Summary The light environment, photosynthetic dynamics and steady-state net photosynthetic rates of lateral branch shoots of Pseudotsuga menziesii var. glauca (Beissn.) Franco seedlings growing in the open and in the forest understory were investigated in situ. Mean incident photosynthetic photon flux density (PPFD) was 702.5 µmol m−2 s−1 on open-grown branches and 52.0 µmol m−2 s−1 on understory-grown branches. Mean daily durations of PPFD greater than 500, 200, and 50 µmol m−2 s−1 were 8.5, 31.5, and 270.3 min, respec-tively, on understory-grown branches, and 559.1, 700.7, and 803.3 min, respectively, on open-grown branches. Sunflecks accounted for 32.4% of total daily photosynthetically active radiation incident on understory branches. Following 10 min at a PPFD of 50 µmol m−2 s−1, the induction time required for net photosysnthesis to reach 50 and 90% of steady-state rates was shorter at a PPFD of 200 than at a PPFD of 500 µmol m−2 s−1 and shorter in understory-grown branches than in open-grown branches. On a leaf area basis, dark respiration rates of under-story-grown branches were lower and net photosynthetic rates were higher than those of open-grown branches exposed to low PPFD. However, at high PPFDs, understory-grown branches had lower photosynthetic rates than open-grown branches. When measurements were expressed on a leaf dry mass basis, there was no difference in dark respiration rates between un-derstory branches and open-grown branches, but net photosyn-thetic rates of understory branches were equal to or higher than those of open-grown branches at all PPFDs.
Keywords: dark respiration, light acclimation, net photosyn-thesis, photosynthetic induction, sunflecks.
Introduction
Photosynthetic acclimation to a changing light environment has been studied in some tropical shrubs (Chazdon 1988, Chazdon 1992, Chazdon and Kaufmann 1993), agricultural crops (Pons et al. 1992, Intrieri et al. 1995), herbs (Young and Smith 1980, Koizumi and Oshima 1993, Pearcy and Sims 1994, Pearcy et al. 1994, Sims and Pearcy 1994), and broadleaf tree species (Ellsworth and Reich 1992, Kitajima 1994, Letho and Grace 1994, Tang et al. 1994, Tinoco-Ojanguren and Pearcy 1995). In general, leaves of shade plants have a lower dark respiration rate and a lower light-saturated photosynthetic
rate (Amax) on a leaf area basis than sun leaves (Chazdon 1992,
Chazdon and Kaufmann 1993, Walters et al. 1993, Kitajima 1994, Letho and Grace 1994, Sims and Pearcy 1994, Intrieri et al. 1995). Photosynthesis that is dependent on sunflecks ac-counts for up to 50% of net carbon gain in some understory species of tropical rain forests (Chazdon 1988, Chazdon and Pearcy 1991). Simulations of dynamic photosynthetic re-sponses in controlled laboratory conditions indicate that shade plants achieve higher light-use efficiencies during sunflecks than sun plants because of faster photosynthetic induction and greater post-illumination assimilation (Pons et al. 1992, Tang et al. 1994). There have been few studies of photosynthetic plasticity to sun and shade conditions in conifers (Brooks et al. 1994, Leverenz 1995, Stenberg et al. 1995). Furthermore, few studies of the relationship between light acclimation and pho-tosynthetic behavior have been carried out under natural light conditions (Young and Smith 1980, Chazdon 1992, Ellsworth and Reich 1992, Chazdon and Kaufmann 1993), thus little is known about the dynamic responses of net photosynthesis to light acclimation under field conditions.
In central British Columbia, interior Douglas-fir (Pseudot-suga menziesii var. glauca (Beissn.) Franco) is considered a late-successional, moderately shade-tolerant species of mon-tane forests that is capable of regenerating under its own canopy. The sun leaves are distributed around the branches in a whorled pattern, whereas the shade leaves are oriented in a nearly horizontal plane. A previous study on naturally regen-erated Douglas-fir saplings showed that, with decreasing light availability, (i) specific leaf area increases and (ii) the ratio of the terminal shoot increment to lateral branch increment de-creases (Chen et al. 1996). Both changes likely make saplings more efficient in capturing light energy in a light-limiting environment. Whether Douglas-fir seedlings grown in a low-light environment have lower dark respiration rates and higher photosynthetic light-use efficiencies must be investigated to understand how this species maintains vigorous growth in the forest understory. A higher light-use efficiency, if it exists, may be attributed to (i) faster induction responses and (ii) higher net photosynthetic rates under conditions of constant irradiation.
