www.elsevier.com/locate/jinsphys
Effect of helicokinins and ACE inhibitors on water balance and
development of
Heliothis virescens
larvae
qA. Seinsche
a,*, H. Dyker
b, P. Lo¨sel
a, D. Backhaus
b, J. Scherkenbeck
baCentral Research, Bayer AG, LWZ Monheim, Geb. 6220, Alfred-Nobel-Strasse 50, 40789 Monheim, Germany bCentral Research, Bayer AG, Q18, 51368 Leverkusen, Germany
Received 20 January 2000; received in revised form 15 March 2000; accepted 16 March 2000
Abstract
The diuretic activity of the helicokinins I (YFSPWG-amide), II (VRFSPWG-amide) and III (KVKFSAWG-amide) was tested onHeliothis virescenslarvae. All three kinins increased fluid secretion in isolated Malpighian tubules in a dose-dependent manner. Injections into the haemolymph caused a significant reduction in weight gain after 24 h and, in the case of helicokinin I, led to an increased mortality of 43% within 6 days. When truncated analogues of helicokinin I were tested in vitro, only the pentapeptide (FSPWG-amide) stimulated fluid secretion. Tested in vivo the pentapeptide did not influence normal development of the larvae. An alanine scan of helicokinin I showed that the substitution of phenylalanine, tryptophan and glycine led to a massive decrease or even loss of diuretic activity. The substitution of the other amino acids had no effect in vitro. The ACE inhibitors captopril, enalapril-maleate and lisinopril were tested for their influence on the development of the larvae. In combination with one of the helicokinins the in vivo injection of the ACE inhibitors led to increased rates of mortality and/or reductions in pupal weight. 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Water-balance; Neuropeptides; Helicokinins; Malpighian tubules; Ramsay Assay
1. Introduction
The regulation of water balance is a crucial aspect of homeostasis in terrestrial insects involving excretion of excess water via the Malpighian tubules and resorption in posterior regions of the hindgut. The processes of urine production has been shown to be under endocrine control. A number of peptides have been shown to pos-sess diuretic activity by their ability to stimulate fluid secretion in isolated Malpighian tubules in vitro. Most of them belong to two distinct families: the CRF-related peptides, which, as their name implies, show high sequence homologies with the vertebrate corticotropin/sauvagine/urotensin I family; and the insect kinins (for review see Coast 1996, 1998). The CRF-related peptides are 30–47 amino acids in length and, except for the peptides isolated from Tenebrio molitor
q Dedicated to Dr Pol Bamelis on the occasion of his 60th anniver-sary.
* Corresponding author. Tel.: +49-2173-384836; fax: + 49-2173-384932.
0022-1910/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 0 2 2 - 1 9 1 0 ( 0 0 ) 0 0 0 6 5 - 2
(Furuya et al. 1995, 1998), are amidated at the C-ter-minus. CRF-related peptides so far have been identified inManduca sexta(Kataoka et al., 1989; Blackburn et al., 1991), Acheta domesticus (Kay et al., 1991a), Locusta migratoria (Kay et al., 1991b; Lehmberg et al., 1991),
Musca domestica and Stomoxys calcitrans (Clottens et al., 1994), Periplaneta americana (Kay et al., 1992),
Tenebrio molitor (Furuya et al. 1995, 1998) and Hyles lineata (Furuya et al., 2000). Their widespread occur-rence suggests that they may be ubiquitous throughout the Insecta (for review see Coast 1996, 1998). These peptides activate cation transport mechanisms through the cells of the Malpighian tubule mediated by the second messenger cAMP.
The kinins are smaller molecules (6–15 amino acids) and share a common C-terminal sequence F-X1-X2
-W-G-amide with X1=S, H, N and Y and X2=S, P and A.
They are potent stimulators of in vitro secretion with EC50 values in the range 0.3–0.02 nM (Coast et al.,
character-ised as myotropic factors on the basis of the bioassay employed. Later, their ability to stimulate fluid secretion and/or to depolarise the transepithelial potential of Mal-pighian tubules ofAedes aegyptiandA. domesticuswas shown (Hayes et al., 1989; Coast et al., 1990). They also seem to be involved in the regulation of lipid concen-tration in the haemolymph and protein synthesis in the fat body (Goldsworthy et al., 1992). So far, kinins have been isolated fromA. domesticus(Holman et al., 1990),
L. migratoria (Schoofs et al., 1992), Culex salinarius
(Hayes et al., 1994),A. aegypti(Veenstra, 1994), Helico-verpa zea(Blackburn et al., 1995),P. americana(Predel et al., 1997), M. domestica (Holman et al., 1998) and
Drosophila melanogaster (Terhzaz et al., 1999). It is the C-terminal pentapeptide sequence common to all insect kinins that seems to be necessary to elicit both the diuretic and the myotropic response in vitro. In the cricketA. domestica the active core sequence FYPWG-amide is equipotent with the parent peptide in both the hindgut and Malpighian tubule assays (Holman et al., 1987a,b; Coast et al., 1990). Within this sequence the conformation of the aromatic residues Phe (residue 1) and Trp (residue 4) seems to play a key role in both activities. By contrast, position 2 tolerates wide vari-ations in the composition of the side-chain, although compounds containing aromatic residues were the most potent in bioassays (Nachman et al. 1991, 1994; Nach-man et al., 1993a).
