Agrobacterium tumefaciens
– mediated transformation of
Cyclamen
persicum
Mill.
Ryutaro Aida *, Yukio Hirose
1, Sanae Kishimoto, Michio Shibata
National Research Institute of Vegetables,Ornamental Plants and Tea,Ano,Mie514-2392,Japan
Received 27 October 1998; received in revised form 15 April 1999; accepted 23 April 1999
Abstract
A method forAgrobacterium-mediated transformation ofCyclamen persicumMill. is reported. Etiolated petiole segments were infected withAgrobacterium tumefaciensstrain AGL0 or LBA4404. These strains have a binary vector plasmid, pIG121Hm, that includes theb-glucuronidase (GUS) gene with an intron as reporter gene, and the neomycin phosphotransferase II gene and the hygromycin phosphotransferase gene as selection markers. Explants were cultured on Murashige and Skoog medium supple-mented with 1.0 mg/l thidiazuron, 1.0 mg/l 2,4-dichlorophenoxyacetic acid, 300 mg/l ticarcillin, and 5 mg/l hygromycin or 100 mg/l kanamycin (selection medium) for regeneration. Transformation was confirmed by histochemical assays of GUS activity in plant tissues, and by Southern blot analysis of the GUS gene. Through five experiments, 103 independent GUS-positive plants were obtained from 920 explants. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cyclamen persicumMill.; Transformation; Ornamental plants;Agrobacterium tumefaciens; Regeneration
www.elsevier.com/locate/plantsci
1. Introduction
Genetic transformation can create novel culti-vars. Several attempts have been made to improve ornamental plants by genetic engineering [1 – 3]. Transformation of ornamental plants is important, especially for the modification of ornamental char-acteristics such as flower color, shape and longevity.
The genus Cyclamen, the family Primulaceae, contains about 19 species, most of which occur in the Mediterranean region [4]. C. persicum Mill., commonly known as cyclamen, is the only com-mercially important species in the genus and is one of the most important ornamental pot plants in the world. Although tissue culture of cyclamen has
been well reported [5], and transgenic cyclamen plants have been obtained byAgrobacterium rhizo-genes-mediated transformation (Dr Masahiro Mii, Chiba University, personal communication), transformation of cyclamen has not been docu-mented until now.
A transformation system for cyclamen would be useful for breeding; for example, to modify flower color or to improve disease resistance. Flower colors of cyclamen range from white to scarlet, salmon, and pale pink [4]. A new color, pale yellow, has been reported recently [6], but blue cyclamens do not exist. Genetic transformation would be a powerful tool for producing deep yellow- or blue-flowered cyclamens. Resistance to diseases could also be introduced by genetic trans-formation. In this paper, we describe an Agrobac-terium tumefaciens-mediated transformation system for cyclamen, the first formal report of the transformation of cyclamen.
* Corresponding author. Fax: +81-59-2681339.
E-mail address:[email protected] (R. Aida)
1Present address: Ehime Agricultural Experiment Station, Hojo,
Ehime 799-2405, Japan
2. Materials and methods
2.1. Plant materials for transformation
Cyclamen cv. ‘Anneke’ was used as plant mate-rial. This cultivar is known to have high embryo-genic potential [7]. Seeds were soaked in 70% ethyl alcohol for 15 sec, surface-sterilized with 1% sodium hypochlorite for 30 min, and then rinsed twice with sterilized distilled water for 10 min each time. They were germinated on Murashige – Skoog medium with half-strength minerals (1/2 MS) [8], solidified with 0.2% (w/v) gellan gum. Cultures were maintained in the dark at 20°C to obtain etiolated petioles, which have high regenerative potential [9]. Two to three months after germina-tion without any subculture, etiolated petioles were cut into 8-mm segments and used as explants for transformation experiments.
2.2. Bacterial strain and 6ector plasmids
A. tumefaciens strains AGL0 [10] and LBA4404 (Clontech, Palo Alto, CA, USA), both of which harbor the binary vector plasmid pIG121Hm (Fig. 1) [11], were used for experiments. pIG121Hm contains the neomycin phosphotransferase II (NP-TII) gene (nos promoter), the b-glucuronidase (GUS) gene with a modified intron from the castor bean catalase gene [12] (35S promoter), and the hygromycin phosphotransferase (HPT) gene (35S promoter).