The objectives of this study were to: (i) characterize the light environment of plantation-grown Douglas-fir seedling branches, (ii) examine the time-courses of photosynthetic induction re-sponses to various irradiances in seedlings grown in full-light
Light availability and photosynthesis of Pseudotsuga menziesii
seedlings grown in the open and in the forest understory
HAN Y. H. CHEN and KAREL KLINKA
Department of Forest Sciences, University of British Columbia, 270-2357 Main Mall, Vancouver, B.C. V6T 1Z4, Canada
Received January 30, 1996
environments and in the forest understory, and (iii) determine whether the photosynthetic light-use efficiency of understory-grown seedlings is higher than that of open-understory-grown seedlings.
Materials and methods
Study site and plant materials
The study site was located in the dry mild interior Douglas-fir subzone (Meidinger and Pojar 1991), near Okanagan Falls, British Columbia (50°05′ N, 119°40′ W). The area receives an annual average of 600 mm total precipitation. The wettest and driest months are June and April, respectively. The mean tem-peratures for the coldest and the warmest month are --10.7 and 15.8 °C, respectively. The length of the growing season is between 4 and 5 months extending from late April to early September (Meidinger and Pojar 1991).
In April 1993, one-year-old container-grown P. menziesii seedlings of a local provenance were obtained from a local nursery and planted in a recently created 2-ha clearcut and in an adjacent naturally regenerated, immature stand with a semi-open canopy. The stand composition was 70% interior Douglas-fir, 20% western larch (Larix occidentalis Nutt.), and 10% ponderosa pine (Pinus ponderosa Dougl. ex Laws.). Mean height of the stand was 15 m; mean age of the canopy trees was 30 years; and stand basal area was 15 m2 ha−1. To minimize the substrate effect, all seedlings were planted in mineral soil. Based on the method described by Green and Klinka (1994), both the understory and clearcut portions of the study site were considered to be moderately dry with medium nitrogen availability. After 17 months, light measurements were made in the vicinity of 16 randomly chosen seedlings (eight from the forest understory and eight from the clearcut). Only the uppermost lateral branches with well-expanded cur-rent-year needles were selected for light and gas exchange measurements. These branches were approximately 25 to 35 cm above ground. To eliminate the possible influence of soil water deficit, the root zones of sampled seedlings were maintained at field capacity by watering on three successive days before gas exchange was measured.
Light measurements
Photosynthetic photon flux density (PPFD) at the center of sampled branches was measured with a PPFD sensor (LI-190 SA quantum sensor, Li-Cor, Inc., Lincoln, NE) connected to a datalogger (Li-Cor LI-1000 datalogger) monitored at 5-s inter-vals from before sunrise to after sunset. Another PPFD sensor and datalogger were placed in the center of the clearcut. The two datalogger clocks were synchronized before taking PPFD measurements. When the PPFD sensors were placed side by side and compared over a PPFD range from 10 to 1500 µmol m−2 s−1, their readings were found to differ by less than 0.5%. The dataloggers were set to average 12 instantaneous PPFD recordings each minute. All measurements were made under clear skies from July 28 to August 15, 1994. Light availability for the branches of open-grown seedlings was considered equivalent to full light even though some self-shading by terminal shoots may have occurred.
