromatogr. A Briggs, Food Chem. 63 (1998) 577.
707 (1995) 205. [27] J . Shi, M. Le Maguer, Crit. Rev. Food Sci. Nutr. 40 (2000) [17] C .Y. Tai, B.H. Chen, J. Agric. Food Chem. 48 (2000) 5962. 1.