Plasmid-bearingAgrobacteriumcells were inocu-lated into liquid YEB medium (sucrose 5 g/l, beef extract 1 g/l, yeast extract 1 g/l, peptone 1 g/l) containing 50 mg/l kanamycin and shaken for 48 h at 28°C. The cells were pelleted by centrifugation and resuspended in 10 mM magnesium sulfate solution to a density of 1.0×108cells/ml for plant
infection.
2.3. Preculture and coculture
Precultures and cocultures of the Cyclamen ex-plants were maintained in the dark at 20°C. Seg-ments of etiolated petioles were precultured on MS medium solidified with 0.2% (w/v) gellan gum and containing 1.0 mg/l thidiazuron (TDZ), 1.0 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D), and 100 mM acetosyringon for 6 days. Acetosyringon activates the virulence genes of Agrobacteriumand enhances the transfer of foreign genes into a plant genome [13]. After preculture, the explants were incubated in the Agrobacterium suspension for 5 min, then blotted dry on sterilized filter paper. Explants were placed on another sterilized filter paper on the same medium for 6 days.
2.4. Selection and growing culture
After cocultivation, the explants were trans-ferred to solid MS medium (0.2% gellan gum) containing 1.0 mg/l TDZ, 1.0 mg/l 2,4-D, 300 mg/l ticarcillin, and 5 mg/l hygromycin or kanamycin (selection medium) for regeneration. The selection medium was changed every 2 weeks. Ando and Murasaki [9] reported the stimulative effects of darkness during culture of etiolated petioles on shoot regeneration, so we maintained cultures in the dark at 20°C.
About 3 months after infection, explants that formed shoots were transferred to solid 1/2 MS medium (0.2% gellan gum) containing 300 mg/l ticarcillin (growing medium) and cultured under a 16-h photoperiod regime under fluorescent light (70 mmol/m2/s) at 20°C for regenerated plants to
grow. After 2 to 3 months’ culture on the growth medium, a single regenerated plant was excised from each explant and cultured on sterilized Metro-Mix 350 (bark ash product; Scotts-Sierra Horticultural Products Company, Marysville, OH, USA) containing 1/2 MS solution with 300 mg/l carbenicillin (Metro-Mix medium) for rooting and further growth (16 h light, 20°C).
Fig. 1. Structure of the binary vector pIG121Hm [11]. The chimeric genes were inserted between the right and left border sequences of T-DNA. The length in the figure does not correspond to the actual length. The GUS probe was used for Southern blot analysis. BR and BL=right and left border sequences of T-DNA; Pnos and Tnos=promoter and termi-nator of nopaline synthase gene; 35S=promoter of CaMV 35S RNA gene; NPTII=coding region of neomycin phos-photransferase II gene; Intron-GUS = coding region of
b-glucuronidase gene with an intron; HPT=coding region of hygromycin phosphotransferase gene; H, X, B, S, and Sc=
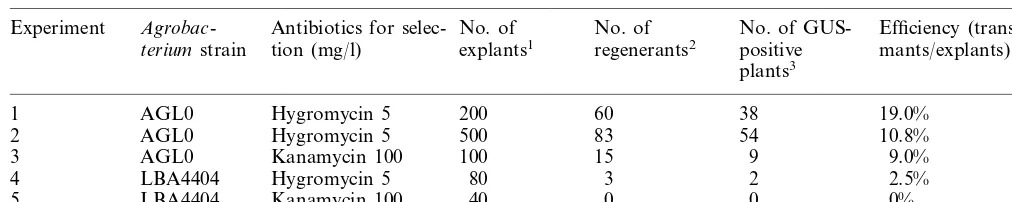
Table 1
Transformation efficiency inCyclamenwith binary vector pIG121Hm
Agrobac- Antibiotics for
selec-Experiment No. of No. of No. of GUS- Efficiency
(transfor-regenerants2
teriumstrain tion (mg/l) explants1 positive mants/explants) plants3
Hygromycin 5 200 60 38 19.0%
1 AGL0
Hygromycin 5 500 83
AGL0 54
2 10.8%
AGL0
3 Kanamycin 100 100 15 9 9.0%
4 LBA4404 Hygromycin 5 80 3 2 2.5%
Kanamycin 100 40 0 0
LBA4404 0%
5
1Segments of etiolated petiole were used as explants.