Gas exchange measurements
We measured gas exchange of lateral branch shoots of five or eight randomly chosen seedlings. We also measured light availability in the vicinity of the same shoots. Measurements were made in the field under a tent on attached lateral shoots using an artificial light source and an open portable photosyn-thesis system (ADC LCA-3, Analytical Development Com-pany Ltd., Herts, U.K.) with a conifer cuvette. The light source consisted of four 20-W, 12-V tungsten-halogen lamps set in a 2 × 2 arrangement to provide uniform irradiation of the cuvette. Photosynthetic photon flux was varied between 50 and 1800 µmol m−2 s−1 by varying the distance between the cu-vette and light source. Nominal irradiance was ± 5% of actual PPFD with the beam perpendicular to the plane of the meas-ured shoot. The tent and additional black cloth screened out most natural light. To reduce the seasonal variation in photo-synthesis caused by needle aging (Brooks et al. 1994), gas exchange measurements were taken between August 8 and August 25, 1994 on the uppermost branches with current-year, well-expanded needles. An air mast (Analytical Development Company) was used to draw fresh air from outside the tent at a height of 1.2 m. With a flow rate of 300 cm3 min−1 through the cuvette, it took the system approximately 35 s to detect a 95% step change in vapor pressure and CO2 concentration in
the cuvette.
After sunset the day before measurements, one seedling, selected alternately from the forest understory or clearcut, was covered by a dark plastic can (ventilated with several small holes) to prevent exposure to diffuse, early-morning light. The photosynthesis system was adjusted for study site conditions before gas exchange measurements were taken each morning. To avoid the more extreme daytime differences in air tempera-ture, ambient humidity, and soil water conditions, gas ex-change measurements were taken every 20 s from before sunrise (0515 ± 0010 h) to 1000 h. Dark respiration rates were measured at 0515 ± 0015 h. Photosynthetic induction re-sponses were observed at 200 and 500 µmol m−2 s−1 after the branch had first been completely induced at a PPFD of 500 µmol m−2 s−1 and then exposed to a PPFD of 50 µmol m−2 s−1 for 10 min. A light-response curve for each branch was constructed following stepwise PPFD increases until photoin-hibition occurred. After gas exchange measurements, branches were removed and stripped of needles to measure total one-sided projected leaf area with a Li-Cor LI-3100 area meter. Needles were then dried at 65 °C for 24 h and weighed.
During gas exchange measurements, ambient CO2
concen-tration, temperature, and relative humidity were monitored. Ambient conditions varied from early morning to 1000 h. Carbon dioxide concentration decreased from 370 ± 10 to 350
Calculations
Direct radiation in the forest understory was subjectively de-fined as PPFD greater than 50 µmol m−2 s−1 (Chazdon and Pearcy 1991, Koizumi and Oshima 1993, Tang et al. 1994). The sunfleck contribution was calculated as:
Sunfleckcontribution =
∫
TrTs
(PPFD− 50)dt
∫
TrTs
PPFD dt
, (1)
where if PPFD < 50, let (PPFD−50) equal zero, and Tr and Ts
are the times of sunrise and sunset when the PPFD was > 1
µmol m−2s−1 and < 1 µmol m−2 s−1, respectively.
Photosynthetic induction state (ISt, normalized net
photo-synthetic rate, %) was based on the formula of Chazdon and Pearcy (1986):
ISt=
Pt− PL PH−PL
100, (2)
wher e Pt is the net photosynthetic rate at time t, PL is
steady-state assimilation rate at 50 µmol m−2 s−1, and PH is
steady-state net photosynthetic rate at either 200 or 500 µmol m−2 s−1. To reduce signal noise in the measurement system, both PL and
PH were averaged from five readings.
Light-response curves for both understory- and open-grown seedlings were fitted to a rectangular hyperbola (Long and Hällgren 1993). When possible photoinhibition occurred at a PPFD, measurements were not included in the final analysis. One-tailed Student’s t-test was used to test the difference between means of open- and understory-grown seedlings.
Results
Light availability
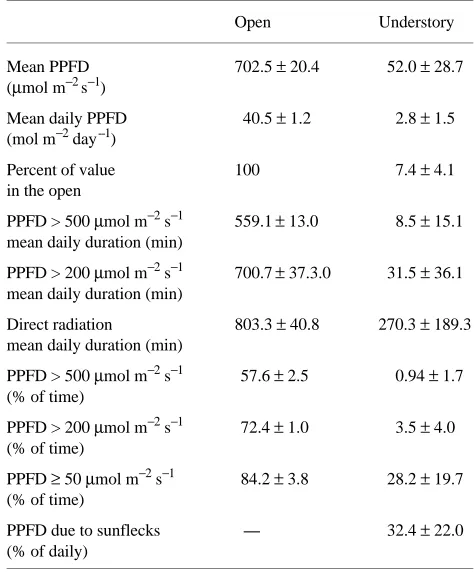
Branches of open-grown seedlings received a mean PPFD of 702.5 µmol m−2 s−1 and a mean daily PPFD of 40.5 mol m−2 day−1, whereas understory-grown seedlings received a mean PPFD of 52 µmol m−2 s−1 and a mean daily PPFD of 2.8 mol m−2 day−1 (Table 1). Branches of understory-grown seedlings received a mean of 7.4% full sun PPFD, with sunflecks con-tributing 32.4% of the daily photon flux (Table 1). Repre-sentative daily courses of PPFD in the forest understory and the open are shown in Figure 1.
