Agricultural Science Research Journal Vol. 2(4) pp. 134 – 139, April 2012 Available online http://www.resjournals.com/ARJ
ISSN-L: 2026-6073 ©2012 International Research Journals
Full Length Research paper
Exogenous application of tryptophan and indole acetic
acid (IAA) to induce root nodule formation and increase
yield of soybean
Sudadi
Department of Soil Science, Faculty of Agriculture, Sebelas Maret University, Ir. Sutami road 36th A Kentingan, Surakarta, Central Java, Indonesia 57126.
Author Email: [email protected]
Abstract
This study aimed at understanding to what extent extracellular tryptophan and indole acetic acid (IAA) induce soybean root nodules formation. Greenhouse experiment was conducted in plastic pots and sterile sand planting medium, arranged in completely randomized design (CRD) with three replications. The factors evaluated were, type of phytohormones (tryptophan and IAA), application time (early planting, vegetative phase 1, vegetative phase 3) and hormone concentrations (0; 0.001; 0.01; 0.1; 1, 0 and 10, 0 ppm). The results showed that both tryptophan and IAA induced root nodules formation. Additionally, the addition of extracellular hormones also increased shoot and root dry weight as well as soybean yields. Highest root nodules number and soybean yield were taken from the treatment of 1.0 ppm tryptophan applied in early planting. It seemed that higher concentrations of tryptophan or IAA are required when applied at early planting, presumably because of its concentration decreases prior to root hairs formed.
Key words: extracellular IAA tryptophan, root nodules, soybean.
INTRODUCTION
The existence and effectiveness of root nodules is very important for soybean plants. Effective root nodules are able to meet up to 75% of the plant’s nitrogen requirement (Jutono, 1985; Ohyama et al., 2009). According to Ohyama et al. (2009), one reason for the low soybean yields compared to its potential are the less effective root nodule. The formation of root nodule is affected by internal factors of the plant and bacteria, including the ability of plant to excrete tryptophan and the capability of bacteria to oxidize tryptophan into IAA (Gray and Williams, 1971; Spaepen, 2007). Phytohormones IAA (auxin) play an important role in early infection process of root nodules (Gray and William, 1971; Spaepen and Vanderleyden, 2010; Verma et al., 2010). Infection process and development of root nodules were induced by auxin (Libbenga et al. cit. Mishra et al.,
1999; Spaepen et al., 2007; Spaepen and Vanderleyden,
2010).
Sudadi 135
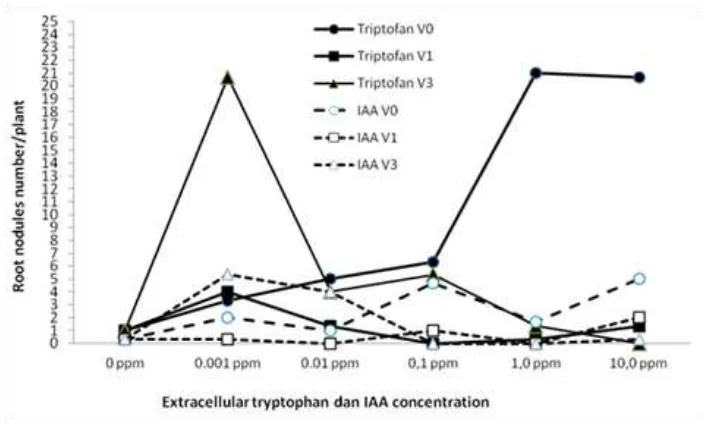
Figure 1. Effect of the type, application time and concentrations of extracellular phytohormones (amino acid tryptophan and IAA) on the number root of nodules of soybean planted on sterile sand planting medium
plants and increased soybean yield.