Several members of the kinin family (Culex depolariz-ing factor II, Lem Kinin I and II and Lom kinin) were shown to be inactivated by an angiotensin-converting enzyme (ACE) from M. domestica through removal of a C-terminal dipeptide amide fragment (Lamango et al., 1997). In mammals, the enzyme, a Zn2+
-metallopeptid-ase, is responsible for the activation of the vasoconstric-tor angiotensin II and the inactivation of the vasodilavasoconstric-tor bradykinin. It also inactivates several amidated peptides by the removal of di- or tripeptide amides, e.g. Leu-/Met-enkephalinamide and substance P. The M. dom-estica ACE-related enzyme is inhibited by several human diuretics such as captopril, enalapril-maleate and lisinopril. The precise role of ACE in insects is not known, but its ability to degrade a variety of different neuropeptides and its high titre in the haemolymph sug-gest that this endopeptidase plays a role in the degra-dation of regulatory peptides in insects. The effects of human ACE inhibitors on larval development of M. sexta (Lamango, 1994) and the lethal phenotype of two AnCE mutants ofDrosophila melanogaster(Tatei et al., 1995), however, indicate that the enzyme is of vital importance for normal development.
For our study, we chose the helicokinins as represen-tatives of the kinin family occurring in an important pest species. The primary aim of the current study was to determine whether synthetic helicokinins based on those isolated fromH. zeacould be shown to affect water
bal-ance in the closely related species Heliothis virescens. A further aim was to investigate the role of ACE in the regulation of these neuropeptides in an economically important species of noctuid.
2. Methods and materials
2.1. Insects
H. virescens were reared on artificial diet and main-tained under natural light conditions and RT. The Mal-pighian tubules (MTs) for the in vitro bioassay were obtained from 2- to 4-day-old fifth-instar larvae.
2.2. Test substances
All peptide sequences tested were synthesized on an automatic peptide synthesizer (Applied Biosystems 431A) using Polystyrene AM RAM amide resin (RAPP-Polymere, Tu¨bingen, Germany) following standard Fmoc/tBu protocols. The resin-cleavage and deprotec-tion steps were performed with trifluoroacetic acid (TFA)/water/thioanisol/1,2-ethanedithiol (10:0.5:0.5:0.25). The crude peptides were precipitated with ether and lyophilized from water. The peptides were further pur-ified by RP–HPLC (acetonitrile/water, 0.1% TFA) using isocratic conditions. The peptides were characterized by ESI–MS.
The ACE inhibitors were obtained from Sigma, Ger-many.
2.3. Bioassays
2.3.1. In vitro
Diuretic activity was measured using a modification of the Ramsay assay (Ramsay, 1954). The MTs were dissected from the larvae and were placed individually in 30µl droplets of Pringle’s saline (154 mM NaCl, 0.27 mM KCl, 0.18 mM CaCl2, 22 mM glucose; Pringle,
equili-bration period the MTs were allowed to secrete without any stimulation for 30 min (control rate), and the diam-eters of secreted fluid droplets were measured using an ocular micrometer. The droplets were then discarded. Fresh saline (30 µl) containing the test substances was added to the saline. After 30 min the diameters of the secreted droplets were measured again.
2.3.2. In vivo
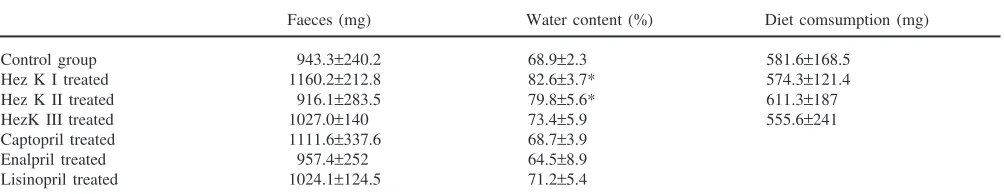
In vivo effects were assessed during the feeding-phase of fifth-instar larvae ofH. virescens(2–3 days after last moult, 250–300 mg of weight). Larvae were temporarily immobilised by cooling on ice before being placed in isolated Petri dishes. Injections were carried out manu-ally using a 10 µl glass syringe with 5 µl of the test compound being applied by inserting a 26-gauge needle through the cuticle at the base of the last abdominal limb. Controls were treated with saline. The larvae were weighed and placed individually on food. They were reweighed at least every 24 h and further development until the pupal stage (12–15 days duration) was recorded. The faeces were collected every day until pupation and weighed. Water contents of the faeces were calculated by subtracting faecal weight after drying (110°C, 24 h).
Differences between control and treatment means were tested for statistical significance using a two-tailed, paired Student’s t-test.