The light environment varied greatly among measured branches from different understory seedlings; mean PPFD ranged from 18.4 to 85 µmol m−2 s−1 and mean daily PPFD ranged from 1.1 to 4.6 mol m−2 day−1. The percent of full sun PPFD had a range of 3 to 12%. The daily cumulated amount of time that branches received a PPFD above 500 µmol m−2 s−1 and 200 µmol m−2 s−1 ranged from 0 to 31 min (0 to 3.2% of day length) and 0 to 83 min (0 to 8.7% of day length), respectively. The daily cumulated amount of time branches received direct radiation ranged from 44 to 485 min (4.6 to
50% of day length). The peak PPFDs due to sunflecks varied from 170 to 1120 µmol m−2 s−1; sunflecks contributed between 6.8 and 58.9% of total PPFD.
Dynamic photosynthetic induction response
There was a larger but slower increase in net photosynthetic rate in open-grown branches than in understory-grown branches exposed to PPFDs of 500 µmol m−2 s−1and 200 µmol m−2 s−1 Table 1. Light conditions for open-grown branches and for branches in the forest understory. Values are means and standard deviations (n = 8). Direct radiation is defined as PPFD values greater than 50
µmol m−2 s−1.
Open Understory
Mean PPFD 702.5 ± 20.4 52.0 ± 28.7 (µmol m−2 s−1)
Mean daily PPFD 40.5 ± 1.2 2.8 ± 1.5 (mol m−2 day--1)
Percent of value 100 7.4 ± 4.1
in the open
PPFD > 500 µmol m−2 s−1 559.1± 13.0 8.5 ± 15.1 mean daily duration (min)
PPFD > 200 µmol m−2 s−1 700.7± 37.3.0 31.5 ± 36.1 mean daily duration (min)
Direct radiation 803.3 ± 40.8 270.3 ± 189.3 mean daily duration (min)
PPFD > 500 µmol m−2 s−1 57.6 ± 2.5 0.94 ± 1.7 (% of time)
PPFD > 200 µmol m−2 s−1 72.4 ± 1.0 3.5 ± 4.0 (% of time)
PPFD ≥ 50 µmol m−2 s−1 84.2 ± 3.8 28.2 ± 19.7 (% of time)
PPFD due to sunflecks ---- 32.4 ± 22.0 (% of daily)
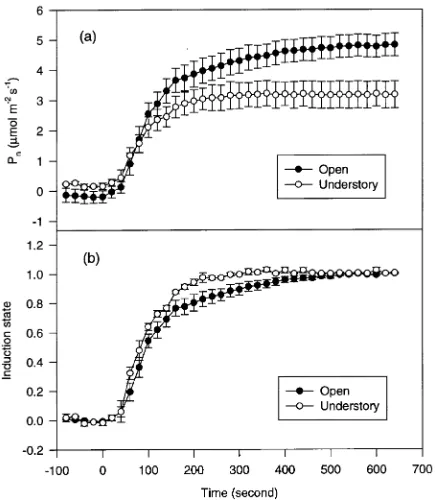
following 10 min at a PPFD of 50 µmol m−2 s−1 (Figures 2 and 3, Table 2). At a PPFD of 500 µmol m−2 s−1, the mean times required by understory-grown branches to reach 50 and 90% of steady-state photosynthetic rate were 85 and 200 s, respectively; corresponding mean times for open-grown branches were 99 and 304 s (Figure 2b, Table 2). At a PPFD of 200 µmol m−2 s−1, the mean times required by understory-grown branches to reach 50 and 90% induction were 63 and 173 s, respectively; corresponding mean times for open-grown branches were 81 and 162 s (Figure 3b, Table 2).