Materials and methods
The experiment was conducted in a greenhouse, using 1 gallon plastic pots with sterile sand planting medium. Local soybean varieties and rhizobia inoculums from Soil and Environmental Microbiology Laboratory, Department of Microbiology, Faculty of Agriculture, Gadjah Mada University, Yogyakarta, Indonesia were used. The experiments were arranged in factorial Completely Randomized Design (CRD) with three factor treatments: i) type of extra cellular phytohormones (amino acids tryptophan = F1 and IAA = F2), ii) application time (early
planting = V0; vegetative phase 1 = V1 and vegetative
phase 3 = V3) and iii) phytohormone concentrations (0;
0.001; 0.01, 0.1; 1, 0 and 10.0 ppm). Each treatment combination was repeated three times. Three soybean seeds were planted in each pot, and thinned to one plant per pot a week after planting. Observed variables included the number of root nodules, shoot and root dry weight and plant yield. Sampling for observation of root nodules and plant dry weight was carried on the growth phase R1 (beginning flowering) while for plant yield at R8
(full mature pods) (Hidayat, 1985; Kamal, 2000). Nitrogen fertilizer was applied at a dose of 20 kg N/ha before planting and 40 kg/ha during pod filling (Afza, et al.,
1987), while P (75 kg/ha P2O5 ), K (100 kg/ha K2O)
(Pasaribu, Suprapto, 1985), and Ca and Mg (200 kg/ha) (Jutono, 1985) were given prior to planting. Micro nutrients were added in the following dosages (Ismunadji, Mahmud, 1985): 10 kg/ha Fe, 10 kg/ha Mn, 4 kg/ha Zn, 10 kg/ha Cu, 0.2 kg/ha Mo and 1.2 kg/ha B. The data
obtained were analyzed statistically by F test at 5% level of confidence, followed by Duncan's multiple range tests in case of significant effects.
Results and discussion
There is an opportunity to utilize extracellular phytohormones to enhance root nodules formation. Mishra et al., (1999) stated that the used of external phytohormones showed a response in the formation of root nodules. Application of exogenous IAA enhanced nodulation on Medicago and Phaseolus vulgaris when added at very low concentrations (up to 10-8 M) to the medium (van Noorden et al. cit. Spaepen et al., 2007). Extracellular auxin or naphtilic acetic acid (NAA) stimulated formation and growth of Medicago sativa root nodules and Arachis hypogaea (Srinivasan and Gopalkrisna cit. Mishra et al. 1999), while 1-napthoxy acetic acid and (p-chlorophenoxy)-isobutyric acid increased root nodules number in nuts (Cartwright cit. Mishra et al. 1999). Kamal (2000) stated that addition of epi-brasinoloid on soybean growth media increased root nodules number per plant. The formation of root nodules could also be induced by other extracellular phytohormones such as cytokinin (Nandwal, Bharti cit.
Mishra et al. 1999) and GA (Bishnoi, Krishnamoorty cit.
136 Agric. Sci. Res. J.
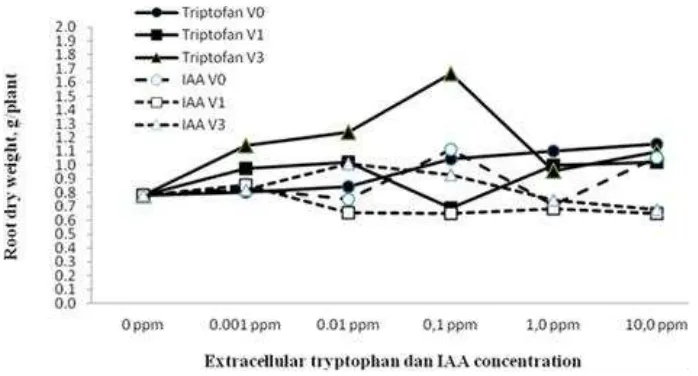
Figure. 2. Effect of the type, application time and concentrations of extracellular phytohormones (amino acid tryptophan and IAA) on root dry weight of soybean planted on sterile sand planting medium.
The number of soybean root nodules were influenced significantly (P = 0.000) by the type, application time and concentration of extracellular phytohormones (tryptophan and IAA). The extracellular addition of tryptophan resulted in higher number of root nodules than IAA. Presumably, this because tryptophan was a precursor of IAA, which was required in root nodules formation processes. Spaepen et al. (2010) stated that application of exogenous tryptophan increased strongly IAA production in various bacteria. IAA was involved in many processes of nodule formation by rhizobia in legume plants such as founder cell specification, nodule initiation and differentiation, nodule number and nitrogen fixation. Many researchers showed that tryptophan played a key role as precursor of IAA biosynthesis in various bacteria (Datta, Basu, 1997; Ghosh, Basu, 1997; Datta, Basu, 1998; Datta, Basu, 2000; Celenza, 2001; Asghar et al., 2002; Bhattacharyya, 2006; Ghosh, Basu, 2006; Spaepen, Vanderleyden, 2010; Chaiharn Lumyong, 2011). Patten and Glick (2002) showed that IAA accumulates in the culture medium of Pseudomonas putida GR12-2 only when grown in the presence of exogenous tryptophan. Tryptophan applied to the soil will be oxidized to IAA, which then stimulated root growth and induced nodules formation. On the other hand, IAA applied into the soil, maybe metabolized or decomposed that decreased its concentration and so its influence on root nodules formation processes. Yang, Saleh (1975) said that IAA was rapidly destroyed in the presence of Mn2+, oxygen and sulfite ion. According to Gregory (2006) root nodules formation was initiated by cortical or pericycle cell divisions and their differentiation are controlled by various hormones, especially auxin and cytokinin. Baca, Elmerich (2007), Spaepen and Vanderleyden (2010) and Verma et al. (2010) stated that
plant hormones including auxin (IAA) play a key role in various physiological processes during plant growth and development. Higher root nodules number were obtained from the treatment combination of (F1 V3 0.001 ppm) as lateral root development thus reducing the number of root nodules per plant because some of soybean root nodules are on it lateral roots.