3. Results
3.1. Ramsay assay
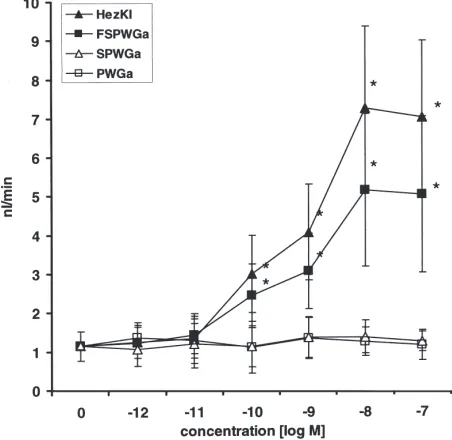
The effect of the three synthetic helicokinins (HezK I–III) on secretory activity of isolated Malpighian tubules from fifth-instarH. virescensis shown in Fig. 1. All three helicokinins stimulated fluid secretion in a dose-dependent manner over a range from 10212to 1027
M with EC50 concentrations of 2.9×10211M (HezK I),
2×10211 M (HezK II) and 3.6×10210 M (HezK III). At
1029 M the kinins increased fluid secretion by 410%
(HezK I), 354% (HezK II) and 207% (HezK III). The highest rates of secretion were induced with concen-trations of 1028M with increases of 6.1 times for HezK
I, 5.3 times for HezK II and 4.2 times for HezK III.
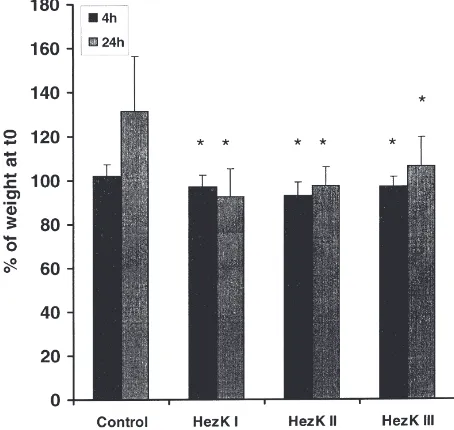
3.2. In vivo microinjections
The injection of the helicokinins led to a reduction in weight gain in all cases (Fig. 2). Four hours after the treatment, the control group gained approximately 2% in weight, while the treated animals lost weight (2.8% HezK I; 7.07% HezK II and 2.6% HezK III). After 24 h the control animals (injected with saline) had generally gained about 29% in weight while the HezK III treated
Fig. 1. Responses of isolated Malpighian tubules from fifth-instarH. virescensto helicokinin I–III. Each point represents the mean (±SD) of 10–15 observations. *Significant differences between control and test means (p#0.005).
Fig. 2. Effects of helicokinin I–III on weight gain of fifth-instar lar-vae ofH. virescens 4 h and 24 h after the treatment. Larvae were injected with 50 pmol of the helicokinin in 5µl saline. The animals were permitted to feed before and after the treatment. The control group was treated with saline. Mean values±SD are shown for groups of 10–15 larvae. *Significant difference (p#0.005).
Table 1
Rates of mortality (in percent) after combined injections with helicokinins (50 pmol) and ACE inhibitors (1 µmol) 5 days after the treatment. Each set of experiments comprised 15–20 larvalH. virescens. The controls were injected with saline. Significant differences are marked with an asterisk (p,0.01)
Injection of saline captopril enalapril-maleate lisinopril
+saline 18.8±3.6 7.3±5.9 4.5±3.6 8.5±2.8
+HezK I 43.8±6.7* 83.3±12.3* 55.5±6.9* 33.3±5.6*
reduced pupal weight
+HezK II 11.1±5.3 22.2±5.7 50±8.3* 45.4±7.2*
reduced pupal weight
+HezK III 16.7±3.7 22.2±4.2 25±9.8 33.3±4.3*
treated groups (Table 2). The faeces from the treated groups, however, contained more water (80–85%) than in the controls (65–75%) (Table 2). Of the HezK I treated animals, 43% died within 6 days after the treat-ment, the levels of mortality in HezK II and III treated larvae did not differ significantly from the controls (Table 1).
3.3. Determination of the active core sequence of HezK I
To determine the active core sequence of HezK I (YFSPWG-amide), truncated sequences were tested in vitro (Fig. 3) over the same concentration range as the original peptide (10212–1027M). Only the pentapeptide
(FSPWG-amide) was able to induce a dose-dependent increase in secretion similar to that found for HezK I (EC50=1.8×10210M). Neither the tetra- (SPWG-amide)
Fig. 3. Responses of isolated Malpighian tubules from fifth-instarH. virescens to helicokinin I and truncated analogues. Each point rep-resents the mean (±SD) of 10–15 observations. *Significant differ-ences (p#0.005).
nor the tripeptide (PWG amide) increased the fluid secretion significantly above the control levels.
Although the C-terminal pentapeptide sequence of HezK I was sufficient to elicit a complete in vitro response, in vivo it was not able to mimic the activity of the parent peptide (data not shown). The treated larvae showed no significant loss of weight or disruption in development.