Both understory- and open-grown branches were slower to reach full induction at a PPFD of 500 µmol m−2 s−1 than at a PPFD of 200 µmol m−2 s−1 (Figures 2b and 3b, Table 2). Times taken for understory- and open-grown branches to reach 100% induction state at a PPFD of 500 µmol m−2 s−1 were 300 and 500 s, respectively, and 240 s for both at a PPFD of 200 µmol m−2 s−1.
Light-response curves
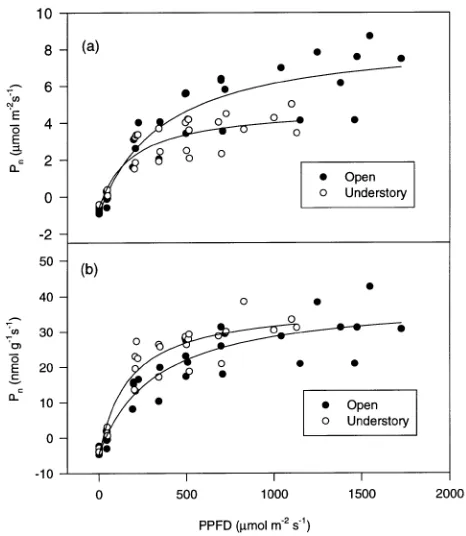
Understory- and open-grown branches had different net pho-tosynthetic responses to steady-state PPFDs (Figures 4a and 4b). Compared to open-grown branches, understory-grown branches had a significantly (P < 0.05) lower dark respiration rate (0.44 ± 0.01 versus 0.77 ± 0.03 µmol m−2 s−1), higher area-based net photosynthetic rate at a PPFD of 50 µmol m−2 s−1, and lower area-based net photosynthetic rates at PPFDs higher than 350 µmol m−2 s−1 (Figure 4a). At PPFDs of 200
and 350 µmol m−2 s−1, no difference in net photosynthetic rates between understory- and open-grown branches was ob-served. At a PPFD of 50 µmol m−2 s−1, the light compensation point was lower for understory-grown branches compared to open-grown branches, as shown by their net photosynthetic rates (0.23 ± 0.02 versus --0.11 ± 0.07 µmol m−2 s−1). Under-story-grown branches reached 90% of their light-saturated net photosynthetic rate at a PPFD of 500 µmol m−2 s−1, whereas open-grown branches did not reach 90% of their light-satu-rated rate even at a PPFD of 1000µmol m−2 s−1. At supersatu-rating PPFD, photosynthetic rates decreased, suggesting the onset of photoinhibition (not shown). Threshold PPFD for Figure 2. (a) Net photosynthetic rates (Pn) and (b) induction responses
to an increase in irradiance from a 10-min PPFD of 50 µmol m− 2 s−1 to a PPFD of 500 µmol m−2 s−1 starting at time 0 for open-grown (closed circles) and understory-grown branches (open circles). Each point is the mean (± SE) of single measurements from five seedlings.
Figure 3. (a) Net photosynthetic rates (Pn)and (b) induction responses
to an increase in irradiance from a 10-min PPFD of 50 µmol m−2 s−1 to a PPFD of 200 µmol m−2 s−1 starting at time 0 for open-grown (closed circles) and understory branches (open circles). Each point is the mean (± SE) of single measurements from five seedlings.
Table 2. Mean (± SE) time (s) required to reach 50 and 90% steady-state net photosynthetic rate for open- and understory-grown seedling branches following 10 min at a PPFD of 50 µmol m−2 s−1. Values followed by the same letter are not significantly different at the same induction state between open- and understory-grown branches (P < 0.05).
PPFD 50% 90%
(µmol m−2 s−1) Open Understory Open Understory
photoinhibition was between 800 and 1400 µmol m−2 s−1 for understory-grown branches and at 1800 µmol m−2 s−1 for open-grown branches.