The effect of tryptophan in root dry weight was more prominent than that of IAA. This is presumably, because amino acid tryptophan is oxidized by rhizobium to IAA which stimulates root growth of soybean plants. Bandurski, Nonhebel (1983), Pandey, Sinha (1996) and Guilfoyle et al., 1998) stated that at cellular level, auxin affects cell division, elongation and differentiation, whereas at plant level auxin affects plant tropism, plant morphology, shoots dominance, leaves senescence, leaf abscission, flowering, fruit formation and maturation, and root growth.
Sudadi 137
Figure 3. Nodules formed at lateral roots of soybean planted on sterile sand planting medium treated with amino acid tryptophan at vegetative (V) growth stage 1 (V1 T0.001 ppm) and V3 (V3 T0.1 ppm).
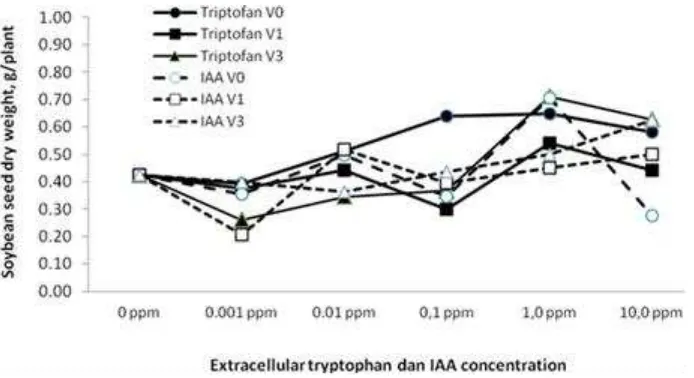
Figure 4. Effect of the type, application time and concentrations of extra-cellular phytohormones (amino acid tryptophan and IAA) on seed dry weight of soybean planted on sterile sand planting medium.
of these extracellular phytohormones (tryptophan and IAA). Highest soybean grain yield (0.711 g / plant) was obtained from the treatment combination of amino acid tryptophan (F1) applied at vegetative growth stage 3 (V3)
with a concentration of 1.0 ppm. This, because extracellular application of the tryptophan at V3 stimulates
root growth and root nodule formation higher than at V0 or
V1, presumably because at V3 the plant root had grown
more extensively and provided more sites for infection and nodulation, so it stimulates higher on N2 fixation and
grain yield. The concentration applied into the soil was 1.0 ppm, to ensure that there was enough tryptophan to produce IAA.
Conclusions and suggestions
138 Agric. Sci. Res. J.
soil, maybe it concentration declined immediately, because of damage or metabolized to another
soybean, groundnut, pea, and others leguminous plants.
Acknowledgement
The author would like to thank to The Director of Research and Community Services, the Directorate General of Higher Education, Ministry of National Education of Indonesia for financial support of the Fundamental research from which this article was written.
Reference
Afza R, G Hardarson, F Zapata (1987). Effect of delayed soil and foliar N fertilization on yield and N2 fixation of soybean. Plant and Soil. 97 : 361 – 368.
Asghar HN, ZA Zahir M, Arshad A Khaliq (2002). Relationship between in vitro production of auxins by rhizobacteria and their growth promoting activities in Brassica juncea L. Bio. Fertility Soil 35: 231-237.