2Only a single regenerant was collected from each explant to obtain independent transformants. 3Transformation was confirmed by histochemical GUS assay.
2.5. GUS assay and Southern blot analysis
Histochemical GUS activity was examined by the procedure reported by Jefferson et al. [14] using 5-bromo-4-chloro-3-indolyl-b-D-glucuronic acid (X-GLUC) as a substrate. The GUS assay buffer used in this experiment contained 20% methyl alcohol to eliminate endogenous GUS ac-tivity, as reported by Kosugi et al. [15]. The sam-ples were incubated at 37° for 16 h.
Total DNA was extracted from leaf tissue with a Phytopure plant DNA extraction kit (Amer-sham, Little Chalfont, England) according to the manufacturer’s instructions. About 20mg of DNA digested with HindIII was electrophoresed in a 0.6% agarose gel and transferred to a nylon mem-brane. HindIII cuts the plasmid at a single site outside the coding region of the GUS gene. The coding region of the GUS gene was used as a probe. Southern hybridization was carried out with a DIG-High Prime and DIG Luminescent Detection Kit for nucleic acids (Boehringer-Mannheim, Germany). Blots were finally washed with 0.2×SSC, 0.1% SDS, at 68°C.
3. Results
3.1. Transient GUS assay after Agrobacterium infection
In a preliminary examination, we compared the ability of A. tumefaciens strains AGL0 and LBA4404 to transfer genes by looking for the transient GUS activity that represents early infec-tion byAgrobacterium. Five explants were assayed
for each strain. All the examined explants, regard-less of strain, showed at least one blue precipita-tion at the cut surface, which corresponds to GUS activity (Fig. 2). GUS activity of explants inocu-lated with AGL0 seemed to be stronger than that of plants inoculated with LBA4404. Vector pIG121Hm contains a modified GUS gene [12] that can be expressed only in plant cells. This result shows that the GUS gene was transferred to the cyclamen cell and was successfully expressed.
3.2. Regeneration
One to two months after Agrobacterium infec-tion, adventitious buds appeared at the cut surface of explants (Fig. 3A). After transfer to the grow-ing medium, the buds became green and grew gradually (Fig. 3B). By 5 months after infection, we obtained 161 independent plants from 920 explants through five experiments (Table 1). Re-generated plants grew normally (Fig. 3C) after being transplanted to the Metro-Mix medium.
3.3. GUS assay
Fig. 2. Transient GUS assay afterAgrobacteriuminfection. After coculture withAgrobacteriumstrain AGL0 (lower) or LBA4404 (upper) harboring a binary vector plasmid, pIG121Hm, that contains the GUS gene with an intron, all explants showed at least one blue precipitation, representing GUS activity, at the cut surface.
Fig. 3. Regeneration of putative transgenic cyclamen. (A) Regenerated buds from explants on solid MS medium (0.2% gellan gum) containing 1.0 mg/l TDZ, 1.0 mg/l 2,4-D, 300 mg/l ticarcillin, and 5 mg/l hygromycin (selection medium). (B) Growing plants on solid 1/2 MS medium (0.2% gellan gum) containing 300 mg/l ticarcillin (growing medium). (C) Normal cyclamen plants growing on the Metro-Mix medium (8 months afterAgrobacteriuminfection).
3.4. Southern blot analysis
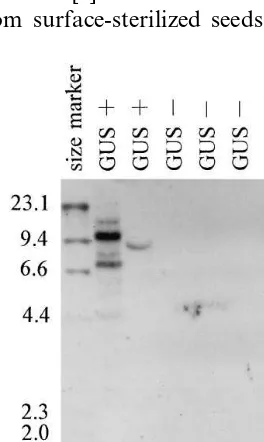
We selected two positive and three GUS-negative plants from experiment one (Table 1) for Southern blot analysis. Analysis showed that the GUS-positive plants had the GUS gene in their genome but that the GUS-negative plants did not (Fig. 5). Digestion of pIG121Hm DNA with HindIII cuts the plasmid at a single site outside the coding region of the GUS gene (Fig. 1). The presence of contaminating plasmid in the tissues should be detected by the presence of a single 15.6-kb band. The GUS-positive plants showed one or more bands of differing sizes, indicating single- or multiple-copy integration of the GUS gene into the genome. We considered the GUS-positive plants to be transformants.