To evaluate the significance of the amino acid side-chains, an alanine scan was carried out. Analogues were synthesised in which one of the six amino acids of the original peptide was substituted by an alanine residue. All peptides were tested in the Ramsay assay over the same concentration range as HezK I. The results are shown in Fig. 4. The exchange of Tyr (residue 1), Ser (residue 3) and Pro (residue 4) had no significant effect on the dose-dependent increase in secretion compared with the results for HezK I. Substitution of Phe (residue 2), Trp (residue 5) and Gly (residue 6), however, led to a complete loss of diuretic activity of the analogues.
Fig. 5. Effects of ACE inhibitors on weight gain of fifth-instar H. virescens 24 h after the injection. The animals were treated with 1 µmol of the ACE inhibitor in 5µl saline. The control group was treated with saline. The animals were allowed to feed before and after the treatment. Mean values of control and test animals (N=10–12) did not differ significantly (p.0.005).
3.4. ACE inhibitors
The Ramsay assay and in vivo microinjections were used to investigate whether the ACE inhibitors captopril, enalapril-maleate and lisinopril could influence in vitro fluid secretion or larval development. In the Ramsay assay the substances were tested over a concentration range from 1027 to 1024 M. None of the three ACE
inhibitors affected the rate of fluid secretion in vitro (data not shown). Microinjection of 1 µmol of these com-pounds had no effect on larval development (Fig. 5). The amount and water content of faeces produced by the treated animals did not differ significantly from the con-trols (Table 2).
In a second set of experiments the larvae were injected with a combination of 50 pmol HezK and 1µmol ACE inhibitor in 5 µl of saline. The results (Table 1) show that 83.3% of the larvae treated with a combination of HezK I and captopril died within 5 days after the
injec-Table 2
Comparison of the amounts of faeces (until pupation)/diet consumption (within 24 h) and water content of faeces between saline-injected larval
H. virescens(control group) and larvae treated with helicokinins (50 pmol) and ACE inhibitors (1µmol). Each set of experiments comprised 15– 20 larvalH. virescens. Significant differences are marked with an asterisk (p,0.01)
Faeces (mg) Water content (%) Diet comsumption (mg)
Control group 943.3±240.2 68.9±2.3 581.6±168.5
Hez K I treated 1160.2±212.8 82.6±3.7* 574.3±121.4
Hez K II treated 916.1±283.5 79.8±5.6* 611.3±187
HezK III treated 1027.0±140 73.4±5.9 555.6±241
Captopril treated 1111.6±337.6 68.7±3.9
Enalpril treated 957.4±252 64.5±8.9
Lisinopril treated 1024.1±124.5 71.2±5.4
tion, compared with 43.3% for HezK I alone (see above). The surviving animals developed to pupae weighing 14.2% less than the controls. A combination of HezK I and enalapril-maleate increased mortality to a level of 55.5%. HezK II and III, which when injected alone did not increase mortality, caused 30–50% mortality in com-bination with the ACE inhibitors. Enalapril-maleate combined with HezK II led to a mortality rate of 50% and a weight reduction of 13% for the surviving pupae. The combination of HezK with lisinopril led to a sig-nificant increase in mortality: 33.3% for HezK III and 45.5% for HezK II.
4. Discussion
This is the first study to investigate the effects of kin-ins (helicokinkin-ins) on the regulation of water balance and development of lepidopteran larvae. While the helicokin-ins used in this study were originally isolated from the noctuid moth H. zea, their diuretic activity has so far only been shown in isolated Malpighian tubules from the adult sphingid M. sexta (Blackburn et al., 1995). They seemed to have no in vivo effects on adultH. zea when tested with a post-injection assay (Blackburn et al., 1995). It is, however, known that larval lepidopteran tissues do contain kinins. In the caterpillar of Agrotis segetum, leucokinin-like immunoreactive fibres were found in the perivisceral organs (Cantera et al., 1992). In the abdominal ganglia of larvalM. sexta, leucokinin-like immunoreactivity is colocated with Mas DP immu-noreactivity (Chen et al., 1994). Furthermore, nerves containing both types of immunoreactive material were found to project into the cryptonephridial complex of the larvae (Chen et al., 1994).
All three synthetic helicokinins stimulated in vitro fluid secretion in Malpighian tubules from larvalH. vire-scensin a dose-dependent manner. The relative potency of the kinins tested, in terms of their EC50 values
2×10211M and 3×10210M. As for the cricket,
Malpigh-ian tubules ofH. virescensseem to be about two orders of magnitude more sensitive to the helicokinins than were the tubules of A. aegypti to leucokinin, where a concentration above 1029 M is necessary to increase
fluid secretion (Hayes et al., 1989). At concentrations less than 1029 M, leucokinin III inhibited the secretion
ofAedestubules (Hayes et al., 1989). No such inhibition was observed for the helicokinins.
The binding affinities of the helicokinins, as reflected by their EC50values, vary in a sequence HezK I.HezK
II.HezK III. For M. sexta the reverse sequence was found: HezK III.HezK II.HezK I (Blackburn et al., 1995).
Coast et al. (1990) showed that the application of ach-etakinins at 1029 M doubled the rate of fluid secretion
in Malpighian tubules of Acheta. For H. virescens the values for HezK I and II were higher (3.1 times for HezK I and 2.5 times for HezK II). The highest rates of secretion were induced with concentrations at 1028 M.