Differences between understory- and open-grown branches were less pronounced when data were expressed on a leaf mass basis (Figure 4b). There was little difference in dark respiration rates between understory- and open-grown branches (P = 0.33). Net photosynthetic rates were significantly higher (P < 0.05) for understory-grown than for open-grown branches at PPFDs of 50 and 200 µmol m−2 s−1. At PPFDs equal to or greater than 350 µmol m−2 s−1, no significant difference in net photosyn-thetic rates was found between open- and understory-grown branches (P > 0.05).
Discussion
Understory light availability
Understory-grown branches received a mean daily PPFD of 2.8 mol m−2 day−1 or 7.4% of full sun, with sunflecks contrib-uting 32.4% of the daily total (Table 1). Light availability under the forest canopy was higher than that reported for low latitude conifer forests and tropical rain forests (Chazdon 1988, Canham et al. 1990). The contribution of sunflecks to daily PPFD was similar to that found in a montane red spruce--balsam fir forest in the Appalachian Mountains of the eastern USA (Canham et al. 1990).
Light availability and sunfleck duration and intensity within the stand varied greatly among branches from different seed-lings. Similar results were reported for understory light envi-ronments of tropical rain forests (Chazdon 1986). Such spatial variability depends on distances from gaps (Canham et al. 1990, Chazdon and Pearcy 1991) and the clumping effect of overstory leaves (Chen and Black 1992, Baldocchi and Col-lineau 1994). Diffuse and direct components were not meas-ured separately in this study; instead, a PPFD of 50 µmol m−2 s−1 was used to discriminate between diffuse light and direct light (e.g., Chazdon 1986, Koizumi and Oshima 1993). However, both the direct component and the diffuse compo-nents may vary spatially and diurnally within a forest stand (Black et al. 1991).
Dynamic and steady-state photosynthesis
Pseudotsuga menziesii seedlings acclimated photosyntheti-cally to their light environments under field conditions. Photo-synthetic induction responses to PPFD changes were quicker in understory-grown branches than in open-grown branches. This pattern is consistent with studies in a tropical forest (Küppers et al. 1996) and in controlled environments on some broadleaf plants (Pons and Pearcy 1992, Tang et al. 1994). Photosynthetic induction responses of P. menziesii branches tended to be faster than those of Quercus serrata Thunb. (Tang et al. 1994) but shorter than those of desiccation-tolerant Poly-podium virginianum L. (Gildner and Larson 1992). However, the time required to reach full photosynthetic induction varies among species (Chazdon 1988) and depends on: (i) length of the period and PPFD of low light condition (Pons et al. 1992, Whitehead and Teskey 1995), and (ii) other ecological factors such as water status. Photosynthetic induction responses tend to be faster in the field than in the laboratory (Gildner and Larson 1992), and water-stressed plants reach their maximum stomatal conductance in less time than unstressed plants (Ti-noco-Ojanguren and Pearcy 1993, Barradas et al. 1994). We also found that the time required to reach full photosynthetic induction depended on PPFD. With increasing PPFD, more time was required for both understory and open-grown P. menziesii branches to reach full photosynthetic induction.
Acclimation of dark respiration under sun versus shade conditions has been studied at the leaf and whole-plant levels (Björkman 1981, Chazdon and Kaufmann 1993, Letho and Grace 1994, Sims and Pearcy 1994, Intrieri et al. 1995, Tinoco-Ojanguren and Pearcy 1995). Plants acclimated to low light environments have low dark respiration rates when expressed on a unit leaf area basis. Similarly, in this study, understory-grown branches of P. menziesii had lower dark respiration rates per unit of leaf area than open-grown branches. On a dry leaf mass basis, however, dark respiration rates did not differ be-tween the understory- and open-grown branches.
Compared to open-grown branches, understory-grown branches were photosynthetically more efficient at low PPFDs but less efficient at high PPFDs (Leverenz 1995). Based on per unit dry leaf mass, net photosynthetic light-use efficiency in understory-grown branches was equal to or higher than that of open-grown branches at all PPFDs except those inducing pho-Figure 4. Photosynthetic light-response curves for open-grown (closed
toinhibition. This pattern has also been observed in some species at the leaf and whole-plant levels (Björkman 1981, Sims and Pearcy 1994, Leverenz 1995). Thus, compared with sun plants, shade plants exhibit no disadvantage in terms of carbon gain per unit dry mass unless photoinhibited.