4. Discussion
There have been many reports of tissue culture of cyclamen. Most early studies used tuber tissue as explant material, but contamination caused by parasitic microorganisms living in the tuber was a serious problem [5]. The use of tissue of seedlings grown from surface-sterilized seeds can overcome
this problem. Ando and Murasaki [9] reported that etiolated petioles of cyclamen could be regen-erated, but that normal (not etiolated) petioles could not. This method also avoids damage to stock plants. We therefore, decided to use etio-lated petioles of aseptic seedlings as explants for the transformation experiments.
We based the characterization of gene transfer
on GUS gene expression. We used vector
pIG121Hm [11], which contains GUS with a modified intron from the castor bean catalase gene in the coding region [12]. Prokaryotes cannot ex-press the intron – GUS combination, so the GUS expression in the explants and regenerated plants was a result of transgene expression in the plant cells. There was no detectable endogenous GUS activity in the cyclamen tissues (Fig. 4, top row). The experiment used selection medium contain-ing TDZ and 2,4-D as plant growth regulators for bud regeneration. TDZ is highly efficient at shoot regeneration in a wide variety of plants [16], and 2,4-D is known to stimulate adventitious organo-genesis from cyclamen tissue [17]. Adventitious buds appeared at the cut surface of explants 1 to 2 months after Agrobacterium infection (Fig. 3A), and regenerated plants grew normally (Fig. 3C). The combination of 1.0 mg/l TDZ and 1.0 mg/l 2,4-D used in this study seemed to be effective for organogenesis of adventitious buds from etiolated petioles of cyclamen. Embryogenesis was never observed under this culture condition. In other experiments, we tried to obtain cyclamen transfor-mants through embryogenic calluses under culture conditions required for embryogenesis, but failed to obtain any embryos on selection medium with antibiotics (data not shown). We considered that kanamycin and hygromycin would have negative effects on cyclamen embryogenesis.
We examined 161 independent regenerated plants for GUS activity; 103 showed GUS activity (Fig. 4), thus showing the existence of the GUS transgene. Two putative transformants and 3 pu-tative non-transformants were subsequently ana-lyzed by Southern blot analysis using the GUS coding region as a probe. The analysis confirmed the integration of the GUS gene into the GUS-positive plants only. However, the GUS-negative plants might be non-transformants – there is a possibility that they have only the HPT (and NP-TII) gene and lack the GUS gene. Further investi-gation will serve to clarify the nature of the
GUS-negative plants. Following the results of the preliminary experiment, we used 5 mg/l hy-gromycin or 100 mg/l kanamycin for selection of transformants, both of which suppressed organo-genesis from non-infected segments of etiolated petioles (data not shown). We obtained transfor-mants with both as selective agents, regeneration of non-transformed escapees could be reduced with more appropriate selective pressure.
Recently, there have been many reports of mod-ification of flower color by genetic transformation; for example, in petunia [18 – 26], Arabidopsis [27], gerbera [28], lisianthus [29,30], chrysanthemum [31], and rose [32]. Our transformation system could be useful for creating cyclamens with new colors, such as deep yellow or blue. Other charac-teristics, such as resistance to disease, could be improved by this system. Tabei et al. [33] reported that transgenic cucumber plants expressing a rice chitinase gene showed increased resistance to gray mold (Botrytis cinerea). This disease is one of the most serious for cyclamen. Introduction of a chiti-nase gene into cyclamen might enhance its resis-tance to B. cinerea. Other disease resistance genes could improve cyclamen in the future.
Acknowledgements
We thank Dr Kenzo Nakamura, Nagoya Uni-versity, for providing the plasmid pIG121Hm, and Dr Robert A. Ludwig, University of California, Santa Cruz, for providing the Agrobacterium strain AGL0. This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries, Japan.