InM. domesticasuch increases (4–5 times) were reached at 1029 M concentrations of muscakinin (Holman et al.,
1998) and 1026 M with other myokinins (Iaboni et al.,
1998). As already mentioned, both the CRF-related pep-tides and the kinins are implicated in the control of diuresis in insect Malpighian tubules. This is particularly well illustrated in the case of the housefly,M. domestica, where representatives of both classes are involved. In this species the stimulatory effect of the tested kinins was comparable with that of the crude extracts of the thoracal-abdominal ganglia. In contrast, the synthetic
Musca-DP, forskolin and cAMP analogues were only able to double the rate of secretion (Clottens et al., 1994). In crickets and locusts the CRF-related peptides have similar diuretic activity to extracts of the corpora cardiaca (Kay et al., 1991b; Coast and Kay, 1994). The results with theMuscatubules suggest that in the house fly entry of Cl2into the tubule lumen is the rate-limiting
step (Iaboni et al., 1998) and this process seems to be mainly regulated by the muscakinin. The only lepidop-teran CRF -related peptides so far isolated were found in M. sexta (Kataoka et al., 1989; Blackburn et al., 1991). To date, there are no reports of such peptides in noctuid species. The results of in vitro bioassays presented here and first tests with 8-bromo cAMP and dibutyryl cAMP (as second messenger of the CRF-related peptides) indicate that inHeliothisthe kinins also may be major regulators of diuresis (unpublished data). In view of the results of the present study which dem-onstrate that the helicokinins affect fluid secretion in iso-lated larval Malpighian tubules of H. virescens, it is tempting to suggest that these kinins could influence the regulation of water balance in the living insect.
Just as water balance in both larval and adultM. sexta
was influenced by CRF-related peptides Mas DP I and II (Kataoka et al., 1989; Blackburn et al., 1995),H.
vire-scens larvae administered Mas DP I fed less and lost weight (Keeley et al., 1992). In our experiments the
Heliothis larvae were injected with 50 pmol of the hel-icokinins. This amount per larva (weight 250–300 mg; haemolymph vol. 50–60 µl) should give a final haemo-lymph concentration of approximately 1µM kinin. Since the in vitro bioassay showed highest increases in urine production with concentrations of only 0.01 µM, more than sufficient amounts of kinins for a physiological response should have been present in the caterpillar’s body. By comparison, the normal haemolymph concen-tration was estimated forA. domesticus andL. maderae
to be in the nanomolar range (Muren et al., 1993; Chung et al., 1994). The lower than normal weight gains follow-ing the injection coupled with increased water loss in the faeces are consistent with the hypothesised action of the tested kinins. The higher excretion of fluid alone does not, however, explain sufficiently the loss of weight observed for helicokinin I and II. Chung et al. (1994) stated that in crickets which had starved for 48 h without access to water the amount of leucokinin-like immunore-activity in the haemolymph increased 10-fold. They speculated that the achetakinins may play a role in energy mobilisation during starvation. This idea is sup-ported by the results of Goldsworthy et al. (1992) who examined the effect of kinins on haemolymph compo-sition of crickets and locusts. Nanomolar doses of ach-etakinins led to increases in haemolymph lipid concen-tration similar to those caused by the adipokinetic hormone and also inhibited haemolymph protein syn-thesis. Based on these findings and our own results we speculate that the injected high doses of kinins induced a “starvation” signal in the Heliothislarvae, resulting in mobilisation of their energy stores. At the same time the digested diet was not efficiently exploited. Together with the increased excretion of fluid this may have led to the massive reduction in weight gain. In the case of helicoki-nin I, this effect seemed to be sufficiently long-lasting to kill some of the treated larvae.
Since helicokinin I produced the most pronounced effects, further work was concentrated on this peptide. A key aim was to obtain more information about which properties of the peptide are essential to induce the diuretic activity.
Our experiments with truncated sequences of helicoki-nin I showed that it is only the pentapeptide fragment FSPWG-NH2 that is necessary for the in vitro activity.
The EC50 values of the key sequence and the parent
sequence are not significantly different. These results correspond with those obtained for the achetakinins and the leucokinins: the fragments FFSWG-NH2 and
FYSWG-NH2are as potent as the parent achetakinin and
(EC50=6.2×10210M) (Nachman et al., 1992). InM.
dom-estica, however, a truncated analogue of the muscakinin was 1000-fold less active than the parent peptide (Holman et al., 1998); here the N-terminus seems to be quite important for an effective binding to the receptor. The pentapeptide was not able to mimic the in vivo effects of the native peptide. This suggests that the N-terminal tyrosine may be necessary for the normal action of the peptide. It may provide protection against degra-dation by peptidases or be essential for the binding to a protective haemolymph protein.