Baca BE, C Elmerich (2007). Chapter 6. Microbial Production of Plant Hormones. In: Elmerich, C, Newton. WE, Associative and Endophytic Nitrogen-fixing Bacteria and Cyanobacterial Associations. Springer, Dordrecht, The Netherlands. pp. 113– 143.
Bandurski RS, HM Nonhebel (1983). Auxins. In: Wilkin, M.B., Advanced Plant Physiology. Glasgow. pp. 1 - 19.
Bhattacharyya RN (2006). Effects of heavy metals on growth
Chaiharn M, S Lumyong (2011). Screening and Optimization of Indole-3-Acetic Acid Production and Phosphate Solubilization from Rhizobacteria Aimed at Improving Plant Growth. Curr Microbiol., 62:173–181.
Datta C, PS Basu (1997). Growth behaviour and IAA production by a Rhizobium sp. isolated from root nodules of a leguminous medicinal herb, Dolichos biflorus. L., in culture.
Datta C, PS Basu (2000). Indole acetic acid production by a
Rhizobium species from root nodules of a leguminous shrub, Cajanus cajan. Microbiol. Res., 155 (2): 122 - 127.
Ghosh AC, PS Basu (1997). Indole acetic acid and its metabolism in the stem nodules of a leguminous emergent hydrophyte, Aeschynomene aspera. Microbiol. Res., 153 (4): 337-340.
Ghosh AC, PS Basu (2006). Production and metabolism of indole acetic acid in roots and root nodules of Phaseolus mungo. Microbiol., Res., 161: 362-366.
Gray TRG, ST William (1971). Soil Micro-Organisms. Longman. London.
Gregory P (2006). Plant Roots. Growth, Activity and Interaction with Soils. Blackwell Publishing Ltd, 9600 Garsington Road, Oxford OX4 2DQ, UK
Guilfoyle T, Hagen G, Ulmasov T, Murfet J (1998). How does auxin turn on genes? Plant Physiol. 118: 341 - 347.
Hidayat OO (1985). Morfologi tanaman kedelai. Dalam: Somaatmadja, S., M. Ismunadji, Sumarno, M. Syam, S.O. Manurung dan Yuswandi (edt.)., Kedelai. PPPTP.Bogor. h. pp.73 – 86
Ismunadji, M Dan, ST Mahmud (1985). Peranan unsur mikro untuk peningkatan produksi kedelai. Dalam: Somaatmadja, S., M. Ismunadji, Sumarno, M. Syams, S.O. Manurung and Yuswandi., Kedelai. PPPTP. Bogor. h. pp.189 - 215
Jutono (1985). Inokulasi Rhizobium. Dalam: Somaatmadja, S., M. Ismunadji, Sumarno, M. Syams, S.O. Manurung and Yuswandi.., Kedelai. PPPTP. Bogor. h. pp. 216 - 230
Kamal M (2000). Response of nodule formation, N2 fixation and sugar content in soybean nodule to epi-brassinolide application. Agrista (4)2: 120 -126. Ito, T Nishiwaki, Y Nagumo, S Ishii, T. Sato (2009). Nitrogen Fixation and Metabolism in Soybean Plants. Chapter 1. Introduction. Nova Science Publishers, Inc. New York. p. 1 -12.
Pandey SN, BK Sinha (1996). Plant Physiology. Part Three-Biochemestry and Metabolism. 17. Nitrogen Metabolism. Vikas Publishing House PVT Ld. Third Revised Edition. pp. 337 -351.
Pasaribu D. Dan Suprapto S (1985). Pemupukan NPK pada Kedelai. Dalam: Somaatmadja, S., M. Ismunadji, Sumarno, M. Syams, S.O. Manurung and Yuswandi.., Kedelai. PPPTP. Bogor. h. 159 - 169 (In Indonesian).
Patten CL, BR Glick (2002). Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can. J. Microbiol., 48 (7) : 635-642.
Reed RC, SR Brady, GK Muday (1998). Inhibition of auxin transport movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118: 1369 – 1378.
Spaepen SJ, Vanderleyden (2010). Auxin and Plant-Microbe Interactions. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a001438 published online November 17, 2010.
Spaepen S, Vanderleyden J, Remans R (2007). Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev,. (2007) 1–24.
Production. Int. J. Agric. Res., 5: 954-983.
Yang SF, MA Saleh (1975). Destruction of indole-3-acetic acid during the aerobic oxidation of sulfite. Phytochem., 12(6) : 1463-1466.