There are qualitative and quantitative differences in light response among individual needles, shoots, and whole plants (Pearcy and Sims 1994, Leverenz 1995, Stenberg et al. 1995). This study describes the dynamic and steady-state photosyn-thetic behavior of P. menziesii at the lateral-shoot level, which integrates both photosynthesis of needles and the structural factor of their distribution. However, photosynthetic behavior at the individual needle and whole-plant levels was not consid-ered. Moreover, computer simulations of dynamic and steady-state photosynthesis in relation to natural light regimes in P. menziesii seedlings may be needed to understand the daily and seasonal carbon balance and the relative importance of dynamic and steady-state photosynthetic plasticity.
Acknowledgments
We especially thank Dr. Robert D. Guy, Department of Forest Sci-ences, University of British Columbia, for his continuous encourage-ment during this study and valuable comencourage-ments on the manuscript. We also thank Dr. T.A. Black for his useful comments on the manuscript. Financial support from the Natural Science and Engineering Research Council of Canada, and B.C. Ministry of Forests, Silviculture Branch is acknowledged.
References
Baldocchi, D. and S. Collineau. 1994. The physical nature of solar radiation in heterogeneous canopies: spatial and temporal attrib-utes. In Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes Above- and Belowground. Eds. M.M. Caldwell and R.W. Pearcy. Academic Press, New York, pp 21--72. Barradas, V.L., H.G. Jones and J.A. Clark. 1994. Stomatal responses
to changing irradiance in Phaseolus vulgaris L. J. Exp. Bot. 45:931--936.
Björkman, O. 1981. Responses to different quantum flux densities. In Encyclopedia of Plant Physiology, New Series, Vol. 12A, Physi-ological Plant Ecology I. Eds. O.L. Lange, P.S. Nobel, C.B. Os-mond and H. Ziegler. Springer-Verlag, Berlin, pp 57--107. Black, T.A., J.M. Chen, X. Lee and R. Sagar. 1991. Characteristics of
shortwave and longwave irradiances under a Douglas-fir forest stand. Can. J. For. Res. 21:1020--1028.
Brooks, J.R., T.M. Hinckley and D.G. Sprugel. 1994. Acclimation responses of mature Abies amabilis sun foliage to shading. Oecolo-gia 100:316--324.
Canham, D.C., J.S. Denslow, W.J. Platt, J.R. Runkle, T.A. Spies and P.S. White. 1990. Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Can. J. For. Res. 20:620--631.
Chazdon, R.L. 1986. Light variation and carbon gain in rain forest understory palms. J. Ecol. 74:995--1012.
Chazdon, R.L. 1988. Sunflecks and their importance to forest under-story plants. Adv. Ecol. Res. 18:1--63.
Chazdon, R.L. 1992. Photosynthetic plasticity of two rain forest shrub across natural gap transects. Oecologia 92:586--595.
Chazdon, R.L. and R.W. Pearcy. 1986. Photosynthetic responses to light variation in rainforest species. I. Induction under constant and fluctuating light conditions. Oecologia 69:517--523.
Chazdon, R.L. and R.W. Pearcy. 1991. The importance of sunflecks for forest understory plants. Bioscience 41:760--766.
Chazdon, R.L. and S. Kaufmann. 1993. Plasticity of leaf anatomy of two rain forest shrubs in relation to photosynthetic light acclima-tion. Funct. Ecol. 7:385--394.
Chen, H.Y.H., K. Klinka and G.J. Kayahara. 1996. Effects of light on growth, crown architecture, and specific leaf area for naturally established Pinus contorta and Pseudotsuga menziesii var. glauca. saplings. Can. J. For. Res. 26:1149--1157.
Chen, J.M. and T.A. Black. 1992. Foliage area and architecture of clumped plant canopy from sunfleck size distributions. Agric. For. Meteorol. 60:249--266.
Ellsworth, D.S. and P.B. Reich. 1992. Leaf mass per area, nitrogen content and photosynthetic carbon gain in Acer saccharum seedlings in contrasting forest light environments. Funct. Ecol. 6:423--435. Gildner, B.S., D.W. Larson. 1992. Photosynthetic response to
sun-flecks in the desiccation-tolerant fern Polypodium virginianum. Oecologia 89:390--396.