The differences at position 2 and 3 of the three core sequences of achetakinins (FFSWG-NH2), leucokinins
(FYSWG-NH2) and helicokinin I (FSPWG-NH2)
indi-cate that these residues are not of great importance for binding at the target side. Especially position 2 in the achetakinins and leucokinins tolerates wide variations in side-chain character ranging from acidic to basic or hydrophobic to lipophobic (Nachman et al., 1990; Nach-man et al., 1993a,b). The results of our alanine scan showed that the substitution of serine at position 3 and proline at position 4 of helicokinin I had little effect on the diuretic activity; the two residues probably function merely as a spacer to keep the correct distances between the side-chains or as a secondary structure element.
Similarly, the exchange of tyrosine for alanine at pos-ition 1 did not decrease the stimulatory effect of the mol-ecule significantly. Results of microinjections with AFSPWG-NH2 and similar analogues suggest that the
tyrosine residue may have a protective role during trans-port through the haemolymph (work in progress).
In contrast, the substitution of phenylalanine, trypto-phan and glycine led to a striking decrease or even a complete loss of diuretic activity. The results for phenyl-alanine and tryptophan agree with those for the achetaki-nins and the leucokiachetaki-nins where the exchange of both residues induced a loss of diuretic and myotropic activity (Nachman et al., 1990; Roberts et al., 1997). For glycine, the results are quite different; for the leucokinins the substitution of this residue by alanine only slightly increased the threshold concentration from 0.1×10210M
to 0.9×10210M in the diuretic bioassay (Roberts et al.,
1997). For helicokinin I this exchange led to a complete loss of diuretic activity. These three amino acids (F, W and G) seem to be essential to allow binding at the tar-get side.
Our results clearly show that the helicokinins are bio-logically active inH. virescenslarvae and that they may be important for normal development. Their amidated C-terminus protects them against degradation by carboy-xpeptidases present in most tissues. Insect enzymes cap-able of removing this C-terminus are of special interest since they may have a key role in regulating titres of amidated neuropeptides. The ACE-related enzyme iso-lated from M. domestica and D. melanogaster is one such enzyme (Lamango and Isaac, 1994; Tatei et al.,
1995). The insect enzyme is susceptible to human ACE inhibitors, such as captopril, lisinopril or fosanopril (Lamango and Isaac, 1994; Lamango et al., 1996). Ten micromoles of captopril inhibited the degradation of [D Ala2, Leu] enkephalin completely (Lamango and Isaac,
1994). When injected into larvae ofH. virescens1µmol of captopril, enalapril-maleate and lisinopril had no obvious effect, as the development of treated larvae did not differ from the control group. Manduca larvae treated with ACE-inhibitor-1 and ACE-inhibitor-2, by contrast, showed reduced growth rates, paralysis, incom-plete shedding of cuticle during the moults and even death as a result of the treatment (Lamango, 1994).
In our tests only the combinations of helicokinins and ACE inhibitors induced weight loss and/or higher mor-tality rates in final instar larvae of H. virescens, but the animals that died showed no signs of paralysis. It appears that there is an ACE-related activity inHeliothis
larvae which is susceptible to human ACE inhibitors and whose affinity to different kinins (Lamango et al., 1997) enables it to break down the injected overdoses. The inhibition of this hydrolysis by ACE inhibitors probably increased the persistency of the high kinin titre which seemed to induce the serious disruptions (see above) in development. The fact that the AnCE gene is expressed throughout all developmental stages and that mutations of this gene have lethal effects indicates that ACE has a vital function in insects.
Our experiments clearly show that the helicokinins are highly potent diuretic substances in vitro and there is indirect evidence in support of an in vivo effect as well. Their effect on this and other functions, such as the con-trol of muscles, demonstrates their key role in important physiological processes in normal development of larval
H. virescensand suggests that insect kinin-regulated pro-cesses may be an interesting target for the development of novel insecticides.
References
Blackburn, M.B., Kingan, T.G., Bodnar, W., Shabanowitz, J., Hunt, D.F., Wagner, R.M. et al., 1991. Isolation and identification of a new diuretic peptide from the tobacco hornworm,Manduca sexta. Biophysical and Biochemical Research Communications 181, 927. Blackburn, M.B., Wagner, R.M., Shabanowitz, J., Kochansky, J.P., Hunt, D.F., Raina, A.K., 1995. The isolation and identification of three diuretic kinins from the abdominal ventral nerve cord of adult
Helicoverpa zea. Journal of Insect Physiology 41 (8), 723–730. Cantera, R., Hanson, B.S., Hallberg, E., Nassel, D.R., 1992.
Postem-bryotic development of leucokinin I-immunoreactive neurons innervating a neurohemal organ of the turnip mothAgrotis segetum. Cell Tissue Research 269, 65–77.
Chen, Y.T., Veenstra, J.A., Hagedorn, H., Davis, N.T., 1994. Leucoki-nin and diuretic hormone immunoreactivity of neurons in the tob-acco hornworm,Manduca sexta, and colocalization of the immuno-reactivity in lateral neurosecretory-cells of abdominal ganglia. Cell Tissue Research 278, 493–507.
tissue titres of achetakinins in the house cricketAcheta domesticus: effect of starvation and dehydration. Journal of Experimental Biology 193, 307–319.