References
[1] J.F. Hutchinson, V. Kaul, G. Maheswaran, J.R. Moran, M.W. Graham, D. Richards, Genetic improvement of floricultural crops using biotechnology, Aust. J. Bot. 40 (1992) 765 – 787.
[2] K.E.P. Robinson, E. Firoozabady, Transformation of floriculture crops, Scientia Horticulturae 55 (1993) 83 – 99.
[3] A. Zuker, T. Tzfira, A. Vainstein, Genetic engineering for cut-flower improvement, Biotechnol. Adv. 16 (1998) 33 – 79.
[4] A.J. Huxley, M. Griffiths, M. Levy, The New Royal Horticultural Society Dictionary of Gardening, Macmil-lan Press Limited, London and Basingstoke, 1992, pp. 792 – 797.
[5] T. Geier, H.W. Kohlenbach, G. Reuther, Cyclamen. Chapter 15, in: P.V. Ammirato, D.A. Evans, W.R. Sharp, Y.P.S. Bajaj (Eds.), Handbook of Plant Cell Culture, Volume 5, Ornamental Species, McGraw-Hill, New York, 1990, pp. 352 – 374.
[6] I. Miyajima, T. Maehara, T. Kage, K. Fujieda, Identifi-cation of the main agent causing yellow color of yellow-flowered cyclamen mutant, J. Japan. Soc. Hort. Sci. 60 (1991) 409 – 414.
[7] T. Takamura, I. Miyajima, E. Matsuo, Somatic embryo-genesis ofCyclamen persicumMill. ‘Anneke’ from aseptic seedlings, Plant Cell Reports 15 (1995) 22 – 25.
[8] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Phys-iol. Plant. 15 (1962) 473 – 497.
[9] T. Ando, K. Murasaki, In vitro propagation of cyclamen by the use of etiolated petioles. Technical Bulletin of the Faculty of Horticulture, Chiba University, 32 (1983) 1 – 5.
[10] G.R. Lazo, P.A. Stein, R.A. Ludwig, A DNA transfor-mation-competent Arabidopsis genomic library in
Agrobacterium, Bio/Technology 9 (1991) 864 – 868. [11] Y. Hiei, S. Ohta, T. Komari, T. Kumashiro, Efficient
transformation of rice (Oryza sati6a L.) mediated by
Agrobacterium and sequence analysis of the boundaries of the T-DNA, Plant J. 6 (1994) 271 – 282.
[12] S. Ohta, S. Mita, T. Hattori, K. Nakamura, Construc-tion and expression in tobacco of a b-glucuronidase (GUS) reporter gene containing an intron within the coding sequence, Plant Cell Physiol. 31 (1990) 805 – 813. [13] S.E. Stachel, E. Messens, M.V. Montagu, P. Zambryski, Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in
Agrobacterium tumefaciens, Nature 318 (1985) 624 – 629. [14] R.A. Jefferson, T.A. Kavanagh, M.W. Bevan, GUS fu-sions: b-glucuronidase as a sensitive and versatile gene fusion marker in higher plants, EMBO J. 6 (1987) 3901 – 3907.
[15] S. Kosugi, Y. Ohashi, K. Nakajima, Y. Arai, An im-proved assay for b-glucuronidase in transformed cells: methanol almost completely suppresses a putative en-dogenous b-glucuronidase activity, Plant Sci. 70 (1990) 133 – 140.
[16] C.A. Huetteman, J.E. Preece, Thidiazuron: a potent cy-tokinin for woody plant tissue culture, Plant Cell. Tissue and Organ Culture 33 (1993) 105 – 119.
[17] T. Takamura, M. Tsutsui, M. Tanaka, Micropropaga-tion of yellow-flowered Cyclamen through adventitious organogenesis in medium containing 2,4-dichlorophe-noxyacetic acid. Technical Bulletin of the Faculty of Agriculture, Kagawa University, 48 (1996) 33 – 38. [18] P. Meyer, I. Heidmann, G. Forkmann, H. Saedler, A
new petunia flower colour generated by transformation of a mutant with a maize gene, Nature 330 (1987) 677 – 678.
[20] C. Napoli, C. Lemieux, R. Jorgensen, Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans, Plant Cell 2 (1990) 279 – 289.