Green, R.N. and K. Klinka. 1994. A field guide to site identification and interpretation for the Vancouver Forest Region. B.C. Min. of For., Victoria, B.C. Land Manage. Handbk. No. 28, 285 p. Intrieri, C., G. Zerbi, L. Marchiol, S. Poni and T. Caiado. 1995.
Physiological response of grapevine leaves to lightflecks. Sci. Hor-tic. 61:47--59.
Kitajima, K. 1994. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419--428.
Koizumi, H. and Y. Oshima. 1993. Light environment and carbon gain of understory herbs associated with sunflecks in a warm temperate deciduous forest. Ecol. Res. 8:135--142.
Küppers, M., H. Timm, K. Orth, J. Stegemann, R. Stöber, H. Schneider, K. Paliwal, K.S.T.K. Karunaichamy and R. Ortiz. 1996. Effects of light environment and successional status on lightflecks use by understory trees of temperate and tropical forests. Tree Physiol. 16:69--80.
Letho, T. and J. Grace. 1994. Carbon balance of tropical tree seedlings: a comparison of two species. New Phytol. 127:455--463.
Leverenz, J.W. 1995. Shade shoot structure of conifers and the photo-synthetic response to light at two CO2 partial pressures. Funct. Ecol. 9:413--421.
Long, S.P. and J-E. Hällgren. 1993. Measurement of CO2 assimilation
by plants in the field and the laboratory. In Photosynthesis and Production in a Changing Environment. Eds. D.O. Hall, J.M.O. Scurlock, H.R. Bolhàr-Nordenkampf, R.C. Leegood and S.P. Long. Chapman & Hall, New York, pp 129--167.
Meidinger, D. and J. Pojar. 1991. Ecosystems of British Columbia. B.C. Min. of For., Victoria, B.C., Special Report Series No. 6, 330 p.
Pons, T.L. and R.W. Pearcy. 1992. Photosynthesis in flashing light in soybean leaves grown in different conditions. II. Efficiency of utilization of lightflecks. Plant, Cell Environ. 15:577--584. Pons, T.L., R.W. Pearcy and J.R. Seemann. 1992. Photosynthesis in
flashing light in soybean leaves grown in different conditions. I. Photosynthetic induction state and regulation of ribulose-1,5-bisphosphate carboxylase activity. Plant, Cell Environ. 15:569--570. Sims, D.A and R.W. Pearcy. 1994. Scaling sun and shade photosyn-thetic acclimation of Alocasia macrorrhiza to whole-plant perform-ance-1. Carbon balance and allocation at different daily photon flex densities. Plant, Cell Environ. 17:881--887.
Stenberg, P., E.H. DeLucia, A.W. Schoettle and H. Smolander. 1995. Photosynthetic light capture and processing from cell to canopy. In Resource Physiology of Conifers: Acquisition, Allocation, and Utilization. Eds. W.K. Smith and T.M. Hinckley. Academic Press, New York, pp 3--38.
Tang, Y., K. Hiroshi, S. Mitsumasa and W. Izumi. 1994. Characteristics of transient photosynthesis in Quercus serrata seedlings grown under lightfleck and constant regimes. Oecologia 100:463--469.
Tinoco-Ojanguren, C. and R.W. Pearcy. 1995. A comparison of light quality and quantity effects on the growth and steady-state and dynamic photosynthetic characteristics of three tropical tree spe-cies. Funct. Ecol. 9:222--230.
Tinoco-Ojanguren, C. and R.W. Pearcy. 1993. Stomatal dynamics and its importance to carbon gain in two rainforest Piper species. I. VPD effects on the transient stomatal response to lightflecks. Oecologia 94:388--394.
Walters, M.B., E.L. Kruger and P.B. Reich. 1993. Growth, biomass distribution and CO2 exchange of northern hardwood seedlings in high and low light: relationships and successional status and shade tolerance. Oecologia 94:7--16.
Whitehead, D. and R.O. Teskey. 1995. Dynamic response of stomata to changing irradiance in loblolly pine (Pinus taeda L.). Tree Physiol. 15:245--251.