Clottens, F.L., Holman, G.M., Coast, G.M., Totty, N.F., Hayes, T.K., Kay, I. et al., 1994. Isolation and characterisation of a diuretic pep-tide common to the house fly and the stable fly. Peppep-tides 15, 971–979.
Coast, G.M., 1996. Neuropeptides implicated in the control of diuresis in insects. Peptides 17, 327–336.
Coast, G.M., 1998. Insect diuretic peptides: structures, evolution and actions. American Zoology 38, 442–449.
Coast, G.M., Kay, I., 1994. The effects of Acheta diuretic peptide on isolated Malpighian tubules from the house cricket,Acheta dom-esticus. Journal of Experimental Biology 187, 225–243.
Coast, G.M., Holman, G.M., Nachman, R.J., 1990. The diuretic activity of a series of cephalomyotropic neuropeptides, the achetak-inins, on the isolated Malpighian tubules of the house cricket, Ach-eta domesticus. Journal of Insect Physiology 36, 481–488. Furuya, K., Schegg, K.M., Wang, H., King, D.S., Schooley, D.A.,
1995. Isolation and identification of a diuretic hormone from the mealworm,Tenebrio molitor. Proceedings of the National Acad-emy Sciences USA 92, 12323–12327.
Furuya, K., Schegg, K.M., Schooley, D.A., 1998. Isolation and identi-fication of a second diuretic hormone fromTenebrio molitor. Pep-tides 19, 619–626.
Furuya, K., Harper, A.H., Schegg, M.S., Schooley, D.A., 2000. Iso-lation and characterization of CRF-related diuretic hormones from the whitelined sphinx mothHyles lineata. Insect Biochemistry and Molecular Biology 30, 127–133.
Goldsworthy, G.J., Coast, G.M., Wheeler, H., Cusinato, O., Kay, I., Khambay, B., 1992. The structural and functional activity of neuro-peptides. In: Crampton, J.M., Eggleston, P. (Eds.), Royal Entomo-logical Society Symposium on Insect Molecular Sciences. Aca-demic Press, London, pp. 205–255.
Hayes, T.K., Pannabecker, T.L., Hinkley, D.J., 1989. Leucokinins, a new family of ion transport stimulators and inhibitors in insect Mal-pighian tubules. Life Sciences 44, 1259–1266.
Hayes, T.K., Holman, G.M., Pannabecker, T.L., 1994. Culekinin depo-larizing peptide: a mosquito leucokinin-like peptide that influences insect Malpighian tubule ion transport. Regulatory Peptides 52, 245–248.
Holman, G.M., Cook, B.J., Nachman, R.J., 1986a. Isolation, primary structure and synthesis of two neuropeptides from Leucophaea maderae: members of a new family of cephalomyotropins. Com-parative Biochemistry and Physiology [C] 84, 205–211.
Holman, G.M., Cook, B.J., Nachman, R.J., 1986b. Primary sequence and synthesis of two additional neuropeptides from Leucophaea maderae: members of a new family of cephalomyotropins. Com-parative Biochemistry and Physiology [C] 84, 271–276.
Holman, G.M., Cook, B.J., Nachman, R.J., 1987a. Isolation, primary structure and synthesis of leucokinin V and VI: myotropic peptides of Leucophaea maderae. Comparative Biochemistry and Physi-ology [C] 88, 27–30.
Holman, G.M., Cook, B.J., Nachman, R.J., 1987b. Isolation, primary structure and synthesis of leucokinin VII and VIII: the final mem-bers of this new family of cephalomyotropic peptides isolated from head extracts ofLeucophaea maderae. Comparative Biochemistry and Physiology [C] 88, 31–34.
Holman, G.M., Nachman, R.J., Wright, M.S., 1990. Comparative aspects of insect myotropic peptides. In: Epple, A., Scanes, C.G., Stetson, M.H. (Eds.), Progress in Comparative Endocrinology. Wiley-Liss, New York, pp. 35–39.
Holman, G.M., Nachman, R.J., Coast, G.M., 1998. Isolation, charac-terization and biological activity of a diuretic myokinin neuropep-tide from the house fly,Musca domestica. Peptides 20, 1–10. Iaboni, A., Holman, G.M., Nachman, R.J., Orchard, I., Coast, G.M.,
1998. Immunocytochemical localisation and biological activity of
diuretic peptides in the housefly, Musca domestica. Cell Tissue Research 294, 549–560.
Kataoka, H., Troetschler, R.G., Li, J.P., Kramer, S.J., Carney, R.L., Schooley, D.A., 1989. Isolation and identification of a diuretic hor-mone from the tobacco hornworm,Manduca sexta. Proceedings of the National Academy of Sciences USA 86, 2976–2980. Kay, I., Coast, G.M., Cusinato, O., Wheeler, C.H., Totty, N.F.,
Gold-sworthy, G.J., 1991a. Isolation and characterisation of a diuretic peptide fromAcheta domesticus: evidence for a family of insect diuretic peptides. Biological Chemistry Hoppe–Seyler 372, 505– 512.
Kay, I., Wheeler, C.H., Coast, G.M., Totty, N.F., Cusinato, O., Patel, M. et al., 1991b. Characterization of a diuretic peptide from
Locusta migratoria. Biological Chemistry Hoppe–Seyler 372, 929–934.