[21] A.R. van der Krol, L.A. Mur, M. Beld, J.N.M. Mol, A.R. Stuitje, Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression, Plant Cell 2 (1990) 291 – 299. [22] T.A. Holton, F. Brugliera, D.R. Lester, Y. Tanaka, C.D.
Hyland, J.G.T. Menting, C.-Y. Lu, E. Farcy, T.W. Stevenson, E.C. Cornish, Cloning and expression of cy-tochrome P450 genes controlling flower colour, Nature 366 (1993) 276 – 279.
[23] Y. Helariutta, P. Elomaa, M. Kotilainen, P. Seppanen, T.H. Teeri, Cloning of cDNA coding for dihy-droflavonol-4-reductase (DFR) and characterization of DFR expression in the corollas of Gerbera hybrida var. Regina (Compositae), Plant Mol. Biol. 22 (1993) 183 – 193.
[24] Y. Tanaka, Y. Fukui, M. Fukuchi-Mizutani, T.A. Holton, E. Higgins, T. Kusumi, Molecular cloning and characterization of Rosa hybrida dihydroflavonol-4-re-ductase gene, Plant Cell Physiol. 36 (1995) 1023 – 1031. [25] M. Bradley, K. Davies, S. Deroles, K. Schwinn, D.
Manson, Colour modification in petunia using the Lc
regulatory gene from maize, Acta Horticulturae 420 (1995) 23 – 25.
[26] K.M. Davies, S.J. Bloor, G.B. Spiller, S.C. Deroles, Production of yellow colour in flowers: redirection of flavonoid biosynthesis in Petunia, Plant J. 13 (1998) 259 – 266.
[27] A.M. Lloyd, V. Walbot, R.W. Davis, Arabidopsis and
Nicotiana anthocyaninproduction activated by maize
reg-ulatorsRandC1, Science 258 (1992) 1773 – 1775. [28] P. Elomaa, J. Honkanen, R. Puska, P. Seppanen, Y.
Helariutta, M. Mehto, M. Kotilainen, L. Nevalainen, T.H. Teeri, Agrobacterium-mediated transfer of antisense chalcone synthase cDNA to Gerbera hybrida inhibits flower pigmentation, Bio/Technology 11 (1993) 508 – 511. [29] S. Deroles, M. Bradley, K. Davies, K. Schwinn, D. Manson, Generation of novel patterns in lisianthus flow-ers using an antisense chalcone synthase gene, Acta Horticulturae 420 (1995) 26 – 28.
[30] K.E. Schwinn, K.M. Davies, S.C. Deroles, K.R. Markham, R.M. Miller, J.M. Bradley, D.G. Manson, N.K. Given, Expression of an Antirrhinum majus UDP-glucose: flavonoid-3-O-glucosyltransferase transgene al-ters flavonoid glycosylation and acylation in lisianthus (Eustoma grandiflorumGrise.), Plant Sci. 125 (1997) 53 – 61.
[31] N. Courtney-Gutterson, C. Napoli, C. Lemieux, A. Mor-gan, E. Firoozabady, K.E.P. Robinson, Modification of flower color in florist’s chrysanthemum: production of a white-flowering variety through molecular genetics, Bio/
Technology 12 (1994) 268 – 271.
[32] F. Souq, P. Coutos-Thevenot, H. Yean, G. Delbard, Y. Mazie`re, J.P. Barbe, M. Boulay, Genetic transformation of roses, 2 examples: one on morphogenesis, the other on anthocyanin biosynthetic pathway, Acta Horticulturae 424 (1996) 381 – 388.
[33] Y. Tabei, S. Kitade, Y. Nishizawa, N. Kikuchi, T. Kayano, T. Hibi, K. Akutsu, Transgenic cucumber plants harboring a rice chitinase gene exhibit enhanced resis-tance to gray mold (Botrytis cinerea), Plant Cell Rep. 17 (1998) 159 – 164.
![Fig. 1. Structure of the binary vector pIG121Hm [11]. Thechimeric genes were inserted between the right and left bordersequences of T-DNA](https://thumb-ap.123doks.com/thumbv2/123dok/1036298.929256/2.612.26.265.481.547/structure-binary-vector-thechimeric-genes-inserted-right-bordersequences.webp)