Kay, I., Patel, M., Coast, G.M., Totty, N.F., Mallet, A.I., Goldsworthy, G.J., 1992. Isolation, characterisation and biological activity of a CRF-related diuretic peptide fromPeriplaneta americanaL. Regu-latory Peptides 42, 111–122.
Keeley, L.L., Chung, J.S., Hayes, T.K., 1992. Diuretic and antifeedant actions byManduca-sextadiuretic hormone in lepidopteran larvae. Experientia 48, 1145–1148.
Lamango, N.S., 1994. Characterization of neuropeptides in the house fly,Musca domestica. Ph.D. thesis, University of Leeds. Lamango, N.S., Isaac, R.E., 1994. Identification and properties of a
peptidyl dipeptidase in the house fly, Musca domestica, that resembles mammalian angiotensin-converting enzyme. Biochemi-cal Journal 299, 651–657.
Lamango, N.S., Sajid, M., Isaac, R.E., 1996. The endopeptidase activity and the activation by Cl2 of angiotensin-converting
enzyme is evolutionarily conserved—purification and properties of an angiotensin-converting enzyme from the house fly,Musca dom-estica. Biochemical Journal 314, 639–646.
Lamango, N.S., Nachman, R.J., Hayes, T.K., Strey, A., Isaac, R.E., 1997. Hydrolysis of insect neuropeptides by an angiotensin-con-verting enzyme from the house fly,Musca domestica. Peptides 18, 47–52.
Lehmberg, E., Ota, R.B., Furuya, K., King, D.S., Applebaum, S.W., Ferenz, H.J. et al., 1991. Identification of a diuretic hormone of
Locusta migratoria. Biochemical and Biophysical Research Com-munications 179, 1036–1041.
Muren, E.J., Lindquist, T.C., Na¨ssel, D.R., 1993. Quantitative determi-nation of myotropic neuropeptide in the nerve system of the cock-roachLeucophaea maderae: distribution and release of leucokinins. Journal of Experimental Biology 179, 289–300.
Nachman, R.J., Roberts, V.A., Holman, G.M., Trainer, J.A., 1990. Consensus chemistry and confirmation of an insect neuropeptide family analogous to tachykinins. In: Epple, A., Scanes, C.G., Stet-son, M.H. (Eds.), Progress in Comparative Endocrinology. Wiley-Liss, New York, pp. 60–66.
Nachman, R.J., Holman, G.M., Haddon, W.F., Vensel, W.H., 1991. An active pseudopeptide analog of the leucokinin insect neuropeptide family. International Journal of Peptide and Protein Research 37, 220–223.
Nachman, R.J., Coast, G.M., Holman, G.M., Haddon, W.F., 1992. A bifunctional heterodimeric insect neuropeptide analog. Inter-national Journal of Peptide and Protein Research 40, 423–428. Nachman, R.J., Holman, G.M., Haddon, W.F., 1993a. Leads for insect
neuropeptide mimetic development. Archives of Insect Biochemis-try and Physiology 22, 181–197.
Nachman, R.J., Holman, G.M., Hayes, T.K., Beier, R.C., 1993b. Acyl, pseudotetrapeptide, tripeptide and dipeptide active-core analogs of insect neuropeptides. International Journal of Peptide and Protein Research 42, 372–377.
Pest Management Agents. ACS Symposium Series, vol. 551. ACS, Washington, DC, pp. 210–219.
Predel, R., Kellner, R., Rapus, J., Penzlin, H., Ga¨de, G., 1997. Isolation and structural elucidation of eight kinins from the retrocerebral complex of the American cockroach, Periplaneta americana. Regulatory peptides 71, 199–205.
Pringle, J.W.S., 1938. Proprioception in insects. A new type of chemi-cal receptor from palps of the cockroach. Journal of Experimental Biology 15, 101–135.
Ramsay, J.A., 1954. Active transport of water by the Malpighian tubules of the stick insect, Dixippus morosus (Orthoptera Phasmidae). Journal of Experimental Biology 31, 104–413. Roberts, V.A., Nachman, R.J., Coast, G.M., Hariharan, J.S., Holman,
G.M., Williams, H. et al., 1997. Consensus chemistry and ß-turn conformation of the active core of the insect kinin neuropeptide family. Chemistry and Biology 4, 105–117.
Schoofs, L., Holman, G.M., Proost, P., van Damme, J., Hayes, T.K., De Loof, A., 1992. Locustakinin, a novel myotropic peptide from
Locusta migratoria, isolation, primary structure and synthesis. Regulatory Peptides 37, 49–57.
Tatei, K., Cai, H., Ip, Y.T., Levine, M., 1995. Race—aDrosophila
homolog of the angiotensin-converting enzyme. Mechanisms of Development 51, 157–168.
Terhzaz, S., O’Connell, F.C., Pollock, V.P., Davies, S.A., Veenstra, J.A., Dow, J.A.T., 1999. Isolation and characterization of a leucoki-nin-like peptide of Drosophila melanogaster. Journal of Experi-mental Biology, submitted.